Abstract
In the present investigation, four species of white rot fungi (Pleurotus), that is, P. flabellatus, P. florida, P. ostreatus and P. sajor-caju were used for decolorization of direct blue 14 (DB14). Among all four species of Pleurotus, P. flabellatus showed the fastest decolorization in petri plates on different concentration, that is, 200 mg/L, 400 mg/L, and 600 mg/L. All these four species were also evaluated for extracellular ligninolytic enzymes (laccase and manganese peroxidase) production and it was observed that the twelve days old culture of P. flabellatus showed the maximum enzymatic activity, that is, 915.7 U/mL and 769.2 U/mL of laccase and manganese peroxidase, respectively. Other three Pleurotus species took more time for dye decolorization and exhibited less enzymatic activities. The rate of decolorization of DB14 dye solution (20 mg/L) by crude enzymes isolated from P. flabellatus was very fast, and it was observed that up to 90.39% dye solution was decolorized in 6 hrs of incubation.
1. Introduction
Azo dyes are the largest class of synthetic dyes used for textile dyeing, paper printing, and other industrial applications. During the dyeing process, 5–20% of the used dyestuffs are released into the processed water [1, 2]. It was estimated that only textile industries alone generate about 4500 million kiloliters of wastewaters annually. As these dyes are synthetic, they contain toxic content in the effluents because partial degradation of these dyes results in aromatic amines that are toxic to aquatic life and mutagenic and carcinogenic to humans [3]. There is a big challenge to remove dye content from industrial effluents. Various physical, chemical, and biological methods have been proposed [4] among these, microbiological and enzymatic decomposition have received much attention in recent years [5–7]. Although, many reports are available for dye decolorization and degradation by bacteria, the reductive products of dye degradation are generally aromatic amines which are potentially hazardous to living organisms [8, 9] and due to larger size of dyes, bacteria are unable to degrade these dyes efficiently.
White rot fungi degrade lignin because they secrete oxidoreductases including lignin peroxidase (1,2-bis (3,4-dimethoxyphenyl) propene-1,3-diol:hydrogen-peroxide-Lip EC 1.1.1.14), manganese peroxidase (Mn(II):hydrogen-peroxide oxidoreductase EC 1.11.1.13), and laccase (benzenediol:oxygen reductase EC 1.10.3.2). These enzymes oxidise in a nonspecific way both phenolic and nonphenolic lignin derivatives and thus are promising candidates for the degradation of environmental pollutants, for example, phenols, anilines, dyes, lignocelluloses [10–14] and highly recalcitrant compounds such as polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs) [15]. Biodegradation of several persistent compounds such as dyes, pesticides and lignin derivatives has been attributed to the oxidative enzymes, especially laccase [16, 17]. The present research work is undertaken to evaluate the potentialities of Pleurotus species for dye decolorization.
2. Materials and Methods
2.1. Cultures and Their Maintenance
The pure cultures of Pleurotus species, that is, P. flabellatus, P. florida, P. ostreatus, and P. sajor-caju were obtained from Directorate of Mushroom Research, Solan (Himachal Pradesh), India. Throughout the study, cultures were maintained on MEA (Malt extract agar) medium at 28°C and subcultured at the regular interval of three weeks.
2.2. Screening of Species for Dye Decolorization
DB14 (direct blue 14) was used as reference dye for group of azo dye. Inoculums of four Pleurotus spp. (P. flabellatus, P. florida, P. ostreatus and P. sajor-caju) were inoculated on MEA plate containing 200 mg/L, 400 mg/L, and 600 mg/L of DB14. These plates were incubated on 28 ± 2°C.
2.3. Production of Enzymes
The medium for enzyme production contained 2% wheat bran and 2.5% malt extract, and the pH was adjusted to 6.0 by using NaOH or HCl. Incubation was carried out at 28°C in biological oxygen demand BOD incubator in cotton-plugged 250 mL Erlenmeyer flasks containing 50 mL of media. Flasks were inoculated with 1 cm2 agar pieces from actively growing fungus on malt extract agar plate.
2.4. Extraction of Extracellular Enzymes
Samples of substrate were collected at regular interval of 3 days and extracted in phosphate buffer (pH 6.0) for ligninolytic enzymes. Filtrate of extraction was used for enzyme assay.
2.5. Enzyme Assay
Laccase activity was determined via the oxidation of o-methoxyphenol catechol monomethylether (guaiacol) as substrate. The reaction mixture contained 1 mL of 1 mM guaiacol in 0.1 M sodium phosphate buffer (pH 6.0) and 1 mL of crude enzyme solution was incubated at 30°C for 10 min. The oxidation was followed by the increase in absorbance at 495 nm. One activity unit was defined as 1 μmol of guaiacol oxidised per minute [18].
