Abstract
Despite recent advances in therapeutic and diagnostic approaches, coronary artery disease (CAD) and its related cardiac disorders represent the most common cause of death in the United States. Nuclear myocardial perfusion imaging (MPI) technologies play a pivotal role in the diagnosis and treatment design for CAD. Recently, in order to develop improved MPI agents for diagnosis of CAD, 99mTc-[bis(dimethoxypropylphosphinoethyl)-ethoxyethyl-amine(PNP5)]-[bis(N-ethoxyethyl)dithiocarbamato(DBODC)]nitride(N-DBODC5)(99mTc-N-DBODC5) with a faster liver clearance than conventional single-photon emission computed tomography (SPECT) imaging agents (technetium 99m sestamibi (99mTc-MIBI) or technetium 99m tetrofosmin) has been introduced. In preclinical and phase I studies, 99mTc-N-DBODC5 has shown characteristics of an essentially ideal MPI tracer. Importantly, however, there is no data to support the use of 99mTc-N-DBODC5 to evaluate myocardial ischemia in patients with suspected CAD. The present study was designed to assess the clinical value of this agent; the findings of stress and rest MPI after the administration of this agent were compared to those of stress and rest 99mTc-MIBI, as well as those of coronary angiography, with respect to the detection of CAD. Our findings indicated the usefulness of 99mTc-N-DBODC5 as a promising MPI agent.
1. Introduction
Coronary artery disease (CAD) remains the single greatest cause of death in men and women in the USA, despite a declining total death rate. Using 2005 data, over 445,000 (or 1 in every 5) deaths in the USA were due to CAD, and it ranked highest among all disease categories in hospital discharges [1]. CAD also remains the third leading cause of death of the Chinese population, creating a significant socioeconomic burden [2]. Therefore, the reduction of the morbidity and mortality due to CAD is of primary importance to physicians and patients.
Nuclear cardiology, cardiovascular magnetic resonance, cardiac computed tomography, position emission computed tomography, and coronary angiography (CA) are imaging modalities that have been used to measure myocardial perfusion, left ventricular function, and coronary anatomy for clinical management and research [3]. Based on current guidelines, invasive CA is a suitable diagnostic procedure for patients with a high pretest likelihood of significant coronary artery disease either with or without troublesome symptoms or clinical findings [4, 5]. In this population, the reported diagnostic yield of invasive CA is 44–48% [6–8]. However, in reality, invasive CA has an even lower diagnostic yield of obstructive CAD of approximately 38% [9]. Noninvasive testing could be of value to defer from invasive diagnostic procedures. Stress myocardial perfusion imaging (MPI) has emerged as an important noninvasive mean of evaluating patients with suspected CAD, with over 8.5 million evaluations performed annually in the USA [10].
The most commonly used imaging modality for this purpose is single-photon emission computed tomography (SPECT) [11]. The advantages of nuclear MPI for the detection of CAD are as follows: first, it enables the simple, safe, and noninvasive assessment of myocardial ischemia and reduction of coronary flow reserve using exercise or pharmacological stress. In addition, it provides left ventricular functional information on coronary arteries, which is different from the morphological information provided by CA [12]. Furthermore, CA is the standard technique for assessing epicardial coronary anatomy, and MPI is the standard technique for assessing myocardial perfusion and is appropriate for the quantitative evaluation of myocardial perfusion conditions [13].
Thallium-201 (201Tl), technetium 99m sestamibi (99mTc-MIBI), and technetium 99m tetrofosmin (99mTc-tetrofosmin) are three traditional, routinely used, MPI tracers and are clinical-validated tracers for evaluation of SPECT MPI [14–16]. Because 201Tl is a cyclotron-produced isotope, it is expensive, and it is not easily available for routine clinical use in many developing countries. Therefore, from a practical standpoint, 99mTc-MIBI or 99mTc-tetrofosmin is the preferred agent for a gated SPECT study. However, tracer activity below the diaphragm is commonly seen with 99mTc-MIBI and 99mTc-tetrofosmin, and this can reduce accuracy in some studies [17, 18].
Recently, 99mTc-N-DBODC5 with a faster liver clearance than conventional SPECT imaging agents has been introduced. It is a new lipophilic, monocationic 99mTc-labeled compound that is currently under clinical investigation as a MPI agent [19–24]. Basic research and phase I studies have shown the safety, excellent biodistribution, and high image quality of this radiopharmaceutical [22, 25, 26]. Like 99mTc-MIBI and 99mTc-tetrofosmin, 99mTc-N-DBODC5, also a monocationic lipophilic complex, is possibly localized in the mitochondrial fraction. Marmion et al. [27] and Bolzati et al. [28] reported that the lipophilic characteristics and electronic charge of the tracer were thought to be important. Importantly, however, the clinical value of diagnosis of CAD has not been fully explored.
In order to evaluate the clinical value of this agent, the findings of stress and rest MPI after the administration of this agent were compared to those of stress and rest 99mTc-MIBI MPI, as well as those of CA, with respect to the detection of CAD.
2. Methods
2.1. Patient Population
Of 120 patients admitted to the hospital because of chest pain from March 2010 to December 2011, 46 (31 males; mean age, 60.08 ± 8.58 years (Table 1)) who underwent stress-rest 99mTc-N-DBODC5 SPECT, 99mTc-MIBI SPECT, and coronary arteriography were included in this study. If the creatine kinase concentration was more than twice the upper normal limit or there was positive troponin T, or if there was evidence of previous myocardial infarction, coronary angioplasty, or a coronary artery bypass graft surgery, the patients were excluded.
Table 1.
Study group.
| Parameter | Value |
|---|---|
| n | 46 |
| M/F | 31/15 |
| Age (yr) | 60.08 ± 8.58 (39–74) |
| Hypertension | 31 |
| Hyperlipidemia | 19 |
| Diabetes mellitus | 14 |
| Smoking | 26 |
| Trigger of chest pain | |
| Effort | 22 |
| Rest | 13 |
| Not specific | 11 |
| ECG abnormality | 11 |
| ST-T elevation | 3 |
| ST-T depression | 8 |
| ≥50% of luminal narrowing | 29 |
| One-vessel disease | 14 |
| Two-vessel disease | 11 |
| Three-vessel disease | 4 |
Data are presented as mean ± SD or number (%) as appropriate.
