Abstract
Esophageal cancer is a highly lethal and aggressive disease and a major public health problem worldwide. The incidence of esophageal cancer in the western hemisphere has increased by 400 % in the past few decades (Posner et al. 2011). Surgery is the mainstay of definitive management of esophageal cancer; however, the results of surgery alone have been dismal, with survival rates of approximately 15 to 20 % at 5 years (Hingorani et al., Clin Oncol 23:696–705, 2011). The last three decades have seen growing interest in various adjuvant and neoadjuvant treatment strategies, with an aim to improve disease control and overall survival. However, due to conflicting and often contradictory results, there was controversy on the ideal treatment paradigm. Recent evidence suggests an improvement in overall survival with neoadjuvant therapy, both chemotherapy and chemoradiotherapy, over surgery. In this review we address various issues concerning multimodality management of locally advanced esophageal cancers: Does neoadjuvant therapy offer a definite benefit over surgery alone? If so, which neoadjuvant strategy? Does the survival benefit outweigh the increased treatment related toxicity/morbidity? Finally, is neoadjuvant treatment the standard of care for locally advanced resectable esophageal cancer?
Keywords: Locally advanced esophageal carcinoma, Squamous cell carcinoma, Adenocarcinoma, Neoadjuvant chemoradiation, Neoadjuvant chemotherapy
Introduction
Esophageal cancer is the eighth most common malignancy and sixth most fatal malignancy in the world [3]. In India, it is currently the fifth most common cause of cancer related death amongst men [4]. It comprises two distinct histopathological types, squamous cell carcinoma (SCC) and adenocarcinoma (AC). The “esophageal cancer belt” [5] from the Middle East through central Asia to north-central China is the highest risk area for squamous carcinoma, whereas the west has had a virtual epidemic of adenocarcinoma in the last few decades. A definite clinical difference exists between the two, in terms of risk factors, patterns of spread and tumour behavior, which may potentially influence patient response to therapy. Most studies mention these as two separate entities; however the overall management is the same and despite the dissimilarities, surprisingly there have been no gross survival differences related to histological type [1]. This may be due to lack of evidence (and limited number of patients) rather than clear evidence that there is no difference.
Surgery remains the primary modality of treatment for localized and locally advanced esophageal cancer. Unfortunately, despite complete resections, overall survival has remained low perhaps due to high rates of locoregional and distant failure. To improve upon the existing treatment, a number of randomized controlled trials (RCTs) were conducted over the past three decades combining chemotherapy and/or radiotherapy in both neo-adjuvant as well as adjuvant settings. Recent cancer statistics [6] show an improving trend in the 5-year relative survival rates for esophageal cancers (5 % in 1970s to 19 % in 2001–2007), the difference in rates reported as statistically significant. Improvement in diagnostic and therapeutic modalities over the years may account for part of this change but this progress could also be probably credited to the multimodality treatment approach.
We reviewed the various RCTs conducted on neoadjuvant chemotherapy (NACT) and neoadjuvant chemoradiotherapy (NACTRT) in locally advanced esophageal cancers, as well as a comparison between the two. Definitive chemoradiation is the standard of care for upper third and locally advanced unresectable esophageal cancers, and the management of locally advanced gastro-esophageal cancer overlaps with that of gastric carcinoma where perioperative chemotherapy is the current recommendation (all of which are not part of this review). The discussion in this review is focused on the treatment of locally advanced, resectable thoracic esophageal cancers.
Locally Advanced Esophageal Carcinoma
Locally advanced esophageal cancers are those restricted to the esophagus or resectable peri-esophageal tissues (>T1, ≤T4a, AJCC/TNM1 system) and/or lymph nodal involvement (N1–3) in the absence of distant metastasis (M0). The latest AJCC/TNM classification schema categorizes tumours involving adjacent pleura, pericardium or diaphragm as resectable (T4a) whereas involvement of other adjacent structures like the aorta, trachea, vertebral body, etc as unresectable (T4b).