Manganese peroxidase (MnP) activity was determined using guaiacol as substrate. The reaction mixture contained 0.2 mL of 0.5 M Na-tartrate buffer (pH 5.0), 0.1 mL of 1 mM MnSO4, 0.1 mL of 1 mM H2O2, 0.25 mL of 1 mM guaiacol and 0.3 mL of crude enzymes. The oxidation of substrate at 30°C was followed spectrophotometrically at (A 465) [19].
2.6. Decolorization of Synthetic Dye by Crude Enzyme Solution
Decolorization of the azo dye direct blue 14 was monitored in a mixture containing 1.4 mL DB14 solution (20 mg L−1), 0.2 mL 0.5 M sodium acetate buffer pH 3.5, 0.2 mL crude enzyme, 0.2 mL of deionised water, 0.1 M H2O2, 0.1 M MnSO4 or 5.5 mM ABTS solutions, at 25 ± 2°C [20].
Visible spectra were recorded with a UV-visible (Elico-SL191) spectrophotometer at λ = 595 nm. The rate of decolorization was expressed as the percentage decrease in absorbance at the peak wavelength. Control tests were conducted with crude enzyme replaced by deionised water. Experiments were performed in triplicate, and results were expressed as the mean values.
3. Results and Discussion
All four species of Pleurotus selected in the present work showed different rate of decolorization of DB14. Among the four species of Pleurotus, P. flabellatus showed the fastest decolorization of DB14 followed by P. florida, P. sajor-caju, and P. ostreatus on 200 mg/L of DB14 (Figure 1). P. flabellatus completely decolorized DB14 within 8 days. P. flabellatus effectively decolorized the dye at the concentration of 400 mg/L and also at 600 mg/L. At 400 mg/L concentration complete decolorization occurred in 10 days and other species took almost the similar time, but the rate of decolorization and mycellial growth were better in case of P. flabellatus. Same results were observed at 600 mg/L concentration. The degrees of decolorization of different dyes such as malachite green, indigo carmine, xylidine ponceau, Bismarck brown and methyl orange using the white rot fungus P. ostreatus were previously evaluated by Neelamegam et al. [21]. This study demonstrated the potentialities of white rot fungi in bioremediation of dye contaminated ecosystems.
Figure 1.
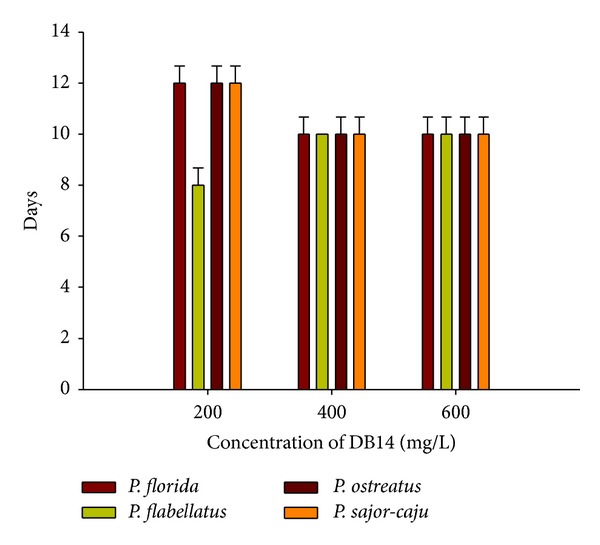
Decolorization of DB14 by Pleurotus spp.
The time course of laccase and MnP activity was followed in the wheat bran supplemented liquid media over a period of 21 days. Initially, it was observed that amongst the four species of Pleurotus, P. flabellatus showed the highest laccase activities on all days evaluated, reaching maximum levels of 915.7 U/mL in 12 days of culture of P. flabellatus on wheat bran containing media. This was followed by P. sajor-caju which showed maximum laccase activity (608.6 U/mL) in 12 days. Subsequently, P. ostreatus and P. florida showed maximum laccase activity, that is, 436.3 U/mL and 353.3 U/mL in 9 days and 21 days, respectively (Figure 2). MnP activities were detected at levels of up to 769.2 U/mL by P. flabellatus in 12 days old culture followed by P. Sajor-caju, P. ostreatus, and P. florida that is, 407.4 U/mL, 241.8 U/mL, and 329 U/mL on 9, 9, and 15 days old culture, respectively (Figure 3).
Figure 2.
