Abstract
Ozone, a pervasive environmental pollutant, adversely affects functional lung growth in children. Animal studies demonstrate that altered lung development is associated with modified signaling within the airway epithelial mesenchymal trophic unit, including mediators that can change nerve growth. We hypothesized that ozone exposure alters the normal pattern of serotonin, its transporter (5-HTT), and two key receptors (5-HT2A and 5-HT4), a pathway involved in postnatal airway neural, epithelial, and immune processes. We exposed monkeys to acute or episodic ozone during the first 2 or 6 months of life. There were three exposure groups/age: (1) filtered air, (2) acute ozone challenge, and (3) episodic ozone + acute ozone challenge. Lungs were prepared for compartment-specific qRT-PCR, immunohistochemistry, and stereology. Airway epithelial serotonin immunopositive staining increased in all exposure groups with the most prominent in 2-month midlevel and 6-month distal airways. Gene expression of 5-HTT, 5-HT2AR, and 5-HT4R increased in an age-dependent manner. Overall expression was greater in distal compared with midlevel airways. Ozone exposure disrupted both 5-HT2AR and 5-HT4R protein expression in airways and enhanced immunopositive staining for 5-HT2AR (2 months) and 5-HT4R (6 months) on smooth muscle. Ozone exposure increases serotonin in airway epithelium regardless of airway level, age, and exposure history and changes the spatial pattern of serotonin receptor protein (5-HT2A and 5-HT4) and 5-HTT gene expression depending on compartment, age, and exposure history. Understanding how serotonin modulates components of reversible airway obstruction exacerbated by ozone exposure sets the foundation for developing clinically relevant therapies for airway disease.
Key Words: bronchial epithelium, lung, Macaca mulatta, serotonin, 5-HT, 5-HT2AR, 5-HT4R, transporter
Ozone (O3) exacerbates respiratory diseases and adversely affects functional lung growth. Ozone levels exceed the established National Ambient Air Quality Standard in many regions of the United States, impacting over 115 million people, a quarter of which are children under 18 years of age (ALA, 2012). Exposure to ozone can contribute to deficiencies in pulmonary function and increase overall respiratory diseases such as asthma (Plopper et al., 2007; Tager et al., 2005). Specifically, ozone induces oxidant stress that may ultimately result in epithelial injury, inflammation, and repair (Hyde et al., 1999; Oslund et al., 2008; Springer et al., 2007). Understanding of how this pattern of injury, inflammation, and repair alters postnatal lung development is critical for evaluating the long-term health risk associated with early-life exposure to ozone.
Children are not simply “little adults” but rather represent a sensitive subpopulation (Selgrade et al., 2008) whose dynamic status of lung growth and maturation results in a spectrum of responses to oxidant stress (Plopper et al., 2007). The concept of “critical windows of susceptibility” arises, and it has been suggested that children may be more vulnerable to air pollution, depending on the extent to which key developmental processes are disrupted and the age at which the exposure occurs (Bearer, 1995; Pinkerton et al., 2007; Selgrade et al., 2008). Lung development spans both the pre- and postnatal periods, and cells mature within the airway tree in a proximal to distal manner (Plopper et al., 1992; Van Winkle et al., 2004). Hence, postnatal proximal airway regions have more mature, inherent metabolic and immunologic capabilities and may be differentially targeted by toxicants compared with more distal regions (Miller et al., 2005; Plopper et al., 1986, 1992). To study the effects of ozone inhalation on the developing lung requires an experimental approach that defines specific airway regions so that both maturation and developmental disruption can be studied at different ages.
Serotonin (5-HT) is the ligand for the 5-HT2AR and 5-HT4R receptors and is a monoamine neurotransmitter that modulates nerve development, airway contractility, and immune response (Fernandez-Madrid, 1977). Serotonin receptors 5-HT2A and 5-HT4 have roles in neuronal excitation (Ptak et al., 2009), smooth muscle contraction (Fink and Göthert, 2007), intracellular calcium modulation, and cytokine release (Bayer et al., 2007; Idzko et al., 2004). Exposure to various immune stimuli (Dürk et al., 2005; Meade et al., 2001; Weisbach et al., 1957) can increase 5-HT that, when bound to 5-HT receptors, induces cytokine release (Bayer et al., 2007) that facilitates enhanced mucin secretion, inflammation, and airway remodeling (Adner et al., 2002; Chen et al., 2003; Müller et al., 2009). Changes in 5-HT can also alter mucociliary clearance (König et al., 2009; Maruyama et al., 1984). Further, 5-HT is present in epithelia as a result of de novo synthesis via tryptophan hydroxylase or via 5-HTT-mediated uptake. Changes in 5-HTT expression or activity may further disrupt the normal epithelial milieu, leading to a more pathological phenotype (Dodson et al., 2004; Wells et al., 2009). Therefore, identifying alterations in the abundance and distribution of 5-HT, its transporter, and receptors in airway epithelia provides insight into how region-specific neuroimmune function is changed with the inhalation of ozone.
Though the relationship between 5-HT, the lung, and airway pathobiology has been suggested for decades (Buckner et al., 1991; Cazzola and Matera, 2000; Kajekar et al., 2007; Pelletier et al., 1981), only recently has the role of 5-HT and its receptors been interrogated in the context of early-life exposure to ozone (Moore et al., 2012). Moore and colleagues recently demonstrated that episodic ozone exposure in the first 6 months of life enhances 5-HT-modulated cholinergic airway smooth muscle contraction that is mediated in part by 5-HT2AR and 5-HT4R in infant rhesus monkeys. This work builds upon clinical research in asthmatics that suggests that 5-HT modifiers, including those that alter 5-HT uptake or 5-HT receptor activity, may be a viable therapeutic approach for managing the severity of asthmatic episodes and decrements in lung function (Lechin et al., 1998; Cazzola and Matera, 2000).
In this study, we examined how ozone exposure alters the localization and relative abundance of key serotoninergic pathway components in the developing airway. We hypothesized that the inhalation of ozone alters the normal distribution and abundance of serotonin, its transporter, and two key receptors (5-HT2A, 5-HT4). The objectives of this study were threefold: (1) to identify the normal early postnatal developmental pattern of serotonin, 5-HTT and these two receptors, (2) to define how acute ozone (AO) exposure impacts the serotonin pathway at two postnatal ages, and (3) to define how this acute response is modified by (a) a prior history of ozone exposure and (b) how age and duration of exposure history modulate the subsequent response to oxidant stress in conducting airways.
MATERIALS AND METHODS
Animals and exposure protocol.
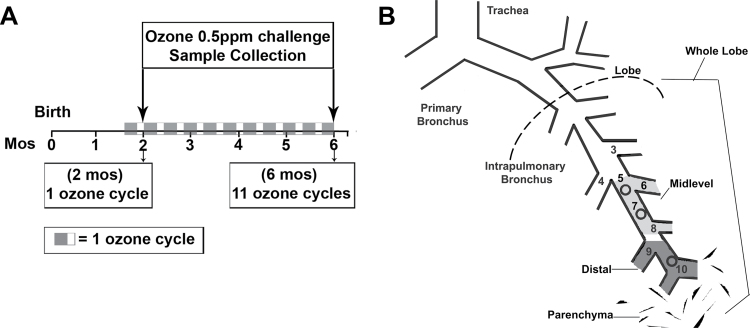
Twenty-four male infant rhesus monkeys were in two age groups, 2 and 6 months. Twelve animals from each age group were assigned to one of three exposure groups: filtered air (FA), FA + acute ozone (O3) challenge, or episodic biweekly O3 exposure cycles + acute O3 challenge (EAO). Animals were housed under FA conditions until exposures began at 1 month of age. Episodic O3 exposure cycle protocols, previously shown to result in remodeled postnatal airways (Fanucchi et al., 2006; Plopper et al., 2007), were as follows: 0.5 ppm O3 × 8h/day × 5 days of exposure, followed by 9 days of FA, repeated over a 14-day cycle (Fig. 1A) (Moore et al., 2012; Schelegle et al., 2003). The 5-days-on and 9-days-off regimens mimic the episodic nature of ozone exposure for humans where (1) patterns of unhealthy ozone often occur for a series of consecutive days then diminish, (2) outdoor laborers would likely incur a 5 days × 8h/day exposure scenario repeatedly, and (3) the 14-day cycle allows for multiple phases of cellular injury and repair to take place concurrently. AO challenge was a single 8-h exposure (0.5 ppm) just prior to necropsy at 2 or 6 months of age. Ozone exposures were administered for 8h overnight. There are six exposure groups (N = 4/group): (1) 2-month FA, (2) 2-month AO, (3) 2-month EAO, (4) 6-month FA, (5) 6-month AO, and (6) 6-month EAO. Additional details are given in Supplementary data.