Standard diagnostic work up includes an upper esophago-gastrointestinal endoscopy with biopsy to confirm the diagnosis, with an endoscopic ultrasonography to differentiate locally advanced from early stage and unresectable disease. Staging investigations also include a contrast enhanced computed tomography of the thorax and upper abdomen, and a fibre-optic bronchoscopy for tumours located in the upper and middle third to assess resectability. There does seem to be a role of PET-CT in ruling out distant metastatic disease, better locoregional staging and assessing response to chemotherapy; however, the evidence to support this is not very strong. Careful patient selection is crucial in the management of esophageal cancers. Optimal treatment, even when evidence-based, should not overlook the patient’s general condition and ability to withstand the therapy, not only for neoadjuvant treatment but surgery as well.
Pros and Cons of Preoperative Chemotherapy and Radiotherapy
Several benefits of preoperative chemotherapy have been proposed. Some of the theoretical advantages are early treatment of micro-metastasis, down-staging of tumour to facilitate surgical resection, potential selection of “chemo-responsive” patients who would benefit from adjuvant chemotherapy, and better tolerance to preoperative as compared to postoperative chemotherapy. The potential benefit of radiotherapy is to achieve better local/locoregional control. Adding concurrent chemotherapy not only deals with the micro-metastasis but also acts as a radio-sensitizer. Concurrent neoadjuvant chemoradiotherapy theoretically combines all the above advantages and results in a higher rate of complete (R-0) resections and complete pathological responses. On the other hand, chemotherapy and radiotherapy related toxicity, morbidity and mortality have been a matter of concern. Non-compliance and intolerance are worrisome as the general health and nutritional status of those with esophageal carcinoma is usually already poor at presentation. Delay in starting definitive treatment (surgery) and more importantly disease progression in non-responders is a major setback for patients who would have otherwise been resectable. There is also a fear amongst surgeons that perioperative morbidity and mortality would be higher after neoadjuvant chemoradiotherapy.
Neoadjuvant Chemotherapy Plus Surgery Versus Surgery Alone
Earlier comparative (observational) studies using historical controls overestimated the benefits of chemotherapy [7], whereas individual RCTs from the 1990s failed to find a significant advantage in terms of overall survival with neoadjuvant chemotherapy. Two major randomized studies, the Intergroup 113 [8] and the Medical Research Council (MRC) [9] studies showed conflicting results—the former showing no difference and the latter showing a benefit with NACT. Table 1 gives an outline of the trials conducted comparing NACT-Surgery to Surgery alone. Various chemotherapy combinations have been used but the most commonly used regimen is cisplatin and infusional 5-fluorouracil. Other drugs that have been used are bleomycin, vinblastin, carboplatin, etoposide, and more recently paclitaxel (as combination regimens).
Table 1.
Randomized controlled trials: NACT-surgery versus surgery alone
| Trial/author | Year | No. of patients | Chemotherapy (cycles) | Histology |
|---|---|---|---|---|
| Roth | 1982 | 39 | Cisplatin/Vindesine/Bleomycin (2) | Squamous |
| Nygaard | 1983 | 81 | Cisplatin/Bleomycin (2) | Squamous |
| Schlag | 1988 | 46 | Cisplatin/5-FU (3) | Squamous |
| Maipang | 1988 | 46 | Cisplatin/Bleomycin (2) | Squamous |
| Law | 1989 | 147 | Cisplatin/5-FU (2) | Squamous |
| Boonstra | 1989 | 169 | Cisplatin/Etoposide (2) | Squamous |
| Kelsen | 1990 | 467 | Cisplatin/5-FU (3) | Squamous/Adeno |
| Ancona | 1992 | 96 | Cisplatin/5-FU (2) | Squamous |
| Allum | 1992 | 802 | Cisplatin/5-FU (2) | Squamous/Adeno |
| Ychou | 1995 | 169 | Cisplatin/5-FU (6 periop) | Adeno |
Adapted with permission from Sjoquist KM et al: survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable esophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12: 681–92
Response Rates with NACT and Correlation with Survival
Clinical chemotherapy response rates (complete plus partial response by direct visualization and imaging) have been reported to range from 19 to 58 % but the pathological complete response (pCR) rates have been much lower (2.5–13 %) [10]. Ancona et al. [11] reported pCR in 12.8 % of patients receiving NACT and found significant improvement in survival in those who had complete response while non-responders had a worse outcome than those who had no chemotherapy: 3-year and 5-year survival rates of 74 % and 60 %, 24 % and 12 % (p = 0.0002), 46 % and 26 % (p = 0.01) respectively. Other studies [12–14] also show that those who have pCR to chemotherapy have better overall survival. Non-responders doing worse may reflect on the inherent “unfavorable biology”, rather than the effect of chemotherapy itself. Clearly, a strategy that has not been adequately explored is to identify responders from non-responders so that neoadjuvant treatment is reserved for patients more likely to respond, with the predicted ‘non-responders’ going on to primary surgery.