2.2. Preparation of 99mTc-N-DBODC5
99mTc-N-DBODC5 was prepared using a lyophilized kit formulation purchased from Beijing Shihong Pharmaceutical Center. The saline solution of sodium pertechnetate (1.0 mL, 1110 MBq) was added to an SDH vial (Vail A: 5.0 mg of succinate dehydrogenase, 5.0 mg of 1,2-diaminopropane-N,N,N′,N′-tetraacetic acid, and 0.05 mg of SnCl2·2H2O). Then, the solution was kept at room temperature for 15 min to form a [99mTcN]2+ intermediate. The other two lyophilized vials (Vail B: 2.0 mg of PNP5, Vail C: 2.0 mg of DBODC) were dissolved with 1.0 mL of saline solution, respectively. And then the saline solutions of Vails B and C were added to the vial of [99mTcN]2+ intermediate (Vail A). The resulting solution was heated at 100°C for 15 min. Before injection, the solution was filtrated through a 0.22-μm membrane. The 99mTc-MIBI was obtained using a lyophilized kit formulation (Beijing Shihong Pharmaceutical Center) in the First Affiliated Hospital of Shanxi Medical University.
Radiochemical purity (RCP) was determined by thin-layer chromatography (TLC). TLC was conducted on polyamide film as the station phase and saline/acetone (v / v = 6 : 1) as the mobile phase. Retention factor (Rf) for 99mTc-N-DBODC5 is 0.3–0.6. The RCP was more than 95% in each experimental study, and the purity was still greater than 95% at least 6 h.
2.3. Exercise Protocol
Figure 1 shows that a two-day protocol was used for both 99mTc-MIBI and 99mTc-N-DBODC5 MPI. Image acquisition procedures conformed to the ASNC imaging guidelines [29, 30], and the aged-adjusted maximal heart rate was the endpoint on an ergometer, but other endpoints included physical exhaustion, uncommon arrhythmia, severe angina, or significant hypotension. At the endpoint of the symptom-limited bicycle ergometer exercise, 740 MBq of 99mTc-N-DBODC5 (mean injected activity, 740.8 ± 11.1 MBq) was injected; exercise was continued for a further 2 min.
Figure 1.
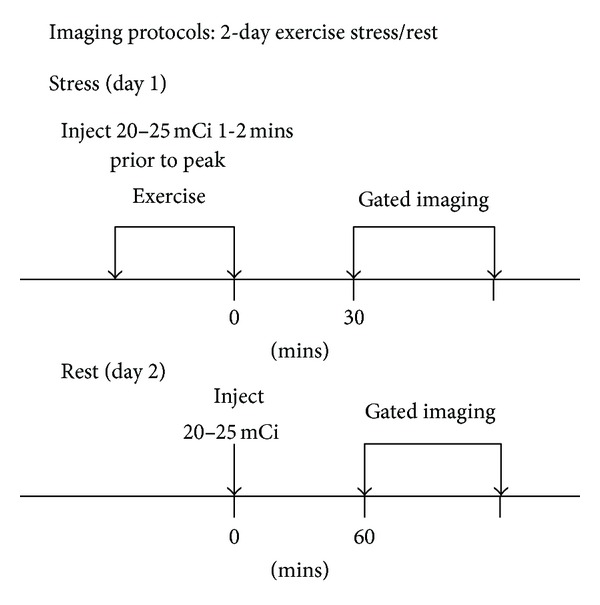
99mTc-N-DBODC5 and 99mTc-MIBI imaging protocols: two-day exercise stress/rest.
Each patient underwent an exercise 99mTc-MIBI study within 7 days of the 99mTc-N-DBODC5 study. For the 99mTc-MIBI (741.5 ± 12.3 MBq) SPECT study, the exercise protocol of patients was identical to that for the 99mTc-N-DBODC5 study. 99mTc-N-DBODC5 and 99mTc-MIBI were used as myocardial perfusion agents in a random sequence.
2.4. Gated SPECT Acquisition and Image Reconstruction
Patients were imaged in the supine position with their arms raised. SPECT images were acquired with a fixed 90° two-headed gamma camera (Infinia VC Hawkeye, General Electric, USA), using a low-energy, high-resolution, parallel-hole collimator, from the 45° right anterior oblique to the 45° left posterior position. Acquisition parameters were as follows: detectors at 90°, 180° rotation at 3° steps with automatic body contouring, 35 s acquisition per step, 64 × 64 matrix, zoom ×1.28, and energy window 140 keV ± 10%. The total acquisition time was approximately 20 min. The same filters were used for both tracers. The raw projection datasets were filtered with a Butterworth filter (cut off frequency 0.50 cycles/pixel and power 6.0 for rest images, cut off frequency 0.50 cycles/pixel and power 5.0 for stress images). No scatter or attenuation correction was performed.
2.5. Heart-to-Organ Analysis
99mTc-N-DBODC5 and 99mTc-MIBI heart-to-organ count ratios were calculated from the anterior projection of each tomographic acquisition. Regions of interests (ROIs) were drawn around the entire left ventricular myocardium, over the hepatic margin adjacent to the inferoapical wall of the left ventricle, excluding the biliary tree, and over the inferior left ventricular wall adjacent large intestine activity. The mean counts per pixel in the three ROIs were normalized to the injected tracer activity after decay correction and to a standard acquisition time of 1 min. Heart-to-liver and heart-to-intestine ratios were then computed [31].
2.6. Image Quality Assessing
For MPI analyses, at first, two experienced observers judged which image sets from 99mTc-MIBI and 99mTc-N-DBODC5 were superior in image quality on the basis of patient motion, statistical noise, tracer activity below the diaphragm, heart-to-organ count ratio, and sharpness, without knowledge of the radiopharmaceutical or patient identity [32].
2.7. MPI Image Interpretation
Both overall qualitative diagnosis and semiquantitative 17 segment with 5-point [33] (0 = normal, 4 = absent tracer uptake) scorings were employed in the independent, blinded read by two expert readers who were experienced in SPECT MPI interpretation. In this model, the left anterior descending artery (LAD) distribution territory comprises seven segments (segments 1-2, 7-8, 13-14, and 17), the left circumflex artery (LCX) comprises five segments (segments 3-4, 9-10, and 15), and the right coronary artery (RCA) comprises five segments (segments 5-6, 11-12, and 16). SPECT stress/rest studies were classified for each myocardial region in the following manner: normal if all the segments were normal after stress; ischemic if at least one segments improved at rest; and scar if no segments improved at rest. In addition, the summed stress (SSS), summed rest (SRS), and summed difference scores (SDSs) were calculated, and ischemia was defined as SDS ≥ 2 [34]. The 99mTc-MIBI and 99mTc-N-DBODC5 images were separately interpreted without knowing the clinical histories, results of coronary angiography, or other radionuclide findings.
2.8. Coronary Angiography Interpretation
All coronary angiograms were interpreted with quantitative CA in a coronary angiography core laboratory blinded to the clinical or imaging results. A coronary stenosis was considered present when there was a stenosis ≥ 50% in diameter in any epicardial coronary artery. The presence of one or more coronary stenoses defined the presence of significant CAD.