FIG. 1.
Experimental design and sampling diagram. (A) Animals from both age groups (2 or 6 months) were randomly assigned to one of three exposure groups: (1) FA, (2) FA + single acute overnight ozone challenge 0.5 ppm × 8h, (3) two-week cycle of ozone 0.5 ppm 8h/day × 5 days then 9 days of FA only (EAO) (2 months—1 cycle, 6 months—11 cycles) + single acute overnight ozone challenge (0.5 ppm × 8h). Ozone cycles began at 1 month of age, and the overnight ozone challenge was administered just prior to necropsy at 2 or 6 months of age. (B) Tissue samples were microdissected from midlevel (generations 5–8) and distal (generations 9 through respiratory bronchiole) intrapulmonary airways, parenchyma, or whole lobe and subjected to gene and protein expression analysis.
Airway microdissection and qRT-PCR.
The right cranial lobe was inflated with Dulbecco’s Modified Eagle’s Medium: Nutrient Mixture F-12 Ham media (Sigma) and microdissected on ice. Midlevel (~2mm thick) and distal (~1mm thick) airway pieces containing intrapulmonary generations ~5–8 and generations 9 respiratory bronchiole, respectively (Fig. 1B), were removed and stored in RNA later solution (Ambion) at −20°C until processed for RNA isolation and qRT-PCR. 5-HT2AR, 5-HT4R, and 5-HT transporter (SLC6A4) gene expression was measured using qRT-PCR in microdissected airway pieces as previously described (Baker et al., 2004). Analysis was conducted using the ddCT method, which allows for fold change comparison relative to not only the internal reference gene but also between relevant groups and compartments when gene-specific data are analyzed under identical CT threshold conditions (Ahn et al., 2008). Samples were run in triplicate with RPL13A as the internal reference gene (sequence: 5′ primer CACGACGTTGGCTGGAAGT, 3′primer TCTTTCCTCTTCTCCTCCAAGGT, and probe CCAGGCAGTGACAGC) (Ahn et al., 2008). The 5-HT2AR, 5-HT4R, and 5-HT transporter reactions used TaqMan inventoried probe/primer assays (CATno. Hs01033524_m1, Hs00410577_m1, and Hs00169010_m1, respectively).
Immunohistochemistry staining.
The left caudal lobe was isolated, cannulated, and fixed in 1% paraformaldehyde at 25cm of hydrostatic pressure. Prior to paraffin embedment, the lobe was partially dissected to allow visualization of the axial pathway and schematic mapping of airway generations. The lobe was then divided into blocks containing 2–3 airway generations each. Blocks containing the defined axial airway generations were paraffin embedded and sectioned (5 µm). Paraffin sections from approximately four animals/treatment group were immunostained for 5-HT (purified rabbit polyclonal antibody 5-HT: S5545, Sigma), 5-HT2AR (purified rabbit polyclonal antibody 5-HT2AR: LS-A1106, Lifespan Biosciences, Inc.), or 5-HT4R (purified goat polyclonal antibody SR-4 (N-16): sc-19154, Santa Cruz Biotechnology, Inc.). Antigen retrieval buffer (BioGenex AR-10) and a decloaking chamber (BioCare Medical) were used for epitope retrieval. Sections from all groups were run together to minimize run-to-run variability following staining optimization and imaging as described in Sutherland et al. (2010).
Morphometric immunohistopathology.
The abundance of serotonin immunoreactive substance in the airway epithelium of midlevel (intrapulmonary generations 5–8) and distal (generations 9 through first alveolar outpockets and terminal/respiratory bronchiole) conducting airways was determined using stereologic assessment of lung structure (Hsia et al., 2010). Paraffin sections (5 µm thick) from 3–4 animals/group/age were immunostained for 5-HT. Whole slides were scanned at ×40 on an Olympus VS110 virtual microscopy system scanner (Olympus, Tokyo, Japan). Scanned images were evaluated at ×40 resolution for volume fraction and at ×10 resolution for surface per volume. The volume fraction of 5-HT-positive cells was estimated using point (P) and intercept (I) counting of bronchial epithelium, utilizing vertical uniform random sine weighted probes and Visiopharm Integrator System software (Visiopharm, Hørsholm, Denmark). Volume fraction was calculated as: Vv 5-HT (epi) = Pp = ΣPn/ΣPt, where Pp is the point fraction of Pn, the number of test points hitting the 5-HT positive epithelial cells, divided by Pt, the total points hitting the reference space (epithelium). To calculate the surface area of epithelial basement membrane per reference volume (Sv) by point and intercept counting, we used the formula: Sv bl (epi)= (2 ΣIbl)/((l/p) ΣPt (epi)), where Ibl is the number of intersections with the reference space (epithelial basal lamina), Pt is the number of points hitting the epithelium, and l/p is the length of cycloid test line per test point on the epithelium. The volume of 5-HT within the epithelial compartment per surface area of basal lamina (μm3/μm2) was calculated using the formula: Vs 5-HT,bl = Vv 5-HT (epi)/Sv bl (epi). To estimate the mean arithmetic mean thickness of airway epithelium, we used the formula 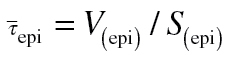 , where V(epi) is the point count estimated volume of the epithelium and S(epi) is the point and intercept estimated surface area of the epithelial basal lamina per volume of epithelium.
, where V(epi) is the point count estimated volume of the epithelium and S(epi) is the point and intercept estimated surface area of the epithelial basal lamina per volume of epithelium.
Statistical analysis.
The total number (N) of animals was 3–4 per group. Data are expressed as mean ± SEM, and statistical outliers are eliminated using the extreme studentized deviate method (Graphpad, La Jolla, CA). When appropriate, multivariate analysis of variance was applied against age, compartment, and exposure factors. Multiple comparisons for factors containing more than two levels were performed using Fisher’s protected least significant difference (PLSD) method. Pairwise comparisons were performed individually using a one-way ANOVA followed by PLSD post hoc analysis using StatView (SAS, Cary, NC). p Values of ≤ 0.05 were considered statistically significant.
RESULTS
5-HT and the Developing Postnatal Lung
First, we assessed the baseline developmental pattern of 5-HT. There were no significant age-related differences in abundance of 5-HT in the epithelium (Table 1) of 2- and 6-month-old FA animals in midlevel (Fig. 2A) or distal (Fig. 3A) airways. However, the spatial distribution of 5-HT in midlevel airway epithelium at 2 months of age (Fig. 2E) is clustered compared with the diffuse staining pattern observed at 6 months (Fig. 2F). In the distal airways, a more diffuse pattern was found at 2 months of age (Fig. 3C), whereas a clustered localization of 5-HT was found at 6 months of age (Fig. 3D). The distal airways had slightly greater overall abundance of 5-HT immunopositive cells compared with midlevel airways.
TABLE 1.