A pathological response grading system, Tumour Regression Grade (TRG) shown in Table 2 has been found to be an important and significant predictor of disease free survival (DFS). Mandard et al [15] report that complete or major (<10 % residual tumour) pathological response (TRG 1–3) has a better DFS as compared to minor (>10 % residual tumour) or no response (TRG 4–5).
Table 2.
Tumour Regression Grade [15]
| TRG 1 | No residual cancer |
| TRG 2 | Rare residual cancer cells |
| TRG 3 | Predominant fibrosis with residual cancer cells |
| TRG 4 | Residual cancer cells outgrowing fibrosis |
| TRG 5 | Complete absence of regression change |
Down Staging of Tumour and R-0 Resection Rates
Down staging of tumour may facilitate surgical resection and improve complete resection (R-0) rates. The MRC trial [9] showed that tumours in the NACT-surgery group were significantly smaller, had less frequent extension into the surrounding tissue and less lymph node involvement. R-0 resection was achieved in 60 % vs. 54 % favouring chemotherapy group. These indicate that neoadjuvant chemotherapy was able to successfully downstage disease. Urschel et al. [16] also reported that surgery after chemotherapy had higher rates of R-0 resection (p = 0.001) due to tumour down staging. A decreasing trend in survival has been seen with the type of resection—a 42.4 % 3-year survival for R-0 resection, 18.0 % for microscopically positive resection and 8.6 % for grossly positive resection [17].
Post Operative Morbidity and Mortality/All-Cause Mortality Rates
Data from most meta analysis [16] (Table 3) have shown similar postoperative morbidity and mortality for neoadjuvant chemotherapy compared to surgery alone; We have had experience in operating 868 patients after NACT and our complication rates and mortality are similar to 579 patients operated without chemotherapy in the same period (unpublished data). From our personal experience, the dissection planes are actually better when operating after NACT and pose no additional intraoperative concerns to the surgeon. Current evidence strongly supports the view that NACT is safe, and contrary to popular belief, is not associated with a higher postoperative complication rate.
Table 3.