2.9. Statistical Analysis
All statistical analyses were performed using the statistical package SPSS 16.0 (Chicago, IL, USA). The results were expressed as the mean value ± SD. The difference in left ventricular function parameters and perfusion scores were compared using a paired Student's t-test. Comparison of the proportion was made with the McNemar test. CA results were used as the “gold standard.” Agreements between CA and SPECT results were defined as the kappa (κ) value. P < 0.05 was considered significant.
3. Results
3.1. Coronary Angiography
Of the 46 patients studied, 29 had ≥50% luminal diameter stenosis in at least one major coronary vessel. Fourteen had single-vessel disease, 11 had two-vessel disease, and four had three-vessel disease; 15 patients showed significant stenosis in the RCA, 19 in the LAD, and 14 in the LCX. The remaining 17 patients had normal or nonsignificantly stenosed coronary arteries.
3.2. Heart-to-Organ Count Ratio
Qualitative analyses of images acquired of 99mTc-N-DBODC5 both at rest and during stress revealed no significant overlap between tracer accumulation in the inferior wall of the ventricle and in the subdiaphragmatic region (Figure 2).
Figure 2.
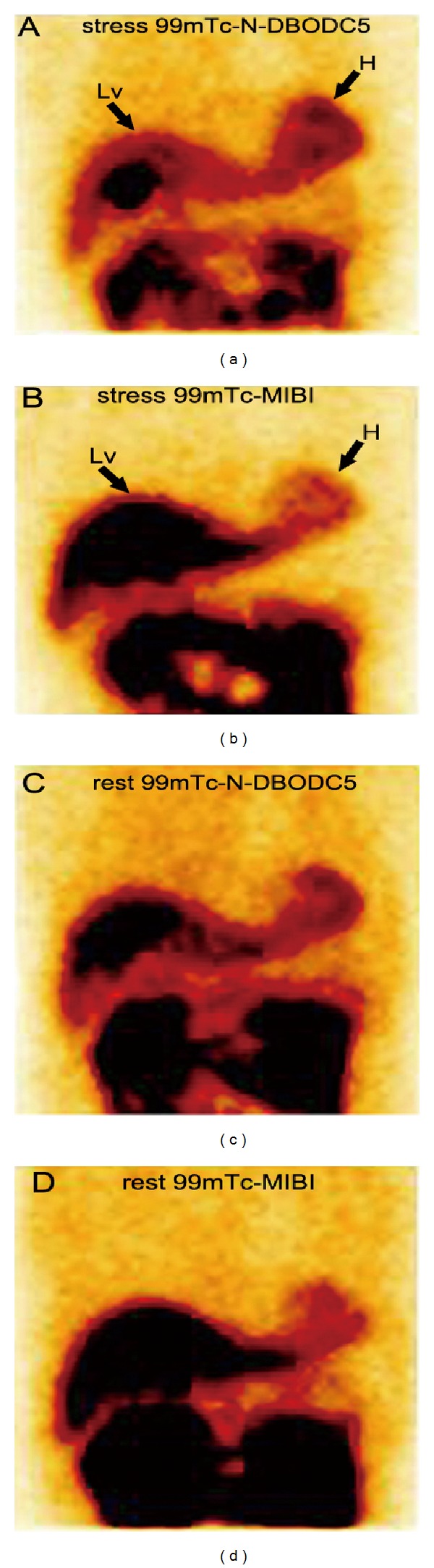
Comparison of liver clearance of the two tracers in anterior tomographic planar images of a patient (as shown in the black arrow; H, heart; Lv, liver). (a) Exercise stress 99mTc-N-DBODC5, (b) Exercise stress 99mTc-MIBI, (c) rest 99mTc-N-DBODC5 and (d) rest 99mTc-MIBI.
Figure 3 depicts the heart-to-liver count ratios (a) and heart-to-intestine count ratios (b) of 99mTc-N-DBODC5 and 99mTc-MIBI in 46 patients. The myocardium-to-liver ratios of 99mTc-N-DBODC5 and 99mTc-MIBI studies were 0.97 ± 0.07 and 0.51 ± 0.02, respectively, at stress. The myocardium-to-liver ratios of 99mTc-N-DBODC5 and 99mTc-MIBI MPI were 0.78 ± 0.04 and 0.62 ± 0.01, respectively, at rest. On the other hand, the mean heart-to-intestine ratios of 99mTc-N-DBODC5 were 2.12 ± 0.01 and 1.36 ± 0.02 for stress and rest images, respectively. The average heart-to-intestine ratios of 99mTc-MIBI were 1.62 ± 0.01 and 0.91 ± 0.00 for stress and rest images, respectively. 99mTc-N-DBODC5 studies had a significantly higher heart-to-organ count ratio compared with 99mTc-MIBI studies (P < 0.05).
Figure 3.
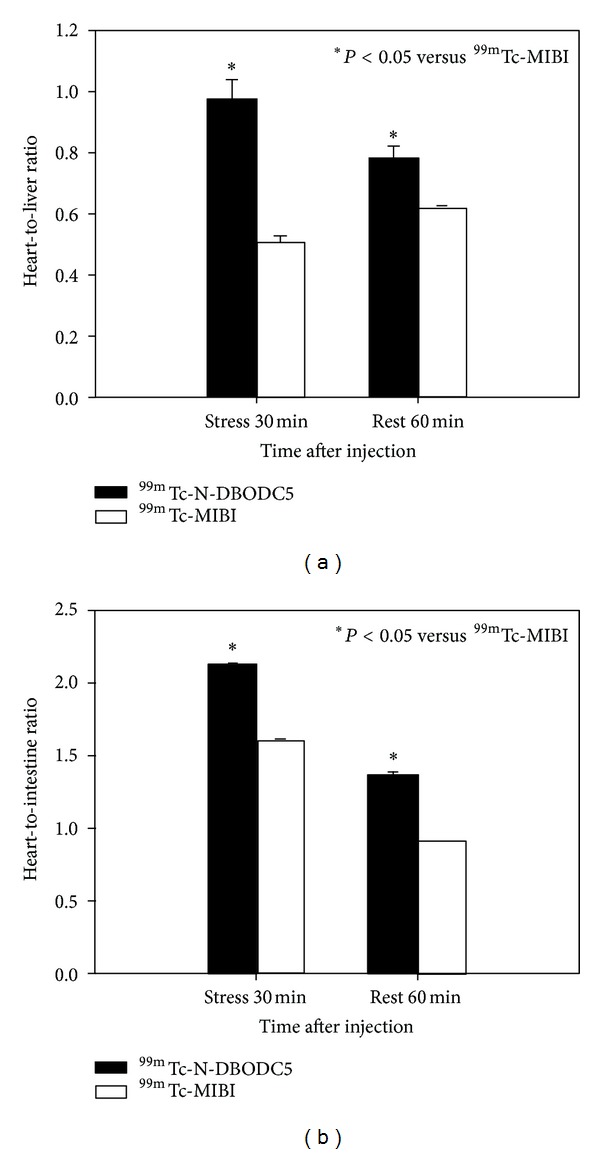
Heart-to-liver ratio and heart-to-intestine ratio measured with an anterior projection images at stress for 30 min and rest for 60 min for 99mTc-N-DBODC5 and 99mTc-MIBI in 46 patients.