Serotonin Pathway Patterns in the Developing Lung
| 5-HT | 5-HTT | 5-HT2AR | 5-HT4R | ||
|---|---|---|---|---|---|
| Midlevel | 2 months | ++ | + | + | + |
| 6 months | ++ | + | +/++ | + | |
| Distal | 2 months | +++ | ++ | + | + |
| 6 months | +++ | +++ | +++ | ++++ |
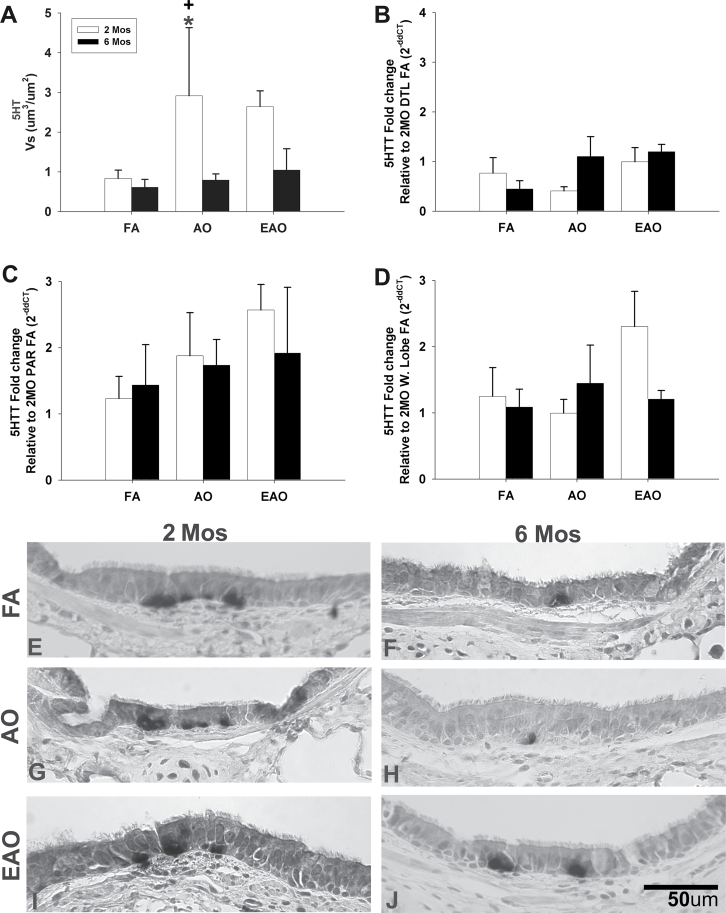
FIG. 2.
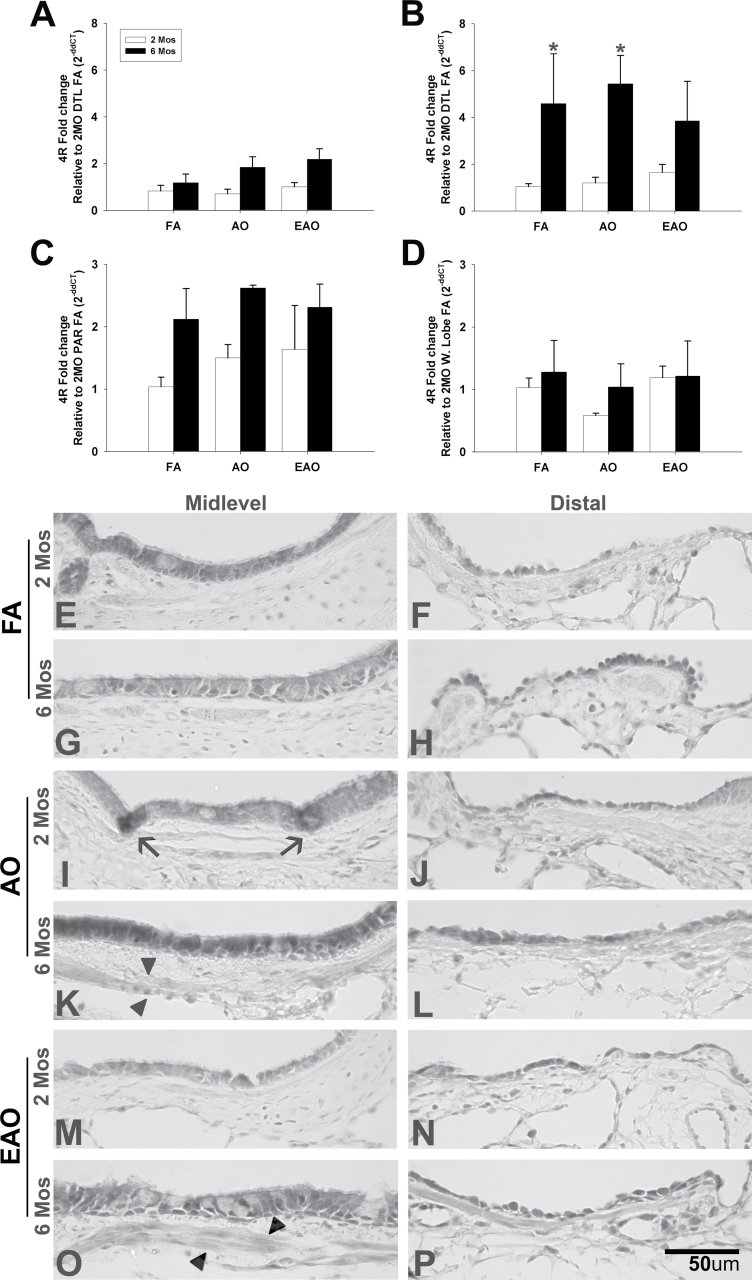
Midlevel airway 5-HT expression. 5-HT transporter gene expression and 5-HT. Volume per surface area, Vs, of serotonin immunopositive midlevel epithelial cells (A). 5-HTT transcription in midlevel airway (B), parenchyma (C), and whole-lobe (D) from 2- or 6-month-old monkeys exposed to FA, AO challenge or cyclic EAO. All transcription values at 6 months are analyzed as fold change relative to the 2-month FA group (e.g., 2-month distal [DTL] FA for midlevel airway, 2-month FA parenchyma [PAR] for parenchyma, and 2-month FA whole lobe [W. Lobe] for whole-lobe). Representative micrographs of 5-HT immunostained midlevel airways from FA control 2-month-old (E) and 6-month-old (F), AO-exposed 2-month-old (G) and 6-month-old (H), and EAO-exposed 2-month-old (I) and 6-month-old (J) animals. Compared with 2-month FA, epithelial 5-HT is increased with 2-month AO or EAO exposure. Compared with 6-month FA, only EAO increases 6-month epithelial 5-HT. Overall, 5-HT is greater in 2-month compared with 6-month midlevel airway epithelia. Data are expressed as mean ± SEM. N = 3–4/group/age, significance is claimed when p < 0.05 (*, different from matched FA group; +, different from treatment-matched 6-month group). Scale bar = 50 µm.
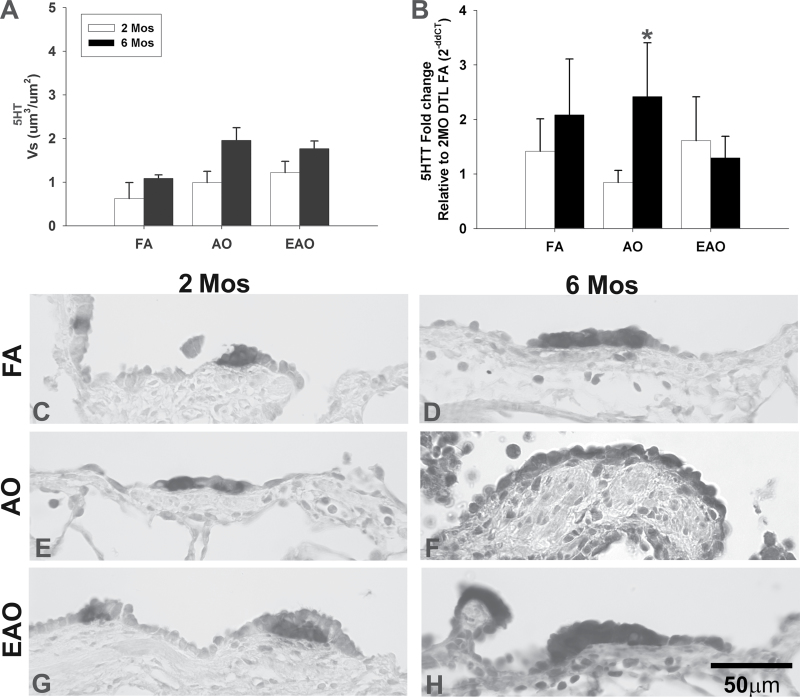
FIG. 3.