List of meta-analyses of Neoadjuvant treatment plus surgery versus surgery alone in esophageal cancer
| Author | Year | No. of RCTs | Conclusions | % change (p value) |
|---|---|---|---|---|
| Urschel [16] | 2002 | 11 NACT | 2 year survival equivalent | OR 0.88 (p = 0.45) |
| Higher R0 resection rate | ||||
| Kaklamanos [31] | 2003 | 7 NACT | No significant survival benefit | 4.4 % difference (p = 0.07) |
| 5 NACTRT | No significan survival benefit | 6.4 % difference (p = 0.86) | ||
| Malthaner [32] | 2004 | NACT/NACTRT/NART/Adjuvant CT/RT | No significant benefit with any | – |
| Fiorica [27] | 2004 | 6 NACTRT | Reduction in 3 year mortality rate | OR 0.53 (95 % CI 0.31–0.93); p = 0.03 |
| Greer [33] | 2005 | 6 NACTRT | No significant survival benefit: NACTRT | RR 0.86 (95 % CI 0.74–1.01; p = 0.07 |
| Gebski [30] | 2007 | 10 NACTRT | Reduction in all cause mortality with NACTRT: | 2 year abs survival benefit −13 % |
| 8 NACT | (Significant: both scc, adeno) | |||
| With NACT: (significant: only adeno) | 2 year abs survival benefit −7 % | |||
| Kranzfelder [34] | 2011 | 9 NACTRT | Overall survival benefit: NACTRT | HR 0.81 (95 % CI 0.70–0.95); p = 0.008 |
| 8 NACT | No survival benefit: NACT | HR 0.93 (95 % CI 0.81–1.08); p = 0.368 | ||
| 3 dCTRT | Better R0 resection: NACT/NACTRT | |||
| Sjoquist [18] | 2011 | 12 NACTRT | Reduction in all cause mortality with NACTRT (scc, adeno) and NACT (only adeno) | HR 0.78 (95 % CI 0.70–0.88); p = 0.001 |
| 9 NACT | HR 0.87 (95 % CI 0.79–0.96); p = 0.005 | |||
| 2 NACTRT vs NACT |
Survival Rates
The most recent and updated meta analysis [18] conclusively showed that there is a definite survival benefit with preoperative chemotherapy in locally advanced esophageal cancers. Earlier trials like the MRC-OE02 RCT [9] developed in the 1990s showed a significant improvement in the disease free survival (DFS) and overall survival (OS) (HR 0.79, p = 0.004), with a median survival benefit of 3.5 months (16.8 vs. 13.3 months) with two cycles of preoperative chemotherapy (Cisplatin + 5-Fluorouracil). The updated analysis of this trial [17] in 2009 reported that the survival benefit was maintained even after a median follow up of 6 years, with absolute 5-year survival of 23 % for NACT compared with 17 % for surgery alone (HR of 0.84, p = 0.03). Besides these, other studies [19, 20] have also shown significant benefit with preoperative chemotherapy (Table 1).
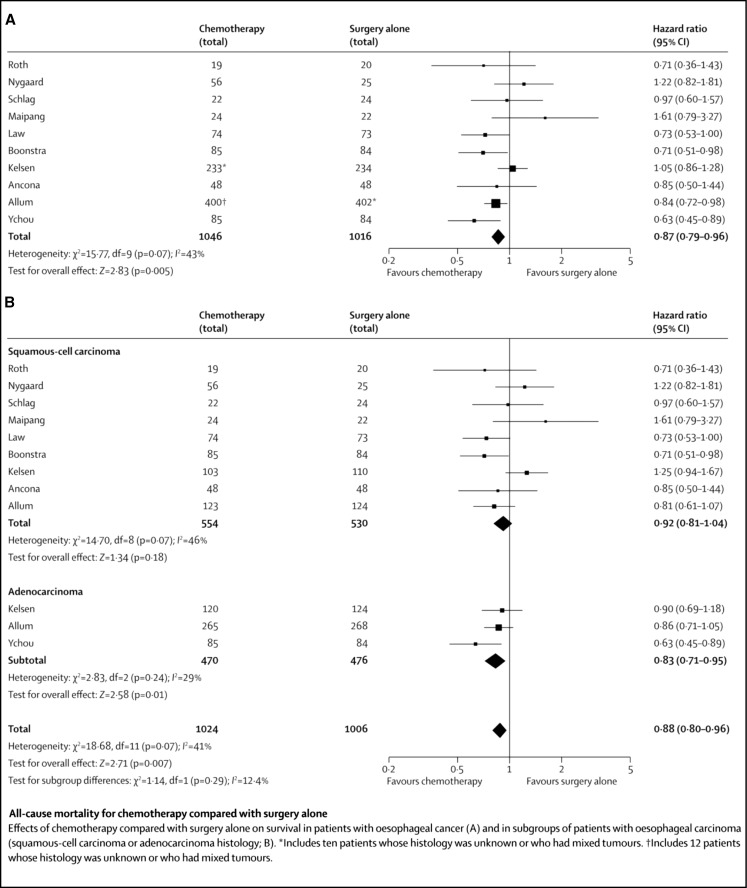
The earlier meta-analyses by Urschel et al. [16] (Table 3) did not include the trials mentioned above and hence failed to show survival benefit with preoperative chemotherapy and suggested ineffectiveness of chemotherapeutic agents as a probable cause. This was more likely inadequate number of patients included in the meta analysis, which was therefore underpowered to detect a clinically important benefit. This is obvious from the fact that despite the chemotherapeutic agents remaining the same, the more recent meta-analysis by Sjoquist et al. [18] found significant survival benefits with NACT. As shown in Fig. 1, this meta-analysis included 10 RCTs and found an absolute survival benefit difference of 5.1 % at 2 years with NACT-Surgery.