3.3. Image Quality
In the stress perfusion imaging, 99mTc-N-DBODC5 MPI was superior in quality to 99mTc-MIBI MPI in 37 of the 46 patients studied. In the remaining nine patients, the MPI was judged to be of equal quality. In the resting perfusion imaging, 99mTc-N-DBODC5 images were superior in all the patients studied. In general, the 99mTc-N-DBODC5 MPI had more counts with less statistical noise, less tracer activity below the diaphragm, and desirable heart-to-organ count ratio compared with 99mTc-MIBI MPI.
3.4. Gated SPECT Findings
Table 2 shows the results of scan segments between the 99mTc-N-DBODC5 and 99mTc-MIBI MPI. Of the 782 segments that could be interpreted by both techniques, 302 were concordantly normal, 119 were concordantly reversible, and 136 were concordantly nonreversible. 99mTc-N-DBODC5 SPECT detected more reversible, defects than did 99mTc-MIBI SPECT (240 versus 140, P < 0.05, McNemar test). 99mTc-MIBI SPECT identified more nonreversible defects than did 99mTc-N-DBODC5 SPECT (312 versus 152, P < 0.001, McNemar test). Seventy-one segments interpreted as normal on 99mTc-N-DBODC5 corresponded to nonreversible defects on 99mTc-MIBI SPECT images. In contrast, 12 normal 99mTc-MIBI segments corresponded to nonreversible defects on 99mTc-N-DBODC5 images. When the patterns of uptake (normal, reversible defect, and nonreversible defect) of 99mTc-N-DBODC5 and 99mTc-MIBI were compared, there was concordance in 71% (557/782) of segments.
Table 2.
The comparison of myocardial perfusion in a total of 782 segments with 99mTc-N-DBODC5 and 99mTc-MIBI SPECT exercise imaging.
| 99mTc-N-DBODC5 | 99mTc-MIBI | Total | ||
|---|---|---|---|---|
| Normal | Reversible defect | Nonreversible defect* | ||
| Normal | 302 (39%) | 17 (2%) | 71 (9%) | 390 |
| Reversible defect* | 16 (2%) | 119 (15%) | 105 (13%) | 240 |
| Nonreversible defect | 12 (2%) | 4 (1%) | 136 (17%) | 152 |
|
| ||||
| Total | 330 | 140 | 312 | 782 |
Data are presented as number; *P < 0.001, 99mTc-MIBI versus 99mTc-N-DBODC5 for nonreversible defect segments; *P < 0.05, 99mTc-N-DBODC5 versus 99mTc-MIBI for reversible defect segments; McNemar test was used.
Table 3 shows the results of left ventricular function parameters in the two techniques used. In this study, the end-diastolic volume (EDV), end-systolic volume (ESV), left ventricular ejection fraction (LVEF), and transient ventricular dysfunction (TID) were assessed by MPI using two tracers. No statistically significant differences in MPI parameters were observed between the 99mTc-MIBI and 99mTc-N-DBODC5 studies. However, the scores of myocardial perfusion defects for the detection of CAD were higher with 99mTc-MIBI than with 99mTc-N-DBODC5 (P = 0.012, for SSS; P = 0.020, for SDS, resp.).
Table 3.
MPI findings of two tracers on left ventricular function parameters and ischemia scores in 46 patients.
| Parameter | 99mTc-MIBI | 99mTc-N-DBODC5 |
|---|---|---|
| LVEF (%) | ||
| Exercise | 54.2 ± 11.3 | 56.7 ± 9.2 |
| Rest | 63.1 ± 8.5 | 64.8 ± 7.9 |
| EDV (mL) | ||
| Exercise | 81.5 ± 18.6 | 83.2 ± 16.3 |
| Rest | 97.3 ± 16.4 | 99.1 ± 13.7 |
| ESV (mL) | ||
| Exercise | 43.2 ± 9.7 | 41.4 ± 12.3 |
| Rest | 59.4 ± 13.6 | 60.2 ± 9.8 |
| SSS | 12.1 ± 1.4★ | 9.6 ± 1.6 |
| SRS | 7.2 ± 0.8 | 7.9 ± 0.9 |
| SDS | 4.2 ± 0.5★ | 3.6 ± 0.4 |
| TID | 0.96 ± 0.06 | 0.94 ± 0.02 |
Data are presented as mean ± SD or number (%) as appropriate; ★statistically significant 99mTc-MIBI versus 99mTc-N-DBODC5 (P < 0.05); paired Student's t-test was used; LVEF: left ventricular ejection fraction; EDV: end-diastolic volume; ESV: end-systolic volume; SSS: summed stress scores; SRS: summed rest scores; SDS: summed difference scores; TID: transient ventricular dysfunction.
3.5. Detection of CAD
Table 4 shows the sensitivity and specificity of two tracers in diagnosing CAD. Based on CA, overall figures for sensitivity and specificity in identification of CAD were 86% (25/29) and 65% (11/17) for 99mTc-MIBI imaging. On the other hand, the overall sensitivity and specificity for the detection of CAD were 86% (25/29) and 88% (15/17) for 99mTc-N-DBODC5 SPECT studies. The concordance of ischemia diagnosis sensitivity of a representative patient for the two tracers is shown in Figure 4. The accuracy was 0.78 for 99mTc-MIBI and 0.87 for 99mTc-N-DBODC5. Angiography agreement was very good for 99mTc-N-DBODC5 (κ = 0.73) and moderate for 99mTc-MIBI (κ = 0.52). Specificity and accuracy were not significantly different but better for the 99mTc-N-DBODC5 group when compared to 99mTc-MIBI SPECT (specificity, 88-65%; accuracy, 87-78%).
Table 4.