Distal airway 5-HT expression. 5-HT transporter gene expression and 5-HT in distal airways from 2- or 6-month-old monkeys exposed to FA, AO challenge, or cyclic EAO. Volume per surface area, Vs, of serotonin immunopositive cells in distal epithelium (A). 5-HTT transcription in distal airway (B), analyzed relative to 2-month distal (DTL) FA. AO exposure significantly reduces distal airway gene expression in 2-month-old animals compared with the treatment-matched 6-month group. Representative micrographs of 5-HT immunostained distal airways from FA control 2-month-old (C) and 6-month-old (D), AO-exposed 2-month-old (E) and 6-month-old (F), and EAO-exposed 2-month-old (G) and 6-month-old (H) animals. Compared with 2-month FA, epithelial 5-HT is relatively unchanged at 2 months with AO or EAO exposure. Compared with 6-month FA, AO or EAO increases epithelial 5-HT at 6 months. Overall, 5-HT is greater in 6-month compared with 2-month distal airway epithelia. Data are expressed as mean ± SEM. N = 3–4/group/age, significance is claimed when p ≤ 0.05 (*, different from treatment-matched 2-month group). Scale bar = 50 µm.
There is a nonsignificant trend for 5-HTT gene expression to increase in a proximal to distal pattern (Table 1), with midlevel epithelial mRNA levels (Fig. 2B) less than distal (Fig. 3B). Parenchyma and whole-lobe 5-HTT gene expression was slightly greater than that in conducting airways (Figs. 2C and D). Neither 5-HTT gene expression nor 5-HT varied significantly with age. Airway epithelial thickness (µm) was comparable between 2 and 6 months of age in each of the regions sampled (Supplementary fig. 2). However, the midlevel airway epithelium is significantly thicker, at nearly double the width (2 months, p = 0.001; 6 months, p = 0.007), compared with age-matched distal epithelium.
Ozone Disrupts Developmental 5-HT Expression
Ozone disrupts the normal developmental pattern of serotonin (Table 2). AO significantly increases the total amount of 5-HT (Fig. 2A) and changes the distribution (Fig. 2G) of 5-HT positive cells in midlevel epithelium of 2-month-old animals compared with age-matched FA controls (Figs. 2A and E) (Vs, p = 0.02). Distal airways at 2 months in AO animals (Figs. 3A and 4E) contained levels comparable to FA controls (Figs. 3A and C). AO has relatively no impact on 5-HT positive cells in midlevel airways of 6 month olds (Figs. 2A and H) but slightly increases 5-HT positive cells in distal airways (Figs. 3A and F). There were significantly greater (Vs, p = 0.03) 5-HT positive cells in midlevel airways of 2-month-old animals compared with 6-month-old animals following AO (Figs. 2A and H); however, there was no significant difference in distal airway 5-HT between 2- and 6-month-old animals. There was a trend (Vs, p = 0.06) for EAO to increase 5-HT in midlevel airways of 2-month-old animals. EAO only slightly increased 6-month midlevel 5-HT (Figs. 2A and J). Distal airways at 2 and 6 months in EAO animals (Figs. 3A and G) contained levels comparable to FA controls (Figs. 3A and C). Ozone, specifically acute exposure, disrupts overall 5-HT in midlevel airways at 2 months of age.
TABLE 2.
Impact of Ozone Exposure on Serotonin Pathway in Developing Lung
| 5-HT | 5-HTT | 5-HT2AR | 5-HT4R | ||
|---|---|---|---|---|---|
| AO | 2 months | ↑ Midlevel | ↓ Midlevel, ↓ Distal |
↑ Midlevel, ↑ Distal |
↑ Midlevel |
| 6 months | ↑ Distal | ↑ Midlevel, ↑ Distal |
↓ Midlevel, ↓ Distal |
↑ Midlevel | |
| EAO | 2 months | ↑ Midlevel | ↑ Midlevel, ↑ Distal |
↑ Midlevel, ↑ Distal |
No change |
| 6 months | ↑ Midlevel, ↑ Distal |
↑ Midlevel, ↓ Distal |
↑ Midlevel, ↑ Distal |
↑ Midlevel |
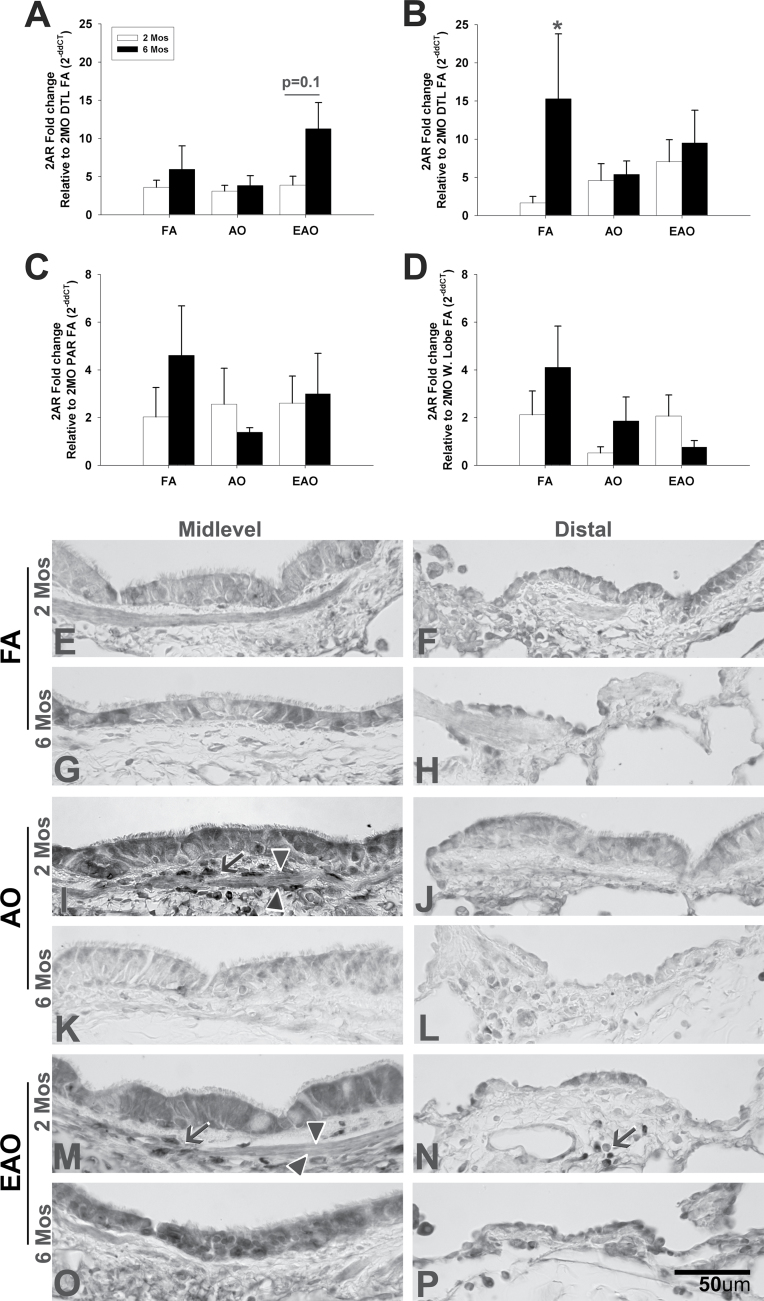
FIG. 4.
5-HT2A receptor expression. 5-HT2A receptor gene (A, midlevel; B, distal; C, parenchyma; D, whole lobe) and protein (E–P) expression in midlevel and distal airways from 2- or 6-month-old monkeys exposed to FA, AO challenge, or cyclic EAO. Representative micrographs of 5-HT2AR immunostained midlevel and distal airways from FA control 2-month-old (E, F) and 6-month-old (G, H), AO-exposed 2-month-old (I, J) and 6-month-old (K, L), and EAO-exposed 2-month-old (M, N) and 6-month-old (O, P) animals. Compared with 2-month FA, AO and EAO increased 5-HT2AR midlevel protein epithelial and airway smooth muscle staining, and EAO exposure increased subepithelial distal staining in 2-month-old animals. Compared with 6-month FA, EAO exposure increased midlevel and distal epithelial and airway staining. Overall, ozone had a greater impact on midlevel airways at 2 months and distal airways at 6 months. Arrowheads denote airway smooth muscle, and arrows note positive nonepithelial compartment staining. All transcription values are analyzed relative to the 2-month FA group (e.g., 2-month distal [DTL] FA for midlevel/distal airways, 2-month FA parenchyma [PAR] for parenchyma, and 2-month FA whole lobe [W. Lobe] for whole lobe). Data are expressed as mean ± SEM. N = 3–4/group/age, significance is claimed when p < 0.05 (*, different from treatment-matched 2-month group). Scale bar = 50 µm.