Fig. 1.
Randomized control trials comparing NACT-surgery to surgery alone: all-cause mortality rates. Reproduced with permission from Sjoquist KM et al: survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable esophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12: 681–92
Neoadjuvant Chemoradiotherapy Plus Surgery Versus Surgery Alone
Several randomized trials (Table 4) have been performed with NACTRT in esophageal cancers and similar to NACT, most of the earlier studies showed divergent results. These contradictory results were probably due to variations in patient population, tumour histology, treatment (chemotherapy regimen/dosage, use of radiotherapy in some patients planned for chemotherapy only, combination CTRT, sequential or concurrent, preoperative or postoperative, surgery), limited number of patients per trial as well as “publication bias” [21]. These studies have also been plagued by large variability in the radiation regimens used—total radiation doses ranging from 20 to 45 Gray (Gy), daily dose from 1.75 to 3.70 Gy per fraction, number of fractions varying from 10 to 30. However, most investigators used cisplatin and 5-Fluorouracil concurrently with a total radiation dose of 35–45 Gy. Notably, the most recently published CROSS trial [22] used taxane-based chemotherapy with five cycles of weekly paclitaxel and carboplatin with a total dose of 41.4 Gy in 23 fractions of 1.8 Gy each.
Table 4.
Randomized controlled trials: neoadjuvant chemoradiotherapy and surgery versus surgery alone
| Trial/author | Year | No. of patients | Chemotherapy (cycles) | Radiotherapy | Histology |
|---|---|---|---|---|---|
| Nygaard | 1983 | 78 | Cisplatin/Bleomycin(2), sequential | 35 Gy/(1.75 Gy/#)/4 weeks | Squamous |
| Apinop | 1986 | 69 | Cisplatin/5-FU(2), concurrent | 40 Gy/(2 Gy/#)/4 weeks | Squamous |
| Le Prise | 1988 | 86 | Cisplatin/5-FU(2), sequential | 20 Gy/10#/12 days | Squamous |
| Urba | 1989 | 100 | Cisplatin/5FU/vinblastine(2), concurrent | 45 Gy/(1.5 Gy/#)/3 weeks | Squamous/Adeno |
| Bosset | 1989 | 293 | Cisplatin (2), sequential | 37 Gy/(3.7 Gy/#)/2 weeks | Squamous |
| Walsh | 1990 | 61 | Cisplatin/5FU(2), concurrent | 40 Gy/15#/3 weeks | Squamous |
| Walsh | 1990 | 113 | Cisplatin/5FU(2), concurrent | 40 Gy/15#/3 weeks | Adeno |
| Burmeister | 1994 | 256 | Cisplatin/5FU(1), concurrent | 35 Gy/15#/3 weeks | Squamous/Adeno |