Sensitivity, specificity, and diagnostic accuracy of scintigraphic perfusion studies and agreement with coronary angiography.
| Overall | LAD | LCX | RCA | |||||
|---|---|---|---|---|---|---|---|---|
| MIBI | DBODC | MIBI | DBODC | MIBI | DBODC | MIBI | DBODC | |
| No. of disease | 25 | 25 | 10 | 12 | 9 | 8 | 13 | 13 |
| Sensitivity (%) | 86 | 86 | 53 | 63 | 64 | 57 | 87 | 87 |
| Specificity (%) | 65 | 88 | 96 | 96 | 94 | 94 | 68 | 87 |
| Accuracy (%) | 78 | 87 | 78 | 83 | 85 | 83 | 74 | 87 |
| ▲Kappa (κ) | 0.53 | 0.73 | 0.52 | 0.63 | 0.62 | 0.55 | 0.48 | 0.71 |
Data are presented as number (%); MIBI = 99mTc-MIBI, DBODC = 99mTc-N-DBODC5; LAD: left anterior descending coronary artery; LCX: left circumflex coronary artery; RCA: right coronary artery. ▲CA was used as the “gold standard” for the calculation of the κ, which is determine between SPECT MPI and CA. If there is no agreement, κ = 0.20–0.39; moderate agreement, κ = 0.40–0.59; very good agreement, κ = 0.60–0.79; excellent agreement, κ = 0.80–1.00.
Figure 4.
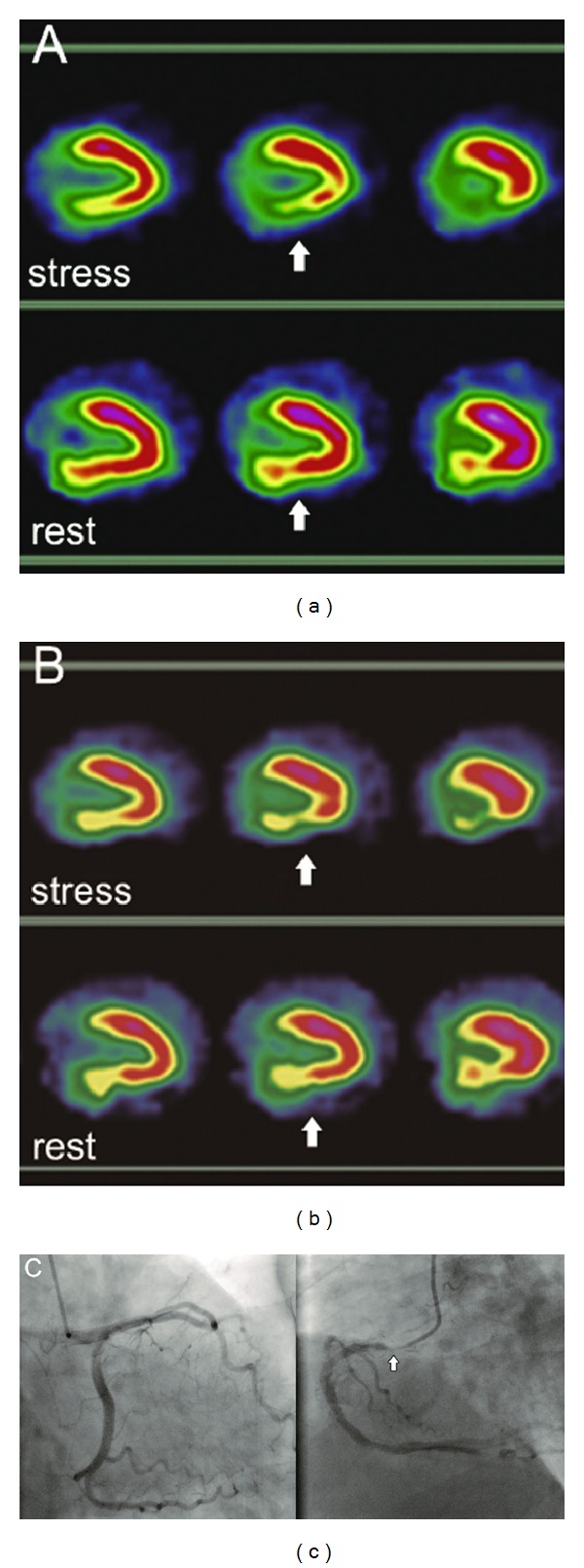
Abnormal MPI in the vertical long-axis slices of a representative patient. Both 99mTc-N-DBODC5 (a) and 99mTc-MIBI (b) images demonstrate an inferoposterior defect (white arrows). The defect is well visualized on two tracer images corresponding to the CA result. This coronary angiography (c) detected a stenosis of 90% in the RCA. The concordance for diagnosis of myocardial ischemia was seen on 99mTc-N-DBODC5 and 99mTc-MIBI studies.
3.6. Detection of Disease in Individual Coronary Vessels
Table 4 also shows the sensitivity and specificity of the two tracers in detecting individual-stenosed vessels. Of a total of 138 arteries in 46 patients, 48 arteries had significant stenoses, and 90 had insignificant lesions or were normal. Overall figures for sensitivity and specificity in identifying individual-stenosed vessels were 67% (32/48) and 86% (77/90) for 99mTc-MIBI imaging; overall sensitivity and specificity to diagnose individual significantly stenosed vessels were 69% (33/48) and 92% (83/90) for 99mTc-N-DBODC5 SPECT studies.
On the other hand, sensitivity and specificity for RCA stenosis vessel lesion detection using 99mTc-MIBI MPI were 87% and 68% compared to 87% and 87% for 99mTc-N-DBODC5 MPI, LAD stenosis (53% sensitivity and 96% specificity for 99mTc-MIBI, 63% sensitivity and 96% specificity for 99mTc-N-DBODC5, resp.), and left circumflex (LCX) (64% sensitivity and 94% specificity for 99mTc-MIBI, 57% sensitivity and 94% specificity for 99mTc-N-DBODC5, resp.). Angiography agreement was very good for 99mTc-N-DBODC5 (κ = 0.63 for LAD; κ = 0.71 for RCA, resp.) and moderate for 99mTc-MIBI (κ = 0.52 for LAD; κ = 0.48 for RCA, resp.). In contrast, the angiography agreement was very good for 99mTc-MIBI (κ = 0.62 for LCX) and moderate for 99mTc-N-DBODC5 (κ = 0.55 for LCX) (Table 4). Overall, the specificity of 99mTc-N-DBODC5 SPECT to detect individual RCA stenosis was better (27/31, 87%) than that of 99mTc-MIBI SPECT (21/31, 68%), despite having no statistical significance.
4. Discussion
These preliminary results demonstrate that stress-rest myocardial perfusion SPECT with 99mTc-N-DBODC5 is a sensitive method for detecting CAD and identifying stenosed coronary arteries. For the detection of CAD, more importantly, 99mTc-N-DBODC5 MPI reached good agreement compared with CA (κ = 0.73).