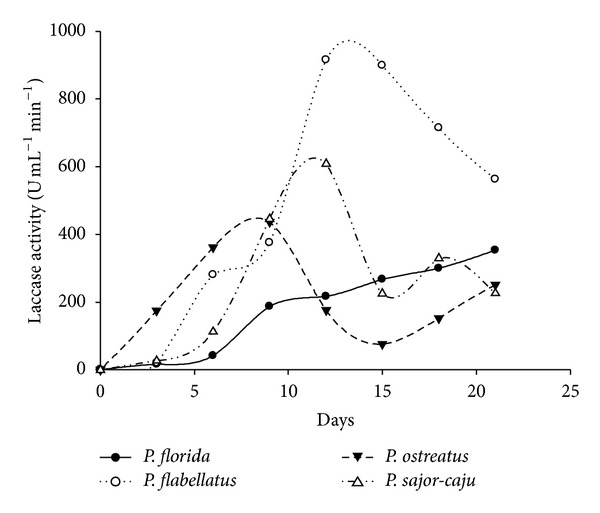
Laccase production by different Pleurotus species in submerged condition.
Figure 3.
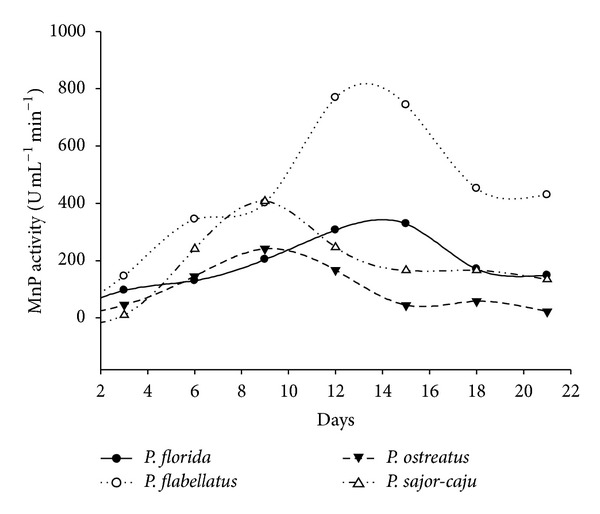
MnP production by different Pleurotus species in submerged condition.
Every species showed maximum enzymatic activity on the 9th day or after the 9th day of incubation, which might be due to the occurrence of initial lag phase when species try to establish it in new medium. When cultures are established in the culture medium, they enter into log phase, and metabolically this is the most active phase where species show maximum enzymatic activity. Cereal bran was reported to increase ligninolytic enzyme production of the white rot fungi Coriolopsis gallica and Bjerkandera adusta [22]. In the beginning of the experiment, on day 3, different Pleurotus species showed low enzymatic activities. This was followed by sharp increase up to 12 days in P. flabellatus and P. sajor-caju, whereas in case P. ostreatus and P. florida it took 9 and 21 days for laccase. P. flabellatus and P. florida, showed maximum MnP activities in 12 days whereas P. ostreatus and P. sajor-caju showed the maximum MnP activities in 9 days, respectively. Laccase and MnP both are oxidative enzyme and have broad range of substrate specificity.
Several authors have discussed the role of lignicolous fungal enzymes in the decolorization of dyes [14, 23, 24]. Crude enzymes obtained from cultures of P. flabellatus on wheat bran containing malt extract media were tested for decolorization of DB14. Figure 4 shows the percent decolorization during various periods. The decolorization of the dye using crude enzyme was 46.49% in the first hour, in present investigation. 90.39% DB14 decolorization was reported in 6 hrs at room temperature (30°C).
Figure 4.
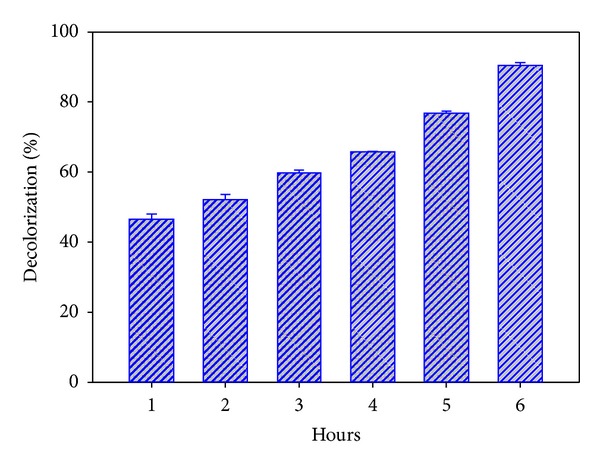
Decolorization of DB14 by crude extracellular enzymes of P. flabellatus.