Similarly, ozone’s effect on 5-HTT gene expression was influenced by age, exposure type, and airway region. All mRNA values were analyzed relative to the 2-month FA group (e.g., 2-month distal FA for midlevel airway, 2-month FA parenchyma for parenchyma, and 2-month FA whole lobe for whole-lobe analysis). Overall, 5-HTT mRNA levels were less in midlevel (Fig. 2B) compared with distal (Fig. 3B) airways. Compared with FA, AO decreased and EAO increased 5-HTT mRNA in 2-month midlevel and distal airways. In 6-month-old animals, both ozone regimens increased midlevel airway mRNA expression but EAO slightly decreased distal 5-HTT expression compared with FA controls. In distal airways, 5-HTT mRNA expression was significantly greater in 6-month-old AO-exposed animals compared with matched 2 month olds (Fig. 3B) (p = 0.05). Analogous to ozone’s effect on 5-HT, AO exerted the greatest effect on 5-HTT. Unlike expression in specific conducting airway compartments, there were no statistically different treatment or age-dependent differences when nonairway tissue (Fig. 2C) or intact whole-lobe (Fig. 2D) tissue was analyzed.
Ozone did not change epithelial thickness (Supplementary fig. 2) except in the midlevel EAO airways of 6-month-old animals where thickness was significantly reduced compared to treatment-matched 2-month-old animals (p = 0.04).
Age, Airway Level, and Exposure Type Modulate 5-HT Receptor Expression
To evaluate how ozone impacts serotonin receptor gene and protein expression in developing postnatal airways, we assessed mRNA expression and relative protein abundance and distribution for receptors 5-HT2A (Fig. 4) and 5-HT4 (Fig. 5) in FA-exposed midlevel and distal airway epithelium (Table 1). Developmentally, gene and protein expression of both receptors appear to be established by 2 months of age in midlevel airways (Figs. 4A, E, and G; Figs. 5A, E, and G). Distally, both 5-HT2AR (Figs. 4B, F, and H) and 5-HT4R (Figs. 5B, F, and H) gene expression are significantly increased at 6 months of age compared with 2-month-old controls (5-HT2AR: p = 0.01, 5-HT4R: p = 0.009).
FIG. 5.
5-HT4 receptor expression. 5-HT4 receptor gene (A, midlevel; B, distal; C, parenchyma; D, whole lobe) and protein (E–P) expression in midlevel and distal airways from 2- or 6-month-old monkeys exposed to FA, AO challenge, or cyclic EAO. Representative micrographs of 5-HT4R immunostained midlevel and distal airways from FA control 2-month-old (E, F) and 6-month-old (G, H), AO-exposed 2-month-old (I, J) and 6-month-old (K, L), and EAO-exposed 2-month-old (M, N) and 6-month-old (O, P) animals. Compared with 2-month FA, AO exposure shifted protein expression from a dispersed to a highly focal pattern in midlevel airways with relatively no distal impact in 2-month-old animals. Compared with 6-month FA, AO or EAO exposure increased midlevel and distal epithelial and midlevel airway smooth muscle staining in 6-month-old animals. Overall, ozone had a greater impact on midlevel airways at 6 months. Distal 5-HT4R gene and protein expression increased developmentally with age, independent of exposure. Arrowheads denote airway smooth muscle, and arrows note focal positive epithelial staining. All transcription values are analyzed relative to the 2-month FA group (e.g., 2-month distal [DTL] FA for midlevel/distal airways, 2-month FA parenchyma [PAR] for parenchyma, and 2-month FA whole lobe [W. Lobe] for whole lobe). Data are expressed as mean ± SEM. N = 3–4/group/age, significance is claimed when p < 0.01 (*, different from treatment-matched 2-month group). Scale bar = 50 µm.
Compared with FA controls, midlevel airway 5-HT2AR gene expression (Fig. 2A) from ozone-exposed 2-month-old animals does not vary. However, midlevel 5-HT2AR protein expression in both acute (Fig. 4I) and episodically (Fig. 4M) exposed animals not only increased in airway epithelium but also localized on subepithelial airway smooth muscle (arrowheads). At 6 months, midlevel 5-HT2AR varied based on exposure type with both gene and protein expression slightly decreased with acute exposure (Figs. 4A and K) and enhanced with episodic exposure (Figs. 4A and O), though not significantly (p = 0.1). In distal airways, both ozone regimens increased mRNA levels of 5-HT2AR at 2 months of age and decreased levels at 6 months of age, relative to 2-month distal FA control (Fig. 4B). Similarly, both acute (Fig. 4J) and episodic (Fig. 4N) ozone increased 5-HT2AR protein expression in 2-month distal airways. 5-HT2AR protein was found adjacent to the airway following episodic exposure (arrow). Akin to the gene expression (Fig. 4B), AO (Fig. 4L) decreased 5-HT2AR protein expression in the distal epithelium of 6 month olds compared with FA control (Fig. 4H). EAO, however, slightly decreased 5-HT2AR mRNA levels relative to age-matched controls but increased protein expression. There were no statistically relevant differences in 5-HT2AR gene expression in the parenchyma (Fig. 4C) or whole-lobe (Fig. 4D).
Similar to midlevel 5-HT2AR gene expression, ozone did not alter 5-HT4R mRNA expression (Fig. 5A) in 2-month midlevel airways but did change protein epithelial expression in both acute and episodically exposed animals. AO (Fig. 5I) resulted in a more condensed, focal pattern of 5-HT4R protein (arrows) compared with FA controls, whereas episodic exposure (Fig. 5M) slightly decreased it. At 6 months of age, both acute and episodic ozone enhanced not only midlevel gene (Fig. 5A) and protein epithelial 5-HT4R expression (Figs. 5K and O) but also induced subepithelial airway smooth muscle expression as well (arrowheads) compared with FA controls. In the distal airways, ozone had relatively no impact on 5-HT4R gene expression at (Fig. 5B). Compared with age-matched midlevel and 2-month compartment-matched groups, 6-month distal 5-HT4R gene expression was markedly increased with ozone-treated groups showing levels similar to the normal FA developmental pattern (p = 0.009). Distal 5-HT4R protein expression (Fig. 5) slightly increased with age but was relatively unaffected by ozone with staining comparable to that of FA controls (Figs. 5F and H). There were no statistically relevant differences in 5-HT4R transcription in parenchyma (Fig. 5C) or whole-lobe (Fig. 5D). Findings are summarized in Table 2.
DISCUSSION
Ozone affects development of the lung in the postnatal period. This study determined how certain elements of this exposure affect the serotonergic pathways including exposure during a susceptible window (first 6 months of life), acute exposure versus age of development, acute response as modified by previous episodic exposure, and how this acute response is amended by age. We found that ozone targets the serotonergic pathway in conducting airways, but age and history of exposure dictate which airway level is affected. We conclude that the first 6 months of life present a vulnerable window, age and exposure history dictate the effects of ozone on the 5-HT pathway and that distribution of 5-HT within the airway compartment modulates the degree to which ozone disrupts neuroimmune processes in the airway epithelium.
Previous exposure studies on the rhesus monkey have identified the first 6 months of postnatal life as a critical window of ozone susceptibility during which exposure to 0.5 ppm ozone results in a more responsive and nerve-remodeled airway (Kajekar et al., 2007; Larson et al., 2004). Because the rhesus monkey is a heterogeneous population with some animals as “high responders” and some as “low responders” (much like humans), we use a fairly high dose, similar to a bad day in Mexico City (Sánchez, 2008) and 4× the 1-h peak O3 level allowed by U.S. NAAQS (U.S. EPA, 2006), in our studies. Fanucchi et al. (2006) have shown that episodic ozone exposure during this period results in fewer nonalveolarized airway generations, hyperplastic bronchiolar epithelium, and altered orientation of airway smooth muscle in distal bronchioles. Episodic exposure has been shown to result in more airway remodeling effects than a chronic exposure (Barr et al., 1990). We selected the 6-month time point to characterize serotonin pathway expression during this more ozone-responsive period of development. The 2-month time point was selected for multiple reasons including to (1) identify early and late developmental trends in serotonin pathway expression during this susceptible window, (2) evaluate ozone-induced changes at an early versus later time point during this window of susceptibility, and (3) identify differences in response to an acute exposure following a single, 14-day cycle (5-day O3, 9-day FA) (2-month EAO) versus no prior ozone history (2-month AO). As ozone cycles for both age groups began at 1 month, the 2-month time point was appropriate to evaluate effects following one 14-day cycle (5-day O3, 9-day FA).