| Tepper | 1997 | 56 | Cisplatin/5FU(2), concurrent | 50.4 Gy/(1.8 Gy/#)/5.6 weeks | Squamous/Adeno |
| Lv | 1997 | 160 | Cisplatin/Paclitaxel(2), concurrent | 40 Gy/(2 Gy/#)/4 weeks | Squamous |
| Lee | 1999 | 101 | Cisplatin/5FU(2), concurrent | 45.6 Gy/(1.2 Gy/#)/28 days | Squamous |
| Mariette | 2000 | 195 | Cisplatin/5FU(2), concurrent | 45 Gy/25#/5 weeks | Squamous/Adeno |
| Van der Gaast | 2004 | 364 | Carboplatin/Paclitaxel(5), Concurrent | 41.4 Gy/(1.8 Gy/#)/4.6 weeks | Squamous/Adeno |
Adapted with permission from Sjoquist KM et al: survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable esophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12: 681–92
Response Rates with NACTRT and Correlation with Survival
Pathological complete response rates (pCR) after neoadjuvant chemo-radiation ranges from 13 to 49 % [23]. It has been established that a pCR is associated with improved survival [24]. Tumour regression grade (Table 2) of pathological specimens after preoperative CTRT was evaluated by Mandard et al. [15], and complete response or major pathological response (TRG 1–2) was found in 42 %, TRG 3 in 20 % and minor or no pathological response (TRG 4–5) in 33 %. As mentioned earlier, lower TRG (better response) is associated with better disease free survival. Other investigators [25, 26] have quoted similar associations making TRG an important prognosticator.
Downstaging of Tumour and R0 Resection Rates
Downstaging of tumors and improved R-0 resection rates have been seen consistently in most trials evaluating NACTRT. There have been statistically significant downstaging of tumour with preoperative CTRT (OR 0.43, p = 0.001) [27]; there is also a significant improvement in complete resection rates with NACTRT. However this increase does not always translate into a survival benefit [18].
Post Operative Morbidity and Mortality Rates and NACTRT-Related Mortality
The frequently reported toxicities with NACTRT are neutropenia, which may occur in up to 60 % of patients, oesophagitis grade 3/4 in up to 43 % and nausea and/or vomiting, with 6 to 35 % requiring some form of nutritional support (enteral or parenteral) during CTRT [23]. The results of several randomized trials as well as some meta analysis [16, 18, 27, 30–34] show a trend towards higher morbidity and mortality with neoadjuvant chemoradiotherapy compared to surgery alone. Higher dose per