The laccase [16] and MnP [25] play a major role in complete oxidation of DB14. It was observed that laccase and peroxidases can act as starters of a chain reaction which leads to dye degradation by generating highly active free radicals (e.g., Mn3+, lipid, hydroxyl, and peroxy-radicals) [26, 27]. According to Meyer [28], because of the structural variety of azo dyes, they are not uniformly susceptible to biodegradation. It was demonstrated that substituent groups such as nitro and sulpho are frequently recalcitrant to biodegradation, whereas 2-methyl, 2-methoxy, 2,6-dimethyl and 2,6-dimethoxy-substituted 4-(4-sulfophenylazo)-phenol were preferred for azo-dye degradation by peroxidase from Streptomyces spp. and Phanerochaete chrysosporium [29]. The breaking down of the dye into smaller fragments, including the breakage of the azo bond, can lead to a decrease in the absorbance of the visible spectra and in a colorless solution [30].
4. Conclusion
The present investigation suggests that the white rot fungus Pleurotus flabellatus can be used in bioremediation of dye-contaminated ecosystems. This is because of the presence of powerful enzymatic machinery which can effectively degrade the recalcitrant and toxic dyes. Out of four species investigated, P. flabellatus showed the maximum enzymatic activity. Extracellular enzymes extracted from 12 days old culture of P. flabellatus decolorized the DB14 to maximum extent. For better enzyme production the wheat bran can be used as supplement in liquid media. Decolorized effluent after enzymatic treatment can be reused by industries and also in agriculture.
Abbreviations
- DB14:
Direct blue 14
- MnP:
Manganese peroxidase
- hrs:
Hours.
References
- 1.Soares GMB, Costa-Ferreira M, Pessoa de Amorim MT. Decolorization of an anthraquinone-type dye using a laccase formulation. Bioresource Technology. 2001;79(2):171–177. doi: 10.1016/s0960-8524(01)00043-8. [DOI] [PubMed] [Google Scholar]
- 2.Wong Y, Yu J. Laccase-catalyzed decolorization of synthetic dyes. Water Research. 1999;33(16):3512–3520. [Google Scholar]
- 3.Pinheiro HM, Touraud E, Thomas O. Aromatic amines from azo dye reduction: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes and Pigments. 2004;61(2):121–139. [Google Scholar]
- 4.Forgacs E, Cserháti T, Oros G. Removal of synthetic dyes from wastewaters: a review. Environment International. 2004;30(7):953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Chagas EP, Durrant LR. Decolorization of azo dyes by Phanerochaete chrysosporium and Pleurotus sajorcaju . Enzyme and Microbial Technology. 2001;29(8-9):473–477. [Google Scholar]
- 6.Murugesan K, Nam I-H, Kim Y-M, Chang Y-S. Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme and Microbial Technology. 2007;40(7):1662–1672. [Google Scholar]
- 7.Zheng Z, Obbard JP. Oxidation of polycyclic aromatic hydrocarbons (PAH) by the white rot fungus, Phanerochaete chrysosporium . Enzyme and Microbial Technology. 2002;31(1-2):3–9. [Google Scholar]
- 8.Chivukula M, Renganathan V. Phenolic azo dye oxidation by laccase from Pyricularia oryzae . Applied and Environmental Microbiology. 1995;61(12):4374–4377. doi: 10.1128/aem.61.12.4374-4377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill C, Lopez A, Esteves S, Hawkes FR, Hawkes DL, Wilcox S. Azo-dye degradation in an anaerobic-aerobic treatment system operating on simulated textile effluent. Applied Microbiology and Biotechnology. 2000;53(2):249–254. doi: 10.1007/s002530050016. [DOI] [PubMed] [Google Scholar]
- 10.Fahr K, Wetzstein H-G, Grey R, Schlosser D. Degradation of 2,4-dichlorophenol and pentachlorophenol by two brown rot fungi. FEMS Microbiology Letters. 1999;175(1):127–132. doi: 10.1111/j.1574-6968.1999.tb13611.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira-Leitão VS, de Carvalho MEA, Bon EPS. Lignin peroxidase efficiency for methylene blue decolouration: comparison to reported methods. Dyes and Pigments. 2007;74(1):230–236. [Google Scholar]