Moore et al. (2012) demonstrated that serotonin enhances cholinergic-mediated smooth muscle contraction in extrapulmonary airways (trachea and bronchi) from rhesus monkeys episodically exposed to ozone during their first 6 months of life. Further they found that 5-HT2A and 5-HT4 receptor agonists and antagonists exacerbate or mitigate airways responsiveness, respectively. Hence, our study builds upon this work to define 5-HT receptor distribution within the intrapulmonary bronchial tree, a region where changes in airway responsiveness would have a deleterious impact on breathing. It was important to determine whether the airway remodeling previously described for this region would also affect the distribution and abundance of the 5-HT pathway, and, indeed, we found that this remodeling also includes changes in the distribution and abundance of serotonin and serotonin-responsive receptors in distal conducting airways.
Serotonin is present in various lung cell types including platelets and mast cells, as well as airway epithelial basal and pulmonary neuroendocrine cells (PNEC) (Fernandez-Madrid, 1977). Mature levels of 5-HT are established in midlevel airway epithelium by 2 months (Figs. 2 and 3). Airway smooth muscle and 5-HT positive cell types can also express 5-HT receptors (Bayer et al., 2007; Segura et al., 2010). However, the cellular distribution of 5-HT shifts with age. These shifts could be multifactorial including proximity to specific 5-HT receptor subtypes or 5-HT transporter activity. Martel et al. (2003) demonstrated that neuronal 5-HTT immunoreactive cells and transporter activity were concentrated in either the apical or the basolateral aspects of enteric epithelial cells and that uptake activity and direction were driven by select ion concentration and potential as a means to regulate intraluminal versus interstitial serotonin concentration. Serotonin’s role in nerve development is complex and includes regulation of neurotrophic, maturation and neuroimmune processes (Fink and Göthert, 2007; Idzko et al., 2004; Müller et al., 2009). Shifts in the location and distribution of 5-HT positive cells throughout the airway epithelium may have a role in both normal development and airway pathology.
The lung is essential to maintain homeostatic levels of serotonin, and excess levels are removed from the systemic circulation, via its transporter, and transferred to the lower pH pulmonary extravascular compartment (Gaddum et al., 1953; Fernandez-Madrid, 1977). Gene expression levels of 5-HTT increase developmentally in a proximal to distal manner in airway epithelium with slight variation between ages (Figs. 2 and 3). Ozone exposure, whether acute or episodic, generates only slight changes at 2 months but more notable deviations from control at 6 months. Whether the varying volume of epithelial 5-HT observed in this study is partly the result of compensatory changes in 5-HTT regulation with altered cellular uptake due to ozone exposure, a 5-HTT independent change or a change in 5-HT de novo synthesis is beyond the scope of this study. However, increased 5-HT levels in plasma have been found in asthmatics during periods of exacerbation (Lechin, 1996). It is worth considering that site-specific changes in 5-HT transporter activity may lead to abnormal concentrations of 5-HT, possibly derived from the circulation.
It is possible that there is a correlation between the native 5-HT distribution in the airway epithelium during a specific developmental stage and the ability of ozone to disrupt the normal pattern and result in a more responsive airway. Interestingly, the compartment-age groupings that are most altered by ozone exposure are those that have a more clustered rather than diffuse 5-HT distribution arrangement at the time of exposure. It is quite probable that the distribution of specific 5-HT positive cell types, such as PNEC’s, and their innate regulatory capabilities at certain stages of development make them more or less vulnerable to ozone-induced changes (Chua and Perks, 1999; Cyphert et al., 2009; Dayer et al., 1985; Fernandez-Madrid, 1977). This may be the case when altered 5-HT levels induce the release of IL6 and IL8 (Bayer et al., 2007), resulting in airway remodeling, altered mucin secretion, and increased leukocyte infiltration (Adner et al., 2002; Chen et al., 2003; Müller et al., 2009). Mucus production and mucociliary clearance are also regulated in part by 5-HT, and disruption of homeostatic levels may result in hypersecretion and aberrant clearance rates, both characteristics of airway disease (König et al., 2009; Maruyama et al., 1984). Future ozone exposure studies evaluating 5-HT receptor activity and pulmonary mechanics during this 2–6 month postnatal window could further elucidate serotonin’s role in a more responsive, asthma-like airway.
Pharmacologic studies in rodents implicate 5-HT2AR and 5-HT4R, as mediators of airways hyperresponsiveness (Adner et al., 2002; Buckner et al., 1991; Cohen et al., 1985; Segura et al., 2010). It is well established that oxidants can hyperexcite nerves that innervate airway smooth muscle and manifest asthma-like bronchospasm (Szarek and Schmidt, 1990). Recently, it was demonstrated that ozone exposure exacerbates serotonin-modulated bronchoconstriction, mediated in part by receptors 5-HT2A and 5-HT4, in a postnatal allergic monkey model of asthma, thus providing a feasible link between ozone, altered postnatal lung response, and serotonin (Moore et al., 2012). Our studies of the distribution of these two receptors during lung development support these findings in that we show both receptors are present in the airway epithelium and within smooth muscle. Ozone disrupts receptor gene and protein expression depending on age, prior exposure history, and airway compartment with the latter being the greatest determinant of developmental or ozone-induced 5-HT receptor expression. Though both receptors have increased ozone-induced protein expression in similar cell types including airway epithelium and airway smooth muscle, peak levels appear to be age and compartment specific with 5-HT2AR protein highest in 2-month midlevel and 6-month distal airways and 5-HT4R highest in 6-month midlevel airways.
Discrepancies between 2-month 5-HT2AR AO gene and protein expression could result from the fact that mRNA expression level is only measured at the end of the 8-h AO exposure. A surge in gene expression could have taken place earlier during exposure and returned to basal levels prior to sampling. For the EAO group, a similar phenomenon could have occurred with an initial transient increase in 5-HT2AR transcription that provoked a persistent increase in protein levels as a result of repeat exposures. At 6 months, 5-HT2AR gene and protein expression in midlevel airways is decreased by AO and increased by EAO. This difference in response to an acute versus episodic history of ozone exposure suggests that the developmental programming has shifted to a more responsive state as a result of repeated oxidant insult. As distal 5-HT2AR is undergoing more developmental changes between 2 and 6 months, it is feasible that ozone has a greater opportunity to disrupt the ongoing programming, making 2-month distal airways more vulnerable to ozone-induced modification than the midlevel compartment.
The ability of ozone to affect midlevel airways at 2 months and distal airways at 6 months may be due to airway size. Developing airways are very small and undergoing key stages of development at 2 months (midlevel) and 6 months (distal) with respect to the 5-HT pathway. Plopper et al. (1998) exposed young adult monkeys to AO (0.4 ppm) and assessed local site–specific ozone concentrations and the degree of epithelial damage. Factors contributing to the degree of epithelial injury and repair observed included resident concentration and utilization of glutathione, and other oxidant-related enzymes. It was evident that the ozone-induced damage was primarily a function of local ozone concentration, with distal conducting airways and respiratory bronchioles having the highest concentrations and greatest damage. It is not known how the smaller airway size and the earlier developmental stage of the airway epithelium of younger animals would alter this distribution of effect.
As ozone induces epithelial damage, including necrotic inflammation, changes in epithelial thickness often result (Barr et al., 1990; Plopper et al., 1998). In the current study, episodically exposed 6-month-old animals had significantly decreased midlevel and slightly decreased distal epithelial thickness compared with treatment- and compartment-matched 2-month-old animals (Supplementary fig. 2). This is likely attributed to reduced cilia length and enhanced sloughing of necrotic cells, common responses to episodic ozone exposure (Barr et al., 1990; Hyde et al., 1999; Jörres et al., 2000). AO exposure at either 2 or 6 months did not result in deviation from the normal epithelial thickness. We observed the trend of decreased epithelial thickness as a result of ozone exposure that is corroborated by other investigators (Plopper et al., 1998). However, we did not observe the same developmental decrease in midlevel epithelial thickness between 2 and 6 months as observed in another rhesus study using high-resolution sections and a different fixation (Van Winkle et al., 2004). This difference is likely due to the differences in how the samples were processed, and the use in this study of more distal airway generations to represent the midlevel compartment where the thickness is smaller, thus small increases are less readily measured.
