Abstract
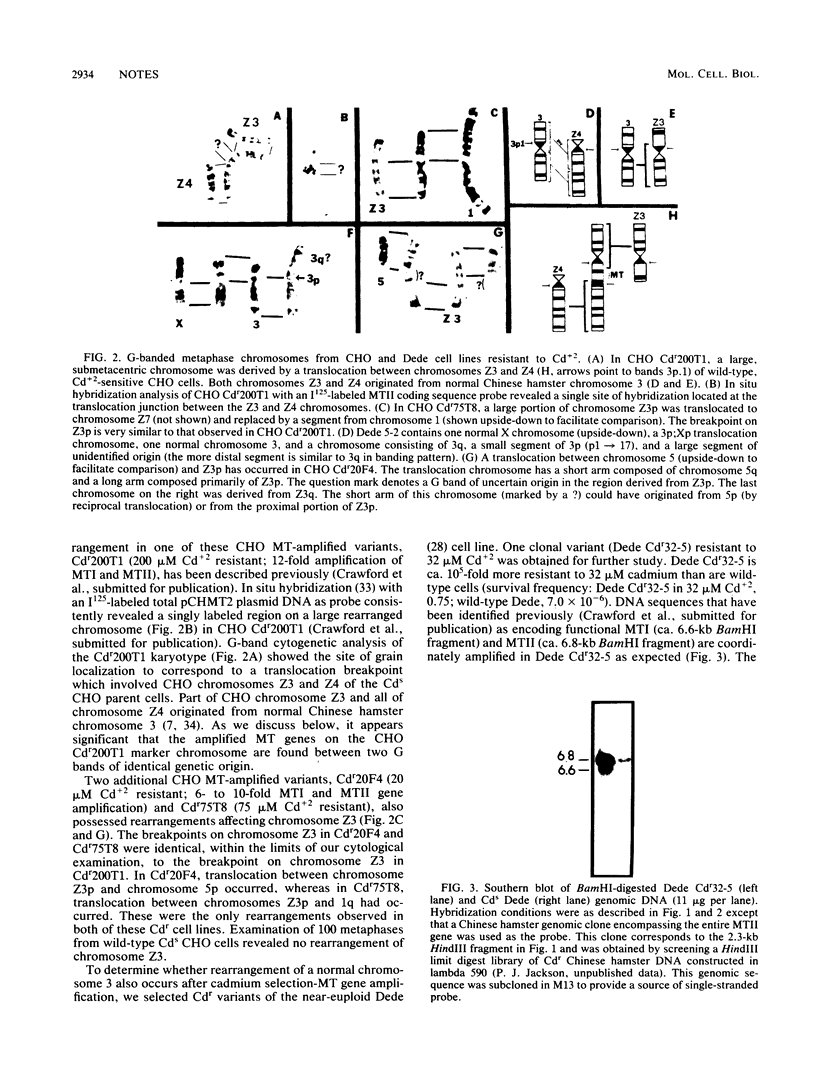
Cadmium resistant (Cdr) variants with coordinately amplified metallothionein I and II (MTI and MTII) genes have been derived from both Chinese hamster ovary and near-euploid Chinese hamster cell lines. Cytogenetic analyses of Cdr variants consistently revealed breakage and rearrangement involving chromosome 3p. In situ hybridization with a Chinese hamster MT-encoding cDNA probe localized amplified MT gene sequences near the translocation breakpoint involving chromosome 3p. These observations suggested that both functionally related, isometallothionein loci are linked on Chinese hamster chromosome 3. Southern blot analyses of DNAs isolated from a panel of Chinese hamster X mouse somatic cell hybrids which segregate hamster chromosomes confirmed that both MTI and MTII are located on chromosome 3. We speculate that rearrangement of chromosome 3p could be causally involved with the amplification of MT genes in Cdr hamster cell lines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Stallings R. L., Friend K. K., Siciliano M. J. Gene mapping and linkage analysis in Chinese hamster: assignment of the genes for APRT, LDHA, IDH2, and GAA to chromosome 3. Somatic Cell Genet. 1983 Jul;9(4):477–487. doi: 10.1007/BF01543048. [DOI] [PubMed] [Google Scholar]
- Andersen R. D., Birren B. W., Ganz T., Piletz J. E., Herschman H. R. Molecular cloning of the rat metallothionein 1 (MT-1) mRNA sequence. DNA. 1983;2(1):15–22. doi: 10.1089/dna.1.1983.2.15. [DOI] [PubMed] [Google Scholar]
- Beach L. R., Palmiter R. D. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2110–2114. doi: 10.1073/pnas.78.4.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Palmiter R. D. The metallothionein-I gene maps to mouse chromosome 8: implications for human Menkes' disease. Hum Genet. 1983;64(1):61–64. doi: 10.1007/BF00289481. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. EFFECT OF HALOGENATED PYRIMIDINES AND THYMIDINE ON GROWTH OF L-CELLS AND A SUBLINE LACKING THYMIDINE KINASE. Exp Cell Res. 1964 Jan;33:19–28. doi: 10.1016/s0014-4827(64)81006-5. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flintoff W. F., Livingston E., Duff C., Worton R. G. Moderate-level gene amplification in methotrexate-resistant Chinese hamster ovary cells is accompanied by chromosomal translocations at or near the site of the amplified DHFR gene. Mol Cell Biol. 1984 Jan;4(1):69–76. doi: 10.1128/mcb.4.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gick G. G., McCarty K. S., Sr Amplification of the metallothionein-I gene in cadmium- and zinc-resistant Chinese hamster ovary cells. J Biol Chem. 1982 Aug 10;257(15):9049–9053. [PubMed] [Google Scholar]
- Griffith B. B., Walters R. A., Enger M. D., Hildebrand C. E., Griffith J. K. cDNA cloning and nucleotide sequence comparison of Chinese hamster metallothionein I and II mRNAs. Nucleic Acids Res. 1983 Feb 11;11(3):901–910. doi: 10.1093/nar/11.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C. E., Griffith J. K., Tobey R. A., Walters R. A., Enger M. D. Molecular mechanisms of cadmium detoxification in cadmium-resistant cultured cells: role of metallothionein and other inducible factors. Dev Toxicol Environ Sci. 1982;9:279–303. [PubMed] [Google Scholar]