fraction appears to be a significant factor predicting postoperative morbidity. As pointed out in the meta-analyses, the only individual study that reached statistical significance was by Bosset et al. [28], which used 3.7 Gy per fraction. Despite operative mortality being higher (10.2 % vs. 3.8 %) [29], the survival benefit of NACTRT is still maintained. At our institute, we have been highly selective with NACTRT, reserving it for young fit patients with locally advanced, resectable esophageal cancer. Our postoperative mortality rates after NACTRT in 47 patients have been similar to those operated per primum and after NACT (unpublished data). However, the small number of patients we have treated with this regime precludes any definitive statement on the issue.
Survival Rates
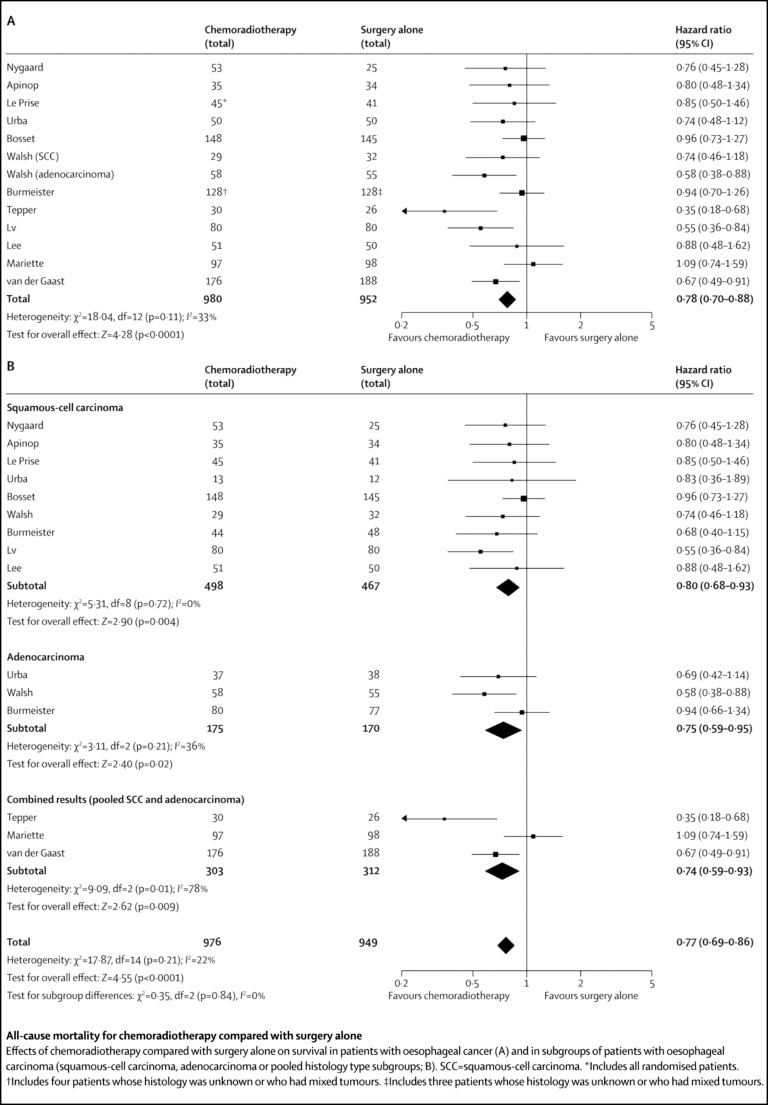
The effect of treatment on overall mortality has consistently been in favour of NACTRT-surgery. Gebski et al. [30] report a 13 % 2-year survival advantage for chemoradiotherapy. A subgroup analyses in an earlier meta-analysis [27] showed significant difference with adenocarcinoma (OR 0.24, p = 0.018) but not with squamous carcinoma (OR 0.81, p = 0.29). However, in an up-dated meta-analysis by Sjoquist et al. [18], where 13 studies (1,932 patients) were included (Fig. 2), they report an absolute survival benefit of 8.7 % at 2 years with NACTRT-surgery, with similar benefits between the two histological types (HR 0.80, p = 0.004 for SCC and HR 0.74, p = 0.02 for AC).
Fig. 2.
Randomized control trials comparing NACTRT-surgery to surgery alone: all-cause mortality rates. Reproduced with permission from Sjoquist KM et al. [18]: survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable esophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12: 681–692
Neoadjuvant CTRT Versus Neoadjuvant CT
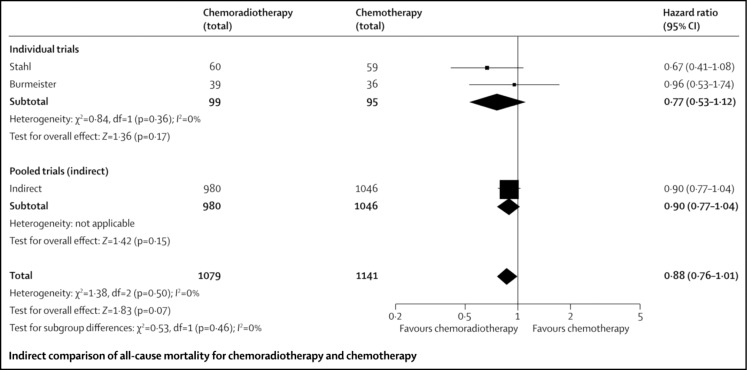
Two trials comparing NACTRT to NACT were analysed by Sjoquist et al. [18] in the meta-analyses. Neither of these trials showed an advantage for one over the other; however, a pooled analysis of all the studies in the meta-analyses found a statistically non-significant trend towards improved survival with NACTRT as compared to NACT (HR 0.88, 0.76–1.01, Fig. 3). Data from pooled analysis derived across trials comparing NACT and NACTRT with surgery being used to compare NACT with NACTRT may not be strictly applicable.