Ozone exacerbates respiratory symptoms, especially in children. Serotonin modulates many of the pathophysiological components associated with these respiratory symptoms including smooth muscle contraction and cytokine-induced inflammatory remodeling. In this study, we examined how the postnatal lung responds to an acute oxidant challenge and how that response is altered by age and cumulative history of previous ozone exposure. We demonstrated that site-specific analysis is critical in understanding the effects of ozone on a key neural pathway within the developing lung. Our work defines the epithelial distribution of serotonin, its transporter, and two key receptors (5-HT2A, 5-HT4) repeatedly implicated to play a role in the triggering or exacerbation of reversible airway obstruction, essentially contributing to a more responsive airway with modified neuroimmune capabilities. Collectively, understanding how serotonin modulates components of reversible airway obstruction exacerbated by ozone exposure sets the foundation for developing clinically relevant therapies for airway disease.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences (ES000628, P51OD011107); U.S. Environmental Protection Agency (FP-91712201-1 to S.R.M.).
Supplementary Material
ACKNOWLEDGMENTS
We thank Patricia Edwards and Frank Ventimiglia for skilled technical assistance. This work is the product of all the efforts of faculty and staff in the California National Primate Research Center Respiratory Diseases Unit. The research described in this article has been funded in part by the U.S. EPA but has not been subject to the agency’s required peer and policy review and therefore does not necessarily reflect the values of the agency, and no official endorsement should be inferred. The authors, with the exception of Dr Van Winkle, declare no competing financial interests. Dr Van Winkle is funded by the American Petroleum Institute (API) to study naphthalene and has received honoraria from API to speak at conferences on naphthalene.
REFERENCES
- Adner M., Rose A. C., Zhang Y., Swärd K., Benson M., Uddman R., Shankley N. P., Cardell L. O. (2002). An assay to evaluate the long-term effects of inflammatory mediators on murine airway smooth muscle: Evidence that TNFalpha up-regulates 5-HT(2A)-mediated contraction. Br. J. Pharmacol. 137, 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K., Huh J. W., Park S. J., Kim D. S., Ha H. S., Kim Y. J., Lee J. R., Chang K. T., Kim H. S. (2008). Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Mol. Biol. 9, 78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALA (2012). The American Lung Association: State of the Air Report 2012 1–179
- Baker G. L., Shultz M. A., Fanucchi M. V., Morin D. M., Buckpitt A. R., Plopper C. G. (2004). Assessing gene expression in lung subcompartments utilizing in situ RNA preservation. Toxicol. Sci. 77, 135–141 [DOI] [PubMed] [Google Scholar]
- Barr B. C., Hyde D. M., Plopper C. G., Dungworth D. L. (1990). A comparison of terminal airway remodeling in chronic daily versus episodic ozone exposure. Toxicol. Appl. Pharmacol. 106, 384–407 [DOI] [PubMed] [Google Scholar]
- Bayer H., Müller T., Myrtek D., Sorichter S., Ziegenhagen M., Norgauer J., Zissel G., Idzko M. (2007). Serotoninergic receptors on human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 36, 85–93 [DOI] [PubMed] [Google Scholar]
- Bearer C. F. (1995). How are children different from adults? Environ. Health Perspect. 103(Suppl. 6),7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner C. K., Dea D., Liberati N., Krell R. D. (1991). A pharmacologic examination of receptors mediating serotonin-induced bronchoconstriction in the anesthetized guinea pig. J. Pharmacol. Exp. Ther. 257, 26–34 [PubMed] [Google Scholar]
- Cazzola M., Matera M. G. (2000). 5-HT modifiers as a potential treatment of asthma Trends Pharmacol. Sci. 21, 13–16 [DOI] [PubMed] [Google Scholar]
- Chen Y., Thai P., Zhao Y. H., Ho Y. S., DeSouza M. M., Wu R. (2003). Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 278, 17036–17043 [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Schenck K. W., Colbert W., Wittenauer L. (1985). Role of 5-HT2 receptors in serotonin-induced contractions of nonvascular smooth muscle. J. Pharmacol. Exp. Ther. 232, 770–774 [PubMed] [Google Scholar]
- Chua B. A., Perks A. M. (1999). The pulmonary neuroendocrine system and drainage of the fetal lung: Effects of serotonin. Gen. Comp. Endocrinol. 113, 374–387 [DOI] [PubMed] [Google Scholar]
- Cyphert J. M., Kovarova M., Allen I. C., Hartney J. M., Murphy D. L., Wess J., Koller B. H. (2009). Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J. Immunol. 182, 7430–7439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer A. M., De Mey J., Will J. A. (1985). Localization of somatostatin-, bombesin-, and serotonin-like immunoreactivity in the lung of the fetal rhesus monkey. Cell Tissue Res. 239, 621–625 [DOI] [PubMed] [Google Scholar]
- Dodson A. M., Anderson G. M., Rhoden K. J. (2004). Serotonin uptake and metabolism by cultured guinea pig airway smooth muscle cells. Pulm. Pharmacol. Ther. 17, 19–25 [DOI] [PubMed] [Google Scholar]
- Dürk T., Panther E., Müller T., Sorichter S., Ferrari D., Pizzirani C., Di Virgilio F., Myrtek D., Norgauer J., Idzko M. (2005). 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int. Immunol. 17, 599–606 [DOI] [PubMed] [Google Scholar]
- Fanucchi M. V., Plopper C. G., Evans M. J., Hyde D. M., Van Winkle L. S., Gershwin L. J., Schelegle E. S. (2006). Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L644–L650 [DOI] [PubMed] [Google Scholar]
- Fernandez-Madrid F. (1977). Serotonin, other biologically active principles, and the lung. Int. Anesthesiol. Clin. 15, 169–188 [PubMed] [Google Scholar]
- Fink K. B., Göthert M. (2007). 5-HT receptor regulation of neurotransmitter release. Pharmacol. Rev. 59, 360–417 [DOI] [PubMed] [Google Scholar]
- Gaddum J. H., Hebb C. O., Silver A., Swan A. A. (1953). 5-Hydroxytryptamine; pharmacological action and destruction in perfused lungs. Q. J. Exp. Physiol. Cogn. Med. Sci. 38, 255–262 [DOI] [PubMed] [Google Scholar]
- Hsia C. C. W., Hyde D. M., Ochs M., Weibel E. R. (2010). An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am. J. Respir. Crit. Care. Med. 181, 394–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde D. M., Miller L. A., McDonald R. J., Stovall M. Y., Wong V., Pinkerton K. E., Wegner C. D., Rothlein R., Plopper C. G. (1999). Neutrophils enhance clearance of necrotic epithelial cells in ozone-induced lung injury in rhesus monkeys. Am. J. Physiol. 277(6 Pt 1), L1190–L1198 [DOI] [PubMed] [Google Scholar]
- Idzko M., Panther E., Stratz C., Müller T., Bayer H., Zissel G., Dürk T., Sorichter S., Di Virgilio F., Geissler M., et al. (2004). The serotoninergic receptors of human dendritic cells: Identification and coupling to cytokine release. J. Immunol. 172, 6011–6019 [DOI] [PubMed] [Google Scholar]
- Jörres R. A., Holz O., Zachgo W., Timm P., Koschyk S., Müller B., Grimminger F., Seeger W., Kelly F. J., Dunster C., et al. (2000). The effect of repeated ozone exposures on inflammatory markers in bronchoalveolar lavage fluid and mucosal biopsies. Am. J. Respir. Crit. Care Med. 161, 1855–1861 [DOI] [PubMed] [Google Scholar]
- Kajekar R., Pieczarka E. M., Smiley-Jewell S. M., Schelegle E. S., Fanucchi M. V., Plopper C. G. (2007). Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir. Physiol. Neurobiol. 155, 55–63 [DOI] [PubMed] [Google Scholar]