4.1. Advantages of 99mTc-N-DBODC5
99mTc-N-DBODC5 is a new lipophilic, monocationic, and nitride 99mTc-labeled tracer that is rapidly cleared from the liver after intravenous injection. It possesses good stability under physiological conditions. Importantly, 99mTc-N-DBODC5 exhibits more rapid liver washout than either 99mTc-MIBI or 99mTc-tetrofosmin. For example, at 60 min after injection in rats, the heart/liver ratio of 99mTc-N-DBODC5 is approximately ten times higher than that of 99mTc-MIBI or 99mTc-tetrofosmin. Preclinical studies have shown that 99mTc-N-DBODC5 SPECT can identify previous ischemia as areas of reduced tracer uptake [25, 26]. Furthermore, the rapid liver clearance and high uptake in the myocardium of 99mTc-N-DBODC5 will allow SPECT images of the left ventricle to be acquired early and with excellent quality [22]. The ratios of heart-to-liver were consistent with the previous report study [22]. These characteristics are suitable, particularly for patients with suspected acute coronary syndromes and without diagnostic electrocardiogram, because early diagnosis is needed in such patients for timely therapeutic decision making. 99mTc-MIBI, on the other hand, may require a wait time of up to 1 h for imaging.
In the present study, the heart-to-organ count ratio of 99mTc-N-DBODC5 MPI was superior to that of 99mTc-MIBI in 46 patients. Compared with 99mTc-MIBI MPI, 99mTc-N-DBODC5 MPI had better image quality in most patients. Furthermore, in this study, exercise stress myocardial images were performed 30 min after 99mTc-N-DBODC5 injection, and excellent MPI with high contrast was possible.
4.2. Clinical Value of 99mTc-N-DBODC5 for the Detection of CAD
This clinical trial assesses the diagnostic value of this agent to detect CAD comparing it with stress 99mTc-MIBI MPI and CA.
In this study, compared with 99mTc-MIBI MPI, 99mTc-N-DBODC5 MPI indicates the same sensitivity and better specificity and accuracy to detect coronary disease in patients, although none of the differences were significant. On the other hand, more importantly, angiography agreement was very good for 99mTc-N-DBODC5 (κ = 0.73) and moderate for 99mTc-MIBI (κ = 0.52); thus, compared with 99mTc-MIBI MPI, it provides a high degree of concordance for the evaluation of CAD, specifically in excluding perfusion abnormalities in patients with suspected CAD. Possible reasons for this difference may be fast liver clearance, favorable heart-to-organ ratio, and high image quality. 99mTc-N-DBODC5 with rapid liver clearance may significantly reduce the photon scatter from the liver into the inferoposterior walls. This reduces the artifactual decreased myocardial perfusion and improves the diagnostic accuracy for the detection of CAD compared with other 99mTc-labeled perfusion agents [24].
Unsurprisingly, Braat et al. [35] and Germano et al. [36] noted that technetium-labeled MPI agents with a fast liver clearance can significantly reduce the photon scatter from the liver into the inferior walls, but the radioactivity may be transferred to the gastrointestinal area, and increased bowel and gastric activity were seen as a problem associated with high liver uptake in the visual and quantitative interpretation of the inferoposterior myocardial walls. In this study, bowel uptake was frequently seen on the 99mTc-N-DBODC5 images, but did not result in any nondiagnostic scans.
For the detection of coronary disease in patients, 99mTc-MIBI produced abnormal results for MPI in four patients who had no angiographically detected stenosis. Some of these false-positive results may be due, in part, to the liver-to-heart artifacts. In this study, we could classify fixed perfusion defects as soft-tissue attenuation artifacts or infarcts by using gated SPECT. Because an artifactual defect would show normal contraction (wall motion or thickening) on a gated image, artifacts can be differentiated from a true infarct [37].
For the detection of individual vessel stenosis, on the other hand, angiography agreement was very good for 99mTc-N-DBODC5 (κ = 0.71 for RCA) and moderate for 99mTc-MIBI (κ = 0.48 for RCA). For LAD and LCX arteries stenosis, the diagnosis of myocardial ischemia on 99mTc-N-DBODC5 and 99mTc-MIBI study had the same specificity; and it was similar to that of two tracer to diagnose sensitivity and accuracy of myocardial ischemia. Abnormal results of myocardial images in individual RCA vessel stenosis (six arteries-stenosed vessels for RCA) were obtained in the 99mTc-MIBI studies, while CA and 99mTc-N-DBODC5 studies showed normal findings in the same individual RCA vessel territories. Some of these false-positive results may be due to photon scatter from the liver and intestine into the inferoposterior walls. 99mTc-MIBI concentration located below the left diaphragm (i.e. liver and bowel) may cause an artifactual perfusion defect in the adjacent myocardial, a phenomenon known as the “liver-heart artifact” [36, 38]. Moreover, the inferior and inferoposterior regions were dominated mainly by RCA territories corresponding to the myocardial short axis and vertical long axis; while arteries regions which LAD and LCX dominated were less interfered from “liver-heart artifact.” Finally, for clinical applications, 99mTc-N-DBODC5 offers better determination of detects, particularly in the inferoposterior wall on myocardial images. A typical example of a false-positive perfusion defect in the inferior wall is shown in Figure 5.
Figure 5.
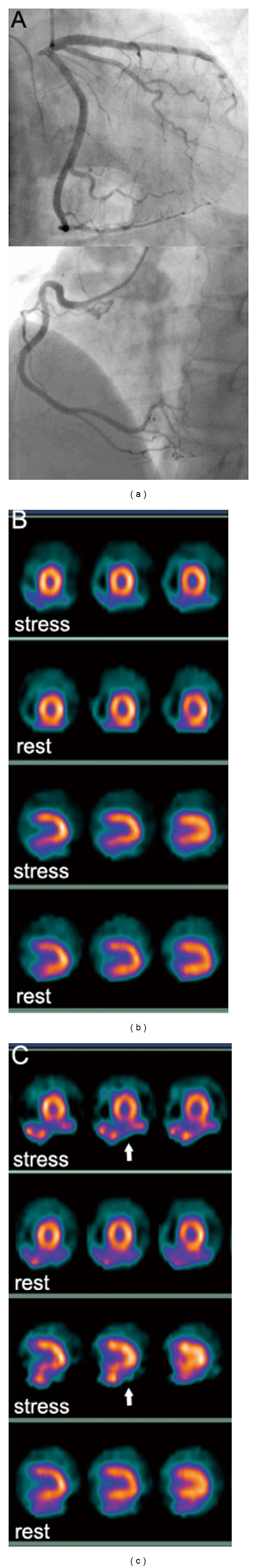
Serial short-axis and vertical long-axis slices of stress-rest 99mTc-N-DBODC5 images (b) and stress-rest 99mTc-MIBI images (c) of a representative patient with normal CA (a). Because of intense uptake of technetium 99mTc-MIBI in the liver, high liver background activity can be observed. Furthermore, a false-positive myocardial perfusion defect was also seen in the inferoposterior wall segments supplied by the RCA territory (white arrows). Importantly, however, at stress and rest, the inferoposterior wall segments of 99mTc-N-DBODC5 images are clearly separated from the subdiaphragmatic activity.