- 12.Pandey VK, Singh MP, Srivastava AK, Vishwakarma SK, Takshak S. Biodegradation of sugarcane bagasse by white rot fungus Pleurotus citrinopileatus . Cellular and Molecular Biology. 2012;58(1):8–14. [PubMed] [Google Scholar]
- 13.Singh MP, Pandey VK, Srivastava AK, Viswakarma SK. Biodegradation of Brassica haulms by white rot fungus Pleurotus eryngii . Cellular and Molecular Biology. 2011;57(1):47–55. [PubMed] [Google Scholar]
- 14.Vishwakarma SK, Singh MP, Srivastava AK, Pandey VK. Azo dye (direct blue) decolorization by immobilized extracellular enzymes of Pleurotus species. Cellular and Molecular Biology. 2012;58(1):21–25. [PubMed] [Google Scholar]
- 15.Svobodová K, Majcherczyk A, Novotný Č, Kües U. Implication of mycelium-associated laccase from Irpex lacteus in the decolorization of synthetic dyes. Bioresource Technology. 2008;99(3):463–471. doi: 10.1016/j.biortech.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Revankar MS, Lele SS. Synthetic dye decolorization by white rot fungus, Ganoderma sp. WR-1. Bioresource Technology. 2007;98(4):775–780. doi: 10.1016/j.biortech.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Riccardi C, Papacchini M, Mansi A, et al. Characterization of bacterial population coming from a soil contaminated by polycyclic aromatic hydrocarbons (PAHs) able to degrade pyrene in slurry phase. Annals of Microbiology. 2005;55(2):85–90. [Google Scholar]
- 18.Dhaliwal RPS, Garcha HS, Khanna PK. Regulation of lignocellulotic enzyme system in Pleurotus ostreatus . Indian Journal of Microbiology. 1991;31(2):181–184. [Google Scholar]
- 19.Putter J. Peroxidases. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 2. NewYork, NY, USA: Academic Press; 1974. pp. 685–690. [Google Scholar]
- 20.Abrahão MC, De Mello Gugliotta A, Da Silva R, Fujieda RJY, Boscolo M, Gomes E. Ligninolytic activity from newly isolated basidiomycete strains and effect of these enzymes on the azo dye orange II decolourisation. Annals of Microbiology. 2008;58(3):427–432. [Google Scholar]
- 21.Neelamegam R, Baskaran V, Dhanasekar R, Viruthagiri T. Decolourization of synthetic dyes using rice straw attached Pleurotus ostreatus . Indian Journal of Chemical Technology. 2004;11(5):622–625. [Google Scholar]
- 22.Pickard MA, Vandertol H, Roman R, Vazquez-Duhalt R. High production of ligninolytic enzymes from white rot fungi in cereal bran liquid medium. Canadian Journal of Microbiology. 1999;45(7):627–631. [Google Scholar]
- 23.Singh MP, Pandey AK, Vishwakarma SK, Srivastava AK, Pandey VK. Extracellular Xylanase Production by Pleurotus species on Lignocellulosic Wastes under in vivo Condition using Novel Pretreatment. Cellular and Molecular Biology. 2012;58(1):170–173. [PubMed] [Google Scholar]
- 24.Singh MP, Pandey VK, Pandey AK, Srivastava AK, Vishwakarma NK, Singh VK. Production of xylanase by white rot fungi on wheat straw. Asian Journal of Microbiology, Biotechnology and Environmental Sciences. 2008;10(4):859–862. [Google Scholar]
- 25.Tsukihara T, Honda Y, Sakai R, Watanabe T, Watanabe T. Mechanism for oxidation of high-molecular-weight substrates by a fungal versatile peroxidase, MnP2+ . Applied and Environmental Microbiology. 2008;74(9):2873–2881. doi: 10.1128/AEM.02080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP) Enzyme and Microbial Technology. 2002;30(4):454–466. [Google Scholar]
- 27.Rabinovich ML, Bolobova AV, Vasil’chenko LG. Fungal decomposition of natural aromatic structures and xenobiotics: a review. Applied Biochemistry and Microbiology. 2004;40(1):1–17. [PubMed] [Google Scholar]
- 28.Meyer U. Biodegradation of synthetic organic colorants. FEMS Symposium. 1981;12:371–385. [Google Scholar]
- 29.Suzuki T, Timofei S, Kurunczi L, Dietze U, Schüürmann G. Correlation of aerobic biodegradability of sulfonated azo dyes with the chemical structure. Chemosphere. 2001;45(1):1–9. doi: 10.1016/s0045-6535(01)00074-1. [DOI] [PubMed] [Google Scholar]
- 30.Zille A, Tzanov T, Gübitz GM, Cavaco-Paulo A. Immobilized laccase for decolourization of reactive Black 5 dyeing effluent. Biotechnology Letters. 2003;25(17):1473–1477. doi: 10.1023/a:1025032323517. [DOI] [PubMed] [Google Scholar]