- König P., Krain B., Krasteva G., Kummer W. (2009). Serotonin increases cilia-driven particle transport via an acetylcholine-independent pathway in the mouse trachea. PLoS One 4, e4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S. D., Schelegle E. S., Walby W. F., Gershwin L. J., Fanuccihi M. V., Evans M. J., Joad J. P., Tarkington B. K., Hyde D. M., Plopper C. G. (2004). Postnatal remodeling of the neural components of the epithelial-mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen. Toxicol. Appl. Pharmacol. 194, 211–220 [DOI] [PubMed] [Google Scholar]
- Lechin F., van der Dijs B., Orozco B., Jara H., Rada I., Lechin M. E., Lechin A. E. (1998). Neuropharmacologic treatment of bronchial asthma with the antidepressant tianeptine: A double-blind, crossover placebo-controlled study. Clin. Pharmacol. Ther. 64, 223–232 [DOI] [PubMed] [Google Scholar]
- Lechin F., van der Dijs B., Orozco B., Lechin M., Lechin A. E. (1996). Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann. Allergy. Asthma Immunol. 77, 245–253 [DOI] [PubMed] [Google Scholar]
- Martel F., Monteiro R., Lemos C. (2003). Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). J. Pharmacol. Exp. Ther. 306, 355–362 [DOI] [PubMed] [Google Scholar]
- Maruyama I., Inagaki M., Momose K. (1984). The role of serotonin in mucociliary transport system in the ciliated epithelium of frog palatine mucosa. Eur. J. Pharmacol. 106, 499–506 [DOI] [PubMed] [Google Scholar]
- Meade C. J., Dumont I., Worrall L. (2001). Why do asthmatic subjects respond so strongly to inhaled adenosine? Life Sci. 69, 1225–1240 [DOI] [PubMed] [Google Scholar]
- Miller L. A., Hurst S. D., Coffman R. L., Tyler N. K., Stovall M. Y., Chou D. L., Putney L. F., Gershwin L. J., Schelegle E. S., Plopper C. G., et al. (2005). Airway generation-specific differences in the spatial distribution of immune cells and cytokines in allergen-challenged rhesus monkeys. Clin. Exp. Allergy. 35, 894–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. D., Hyde D., Miller L., Wong E., Frelinger J., Schelegle E. S. (2012). Allergen and ozone exacerbate serotonin-induced increases in airway smooth muscle contraction in a model of childhood asthma. Respiration. 83, 529–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T., Dürk T., Blumenthal B., Grimm M., Cicko S., Panther E., Sorichter S., Herouy Y., Di Virgilio F., Ferrari D., et al. (2009). 5-hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One 4, e6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslund K. L., Hyde D. M., Putney L. F., Alfaro M. F., Walby W. F., Tyler N. K., Schelegle E. S. (2008). Activation of neurokinin-1 receptors during ozone inhalation contributes to epithelial injury and repair. Am. J. Respir. Cell Mol. Biol. 39, 279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G., Steinbusch H. W., Verhofstad A. A. (1981). Immunoreactive substance P and serotonin present in the same dense-core vesicles. Nature 293, 71–72 [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Balmes J. R., Fanucchi M. V., Rom W. N. (2007). Ozone, a malady for all ages. Am. J. Respir. Crit. Care Med. 176, 107–108 [DOI] [PubMed] [Google Scholar]
- Plopper C., St George J., Cardoso W., Wu R., Pinkerton K., Buckpitt A. (1992). Development of airway epithelium. Patterns of expression for markers of differentiation. Chest 101,(3 Suppl)2S–5S [PubMed] [Google Scholar]
- Plopper C. G., Hatch G. E., Wong V., Duan X., Weir A. J., Tarkington B. K., Devlin R. B., Becker S., Buckpitt A. R. (1998). Relationship of inhaled ozone concentration to acute tracheobronchial epithelial injury, site-specific ozone dose, and glutathione depletion in rhesus monkeys. Am. J. Respir. Cell Mol. Biol. 19, 387–399 [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Smiley-Jewell S. M., Miller L. A., Fanucchi M. V., Evans M. J., Buckpitt A. R., Avdalovic M., Gershwin L. J., Joad J. P., Kajekar R., et al. (2007). Asthma/allergic airways disease: Does postnatal exposure to environmental toxicants promote airway pathobiology? Toxicol. Pathol. 35, 97–110 [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Weir A. J., Nishio S. J., Cranz D. L., St George J. A. (1986). Tracheal submucosal gland development in the rhesus monkey, Macaca mulatta: Ultrastructure and histochemistry. Anat. Embryol. (Berl). 174, 167–178 [DOI] [PubMed] [Google Scholar]
- Ptak K., Yamanishi T., Aungst J., Milescu L. S., Zhang R., Richerson G. B., Smith J. C. (2009). Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J. Neurosci. 29, 3720–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez J. A., Ayala F. J. G. (2008). Recent trend in ozone levels in the metropolitan zone of Mexico city J. Mex. Chem. Soc. 52, 256–262 [Google Scholar]
- Schelegle E. S., Miller L. A., Gershwin L. J., Fanucchi M. V., Van Winkle L. S., Gerriets J. E., Walby W. F., Mitchell V., Tarkington B. K., Wong V. J., et al. (2003). Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol. Appl. Pharmacol. 191, 74–85 [DOI] [PubMed] [Google Scholar]
- Segura P., Vargas M. H., Córdoba-Rodríguez G., Chávez J., Arreola J. L., Campos-Bedolla P., Ruiz V., García-Hernández L. M., Méndez C., Montaño L. M. (2010). Role of 5-HT2A, 5-HT4 and 5-HT7 receptors in the antigen-induced airway hyperresponsiveness in guinea-pigs. Clin. Exp. Allergy 40, 327–338 [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Plopper C. G., Gilmour M. I., Conolly R. B., Foos B. S. (2008). Assessing the health effects and risks associated with children’s inhalation exposures–asthma and allergy. J. Toxicol. Environ. Health. A 71, 196–207 [DOI] [PubMed] [Google Scholar]
- Springer J., Groneberg D. A., Dinh Q. T., Quarcoo D., Hamelmann E., Braun-Dullaeus R. C., Geppetti P., Anker S. D., Fischer A. (2007). Neurokinin-1 receptor activation induces reactive oxygen species and epithelial damage in allergic airway inflammation. Clin. Exp. Allergy 37, 1788–1797 [DOI] [PubMed] [Google Scholar]
- Sutherland K. M., Combs T. J., Edwards P. C., Van Winkle L. S. (2010). Site-specific differences in gene expression of secreted proteins in the mouse lung: Comparison of methods to show differences by location. J. Histochem. Cytochem. 58, 1107–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarek J. L., Schmidt N. L. (1990). Hydrogen peroxide-induced potentiation of contractile responses in isolated rat airways. Am. J. Physiol. 258,(4 Pt 1), L232–L237 [DOI] [PubMed] [Google Scholar]
- Tager I. B., Balmes J., Lurmann F., Ngo L., Alcorn S., Künzli N. (2005). Chronic exposure to ambient ozone and lung function in young adults. Epidemiology 16, 751–759 [DOI] [PubMed] [Google Scholar]
- U.S. EPA (2006). Air Quality Criteria for Ozone and Related Photochemical Oxidants (2006 Final). U.S. Environmental Protection Agency, Washington, DC: [Google Scholar]
- Van Winkle L. S., Fanucchi M. V., Miller L. A., Baker G. L., Gershwin L. J., Schelegle E. S., Hyde D. M., Evans M. J., Plopper C. G. (2004). Epithelial cell distribution and abundance in rhesus monkey airways during postnatal lung growth and development. J. Appl. Physiol. 97, 2355–63; discussion 2354 [DOI] [PubMed] [Google Scholar]
- Weisbach H., Waalkes T. P., Udenfriend S. (1957). Presence of serotonin in lung and its implication in the anaphylaxis reaction. Science 125, 235–236 [DOI] [PubMed] [Google Scholar]
- Wells S. M., Buford M. C., Porter V. M., Brunell H. L., Bunderson-Schelvan M., Nevin A. B., Cardozo-Pelaez F., Holian A. (2009). Role of the serotonergic system in reduced pulmonary function after exposure to methamphetamine. Am. J. Respir. Cell Mol. Biol. 42, 537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.