On a segment-to-segment basis, complete agreement between the two imaging agents occurred in 71% of segments. However, more myocardial segments were nonreversible on 99mTc-MIBI images than on 99mTc-N-DBODC5 images. On the contrary, 99mTc-N-DBODC5 MPI had a higher reversible defect than that of 99mTc-MIBI. A possible reason for this difference may be due, in part, to the “liver-heart artifact.” The presence of high liver activity adjacent to the inferior wall results from oversubtraction of activity from the inferior wall. Therefore, the more “liver-heart artifact” in the inferoposterior wall on myocardial images, the more false nonreversible defects in the inferoposterior wall on myocardial perfusion segmental analysis. In other words, the less reversible defects in the inferoposterior wall were deduced on 99mTc-MIBI MPI segmental analysis.
More importantly, however, the main advantage and clinical value of 99mTc-N-DBODC5 can improve diagnostic specificity and reduce the false-positive diagnosis of patients and associated treatment fees.
5. Limitations
In this study, we did not attempt to assess the absolute diagnostic accuracy of 99mTc-N-DBODC5, because definitive conclusions in this regard can only be drawn by studying larger patient cohort. In addition, although 99mTc-N-DBODC5 did not track flow as well as 201Tl, the magnitudes of the pharmacologic stress-induced perfusion defects were comparable to those previously reported for 99mTc-tetrofosmin. Thus, direct comparison between 99mTc-N-DBODC5 and 99mTc-tetrofosmin should be further proved in the same patient population. Overall, 99mTc-N-DBODC5 MPI will become an important diagnostic tool in the evaluation of myocardial perfusion.
6. Conclusion
This preliminary clinical study showed that 99mTc-N-DBODC5 and 99mTc-MIBI MPI provide comparable diagnostic information for patients undergoing exercise rest for detection of CAD. In addition, 99mTc-N-DBODC5 does not exhibit the disadvantages of 99mTc-MIBI in this study. By contrast, because of its high heart-organ count ratio in comparison to 99mTc-MIBI, it improves high degree of diagnostic concordance in defining or excluding perfusion abnormalities in patients with CAD. Therefore, it can become the agent of choice for the evaluation of myocardial perfusion and ventricular function in patients with CAD.
Ethics Statement
This study was conducted in accordance with the Helsinki Declaration. All subjects provided written informed consent before MPI, acknowledging that they understood their rights and obligations. This study was approved by the Research Ethics Committee of Shanxi Medical University.
Conflict of Interests
The authors declare that they have no conflict of interest.
Acknowledgments
This research is partly supported by Grants from the Youth Science and Technology Research Foundation of Shanxi Province (no. 2009021043-3). The authors acknowledge the members of the participating staff for their contribution to this clinical study.
Abbreviations
- CAD:
Coronary artery disease
- MPI:
Myocardial perfusion imaging
- CA:
Coronary angiography
- SPECT:
Single-photon emission computed tomography
- PET:
Positron emission computed tomography
- 99mTc-MIBI:
Technetium 99m sestamibi
- 99mTc-N-DBODC5:
99mTc(N)(DBODC)(PNP5)
- 201Tl:
Thallium-201
- 99mTc-tetrofosmin:
Technetium-99m-tetrofosmin
- ROI:
Regions of interest
- LAD:
Left anterior descending artery
- LCX:
Left circumflex artery
- RCA:
Right coronary artery
- SSS:
Summed stress score
- SRS:
Summed rest score
- SDS:
Summed difference score
- EDV:
End-diastolic volume
- ESV:
End-systolic volume
- LVEF:
Left ventricular ejection fraction.
References
- 1. National Hospital Discharge Survey, National Health Statistics Report, no. 5, 2006. [PubMed]
- 2.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. The New England Journal of Medicine. 2005;353(11):1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 3.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial sementation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 4.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Journal of the American College of Cardiology. 2003;41(1):159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 5.Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. European Heart Journal. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 6.Jensen JM, Øvrehus KA, Nielsen LH, Jensen JK, Larsen HM, Nørgaard BL. Paradigm of pretest risk stratification before coronary computed tomography. Journal of Cardiovascular Computed Tomography. 2009;3(6):386–391. doi: 10.1016/j.jcct.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Mollet NR, Cademartiri F, Van Mieghem C, et al. Adjunctive value of CT coronary angiography in the diagnostic work-up of patients with typical angina pectoris. European Heart Journal. 2007;28(15):1872–1878. doi: 10.1093/eurheartj/ehl563. [DOI] [PubMed] [Google Scholar]
- 8.Groutars RGEJ, Verzijlbergen JF, Tiel-Van Buul MMC, et al. The accuracy of 1-day dual-isotope myocardial SPECT in a population with high prevalence of coronary artery disease. International Journal of Cardiovascular Imaging. 2003;19(3):229–238. doi: 10.1023/a:1023637804898. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. The New England Journal of Medicine. 2010;362(10):886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iskandrian AE, Garcia EV, editors. Nuclear Cardiac Imaging Priniciples and Applications. 4th edition. New York, NY, USA: Oxford University Press; 2008. Pharmacologic stress testing; pp. 293–315. [Google Scholar]
- 11.Russell RR, III, Zaret BL. Nuclear cardiology: present and future. Current Problems in Cardiology. 2006;31(9):557–629. doi: 10.1016/j.cpcardiol.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Germano G, Kiat H, Kavanagh PB, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. Journal of Nuclear Medicine. 1995;36(11):2138–2147. [PubMed] [Google Scholar]
- 13.Underwood SR, Anagnostopoulos C, Cerqueira M, et al. Myocardial perfusion scintigraphy: the evidence. European Journal of Nuclear Medicine and Molecular Imaging. 2004;31:261–291. doi: 10.1007/s00259-003-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heo J, Iskandrian AS. Technetium-labeled myocardial perfusion agents. Cardiology Clinics. 1994;12(2):187–198. [PubMed] [Google Scholar]