- Hildebrand C. E., Tobey R. A., Campbell E. W., Enger M. D. A cadmium-resistant variant of the Chinese hamster (CHO) cell with increased metallothionein induction capacity. Exp Cell Res. 1979 Dec;124(2):237–246. doi: 10.1016/0014-4827(79)90199-x. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Bonnet J., Li D. W., Ts'o P. O. An alternative view of mammalian DNA sequence organization. I. Repetitive sequence interspersion in Syrian hamster DNA: a model system. J Mol Biol. 1981 Dec 25;153(4):841–864. doi: 10.1016/0022-2836(81)90455-1. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Buck L. B., Axel R. A structure for amplified DNA. Cell. 1983 May;33(1):53–63. doi: 10.1016/0092-8674(83)90334-3. [DOI] [PubMed] [Google Scholar]
- STICH H. F., VANHOOSIER G. L., TRENTIN J. J. VIRUSES AND MAMMALIAN CHROMOSOMES; CHROMOSOME ABERRATIONS BY HUMAN ADENOVIRUS TYPE 12. Exp Cell Res. 1964 Apr;34:400–403. doi: 10.1016/0014-4827(64)90375-1. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H., McBride O. W. Chromosomal location of human metallothionein genes: implications for Menkes' disease. Science. 1984 Jun 8;224(4653):1104–1106. doi: 10.1126/science.6719135. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano M. J., Stallings R. L., Adair G. M., Humphrey R. M., Siciliano J. Provisional assignment of TPI, GPI, and PEPD to Chinese hamster autosomes 8 and 9: a cytogenetic basis for functional haploidy of an autosomal linkage group in CHO cells. Cytogenet Cell Genet. 1983;35(1):15–20. doi: 10.1159/000131830. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Adair G. M., Siciliano M. J. Provisional assignment of MPI, PKM2, PGM3, and ME1 to Chinese hamster chromosome 4. Somat Cell Mol Genet. 1984 Jan;10(1):109–111. doi: 10.1007/BF01534478. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Siciliano M. J. Assignment of ADA, ITPA, AK1, and AK2 to Chinese hamster chromosomes. Genetic and structural evidence for the conservation of mammalian autosomal synteny. J Hered. 1982 Nov-Dec;73(6):399–404. doi: 10.1093/oxfordjournals.jhered.a109687. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Siciliano M. J. Confirmational, provisional, and/or regional assignment of 15 enzyme loci onto Chinese hamster autosomes 1, 2, and 7. Somatic Cell Genet. 1981 Nov;7(6):683–698. doi: 10.1007/BF01538757. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. On the possibility of metabolic control of replicon "misfiring": relationship to emergence of malignant phenotypes in mammalian cell lineages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3673–3677. doi: 10.1073/pnas.78.6.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. Phorbol ester dramatically increases incidence of methotrexate-resistant mouse cells: possible mechanisms and relevance to tumor promotion. Cell. 1981 Aug;25(2):561–572. doi: 10.1016/0092-8674(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Robert de Saint Vincent B., DeRose M. L. Effect of chromosomal position on amplification of transfected genes in animal cells. Nature. 1984 Feb 9;307(5951):516–520. doi: 10.1038/307516a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]