- 15.Zaret BL, Rigo P, Wackers FJT, et al. Myocardial perfusion imaging with 99mTc tetrofosmin: comparison to 201Tl imaging and coronary angiography in a phase III multicenter trial. Circulation. 1995;91(2):313–319. doi: 10.1161/01.cir.91.2.313. [DOI] [PubMed] [Google Scholar]
- 16.Mahmarian JJ, Verani MS. Exercise thallium-201 perfusion scintigraphy in the assessment of coronary artery disease. American Journal of Cardiology. 1991;67(14):2D–11D. doi: 10.1016/s0002-9149(05)80002-5. [DOI] [PubMed] [Google Scholar]
- 17.Miller DD, Younis LT, Chaitman BR, Stratmann H. Diagnostic accuracy of dipyridamole technetium 99m-labeled sestamibi myocardial tomography for detection of coronary artery disease. Journal of Nuclear Cardiology. 1997;4(1):18–24. doi: 10.1016/s1071-3581(97)90045-3. [DOI] [PubMed] [Google Scholar]
- 18.He Z, Iskandrian AS, Gupta NC, Verani MS. Assessing coronary artery disease with dipyridamole technetium-99m-tetrofosmin SPECT: a multicenter trial. Journal of Nuclear Medicine. 1997;38(1):44–48. [PubMed] [Google Scholar]
- 19.Boschi A, Bolzati C, Uccelli L, et al. A class of asymmetrical nitrido 99mTc heterocomplexes as heart imaging agents with improved biological properties. Nuclear Medicine Communications. 2002;23(7):689–693. doi: 10.1097/00006231-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Boschi A, Uccelli L, Bolzati C, et al. Synthesis and biologic evaluation of monocationic asymmetric 99mTc-nitride heterocomplexes showing high heart uptake and improved imaging properties. Journal of Nuclear Medicine. 2003;44(5):806–814. [PubMed] [Google Scholar]
- 21.Bolzati C, Cavazza-Ceccato M, Agostini S, Tokunaga S, Casara D, Bandoli G. Subcellular distribution and metabolism studies of the potential myocardial imaging agent [99mTc(N)(DBODC)(PNP5)]+ Journal of Nuclear Medicine. 2008;49(8):1336–1344. doi: 10.2967/jnumed.108.051482. [DOI] [PubMed] [Google Scholar]
- 22.Cittanti C, Uccelli L, Pasquali M, et al. Whole-body biodistribution and radiation dosimetry of the new cardiac tracer 99mTc-N-DBODC. Journal of Nuclear Medicine. 2008;49(8):1299–1304. doi: 10.2967/jnumed.108.053132. [DOI] [PubMed] [Google Scholar]
- 23.Hatada K, Riou LM, Ruiz M, et al. 99mTc-N-DBODC5, a new myocardial perfusion imaging agent with rapid liver clearance: comparison with 99mTc-sestamibi and 99mTc-tetrofosmin in rats. Journal of Nuclear Medicine. 2004;45(12):2095–2101. [PubMed] [Google Scholar]
- 24.Takeda T, Wu J, Lwin TT. 99mTc-N-DBODC5: a novel myocardial perfusion imaging agent for diagnosis of coronary artery disease, a review. Recent Patents on Cardiovascular Drug Discovery. 2006;1(2):161–166. doi: 10.2174/157489006777442496. [DOI] [PubMed] [Google Scholar]
- 25.Hatada K, Ruiz M, Riou LM, et al. Organ biodistribution and myocardial uptake, washout, and redistribution kinetics of Tc-99m N-DBODC5 when injected during vasodilator stress in canine models of coronary stenoses. Journal of Nuclear Cardiology. 2006;13(6):779–790. doi: 10.1016/j.nuclcard.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Fang W, Li B, Wang X, He Z. Experimental study of [99mTc(PNP5) (DBODC)]+ as a new myocardial perfusion imaging agent. Cardiology. 2009;112(2):89–97. doi: 10.1159/000141013. [DOI] [PubMed] [Google Scholar]
- 27.Marmion ME, Woulfe SR, Neumann WL, Nosco DL, Deutsch E. Preparation and characterization of technetium complexes with Schiff base and phosphine coordination. 1. Complexes of technetium-99g and -99m with substituted acac2en and trialkyl phosphines (where acac2en = N,N′-ethylenebis[acetylacetone iminato]) Nuclear Medicine and Biology. 1999;26(7):755–770. doi: 10.1016/s0969-8051(99)00040-2. [DOI] [PubMed] [Google Scholar]
- 28.Bolzati C, Uccelli L, Boschi A, et al. Synthesis of a novel class of nitrido Tc-99m radiopharmaceuticals with phosphino-thiol ligands showing transient heart uptake. Journal of Inorganic Biochemistry. 2000;78(3):369–374. doi: 10.1016/s0969-8051(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 29.Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao S. Stress protocols and tracers. Journal of Nuclear Cardiology. 2006;13(6):e80–e90. doi: 10.1016/j.nuclcard.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Hansen CL, Goldstein RA, Berman DS, et al. Myocardial perfusion and function single photon emission computed tomography. Journal of Nuclear Cardiology. 2006;13(6):e97–e120. doi: 10.1016/j.nuclcard.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Flamen P, Bossuyt A, Franken PR. Technetium-99m-tetrofosmin in dipyridamole-stress myocardial SPECT imaging: intraindividual comparison with technetium-99m-sestamibi. Journal of Nuclear Medicine. 1995;36(11):2009–2015. [PubMed] [Google Scholar]
- 32.Tamaki N, Takahashi N, Kawamoto M, et al. Myocardial tomography using technetium-99m-tetrofosmin to evaluate coronary artery disease. Journal of Nuclear Medicine. 1994;35(4):594–600. [PubMed] [Google Scholar]
- 33.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Journal of Nuclear Cardiology. 2002;9(2):240–245. doi: 10.1067/mnc.2002.123122. [DOI] [PubMed] [Google Scholar]
- 34.Sharir T, Germano G, Kang X, et al. Prediction of myocardial infarction versus cardiac death by gated myocardial perfusion SPECT: risk stratification by the amount of stress-induced ischemia and the poststress ejection fraction. Journal of Nuclear Medicine. 2001;42(6):831–837. [PubMed] [Google Scholar]
- 35.Braat SH, Leclercq B, Itti R, Lahiri A, Sridhara B, Rigo P. Myocardial imaging with technetium-99m-tetrofosmin: comparison of one-day and two-day protocols. Journal of Nuclear Medicine. 1994;35(10):1581–1585. [PubMed] [Google Scholar]
- 36.Germano G, Chua T, Kiat H, Areeda JS, Berman DS. A quantitative phantom analysis of artifacts due to hepatic activity in technetium-99m myocardial perfusion SPECT studies. Journal of Nuclear Medicine. 1994;35(2):356–359. [PubMed] [Google Scholar]
- 37.Paul AK, Nabi HA. Gated myocardial perfusion SPECT: basic principles, technical aspects, and clinical applications. Journal of Nuclear Medicine Technology. 2004;32(4):179–187. [PubMed] [Google Scholar]
- 38.Nuyts J, Dupont P, Van den Maegdenbergh V, Vleugels S, Suetens P, Mortelmans L. A study of the liver-heart artifact in emission tomography. Journal of Nuclear Medicine. 1995;36(1):133–139. [PubMed] [Google Scholar]