Fig. 3.
Randomized control trials comparing NACT-Surgery to NACTRT-surgery alone: all-cause mortality rates. Reproduced with permission from Sjoquist KM et al. [18]: survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable esophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12: 681–692
Currently, the evidence is not strong enough for one against the other, as seen in the pooled analyses of the above study. More RCTs directly comparing NACT to NACTRT are required to make a definitive statement regarding which is better, and if benefits remain comparable, how to select patients to receive one over the other is yet to be investigated. At our institute, we are presently accruing patients on a randomized trial comparing NACT with NACTRT prior to surgery in this subset of patients.
Conclusion
Based on the existing evidence, neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy both have superior outcomes with a clear survival benefit as compared to surgery alone. NACT followed by surgery has virtually the same postoperative morbidity and mortality as patients undergoing surgery alone; the postoperative morbidity and mortality with NACTRT does seem to be higher than that of surgery alone, but the survival benefit remains, even adjusting for this increased treatment related toxicity. Based on survival data from several RCTs and meta analyses, it is clear that neoadjuvant treatment is the new standard of care for locally advanced resectable esophageal cancers. The unanswered question remains whether neoadjuvant chemoradiotherapy is superior to neoadjuvant chemotherapy alone. Since esophageal cancer patients are already nutritionally compromised with generally poor performance status at the time of diagnosis, pre-chemo/pre-operative enhancement of general condition with nutritional supplementation, hydration and aggressive physiotherapy may help them tolerate better such a treatment with minimal complications, better compliance and maximal benefit.
Footnotes
AJCC: American Joint Committee on Cancer/TNM: Tumour Node Metastasis staging system 7th edition, 2010
References
- 1.Posner MC, Minsky BD, Ilson DH. Cancer of the esophagus. In: Devita VT, editor. Cancer principles & practice of oncology. 9. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 887–923. [Google Scholar]
- 2.Hingorani M, Crosby T, Maraveyas A, et al. Neoadjuvant chemoradiotherapy for resectable oesophageal and gastro-oesophageal junction cancer—do we need another randomised trial? Clin Oncol. 2011;23:696–705. doi: 10.1016/j.clon.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Kamangar F, Dores GM, Anderson WF, et al. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:37–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 4.Dikshit R, Gupta PC, Ramasundarahettige C, et al. Cancer mortality in India: a nationally representative survey. Lancet. 2012;379:1807–1816. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center M, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 7.Bhansali MS, Vaidya JS, Bhatt RG, et al. Chemotherapy for carcinoma of the esophagus: a comparison of evidence from meta-analyses of randomized trials and of historical control studies. Ann Oncol. 1996;7:335–355. doi: 10.1093/oxfordjournals.annonc.a010601. [DOI] [PubMed] [Google Scholar]
- 8.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG Trial 8911 (USA Intergroup113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 9.Medical Research Council Oesophageal Cancer Working Party Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 10.Campbell NP, Villaflor VM. Neoadjuvant treatment of esophageal cancer. World J Gastroenterol. 2010;16(30):3793–3803. doi: 10.3748/wjg.v16.i30.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long-term survival of patients with resectable esophageal squamous cell carcinoma. Cancer. 2001;91(11):2165–2174. doi: 10.1002/1097-0142(20010601)91:11<2165::AID-CNCR1245>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Roth JA, Pass HI, Flanagan MM, et al. Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg. 1992;96:242–248. [PubMed] [Google Scholar]
- 13.Schlag PM. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Arch Surg. 1992;127:1446–1450. doi: 10.1001/archsurg.1992.01420120080015. [DOI] [PubMed] [Google Scholar]
- 14.Law S, Fok M, Chow S, et al. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114:210–217. doi: 10.1016/S0022-5223(97)70147-8. [DOI] [PubMed] [Google Scholar]
- 15.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Urschel JD, Vasan H, Blewett CJ. A meta-analysis of randomized controlled trials that compared neoadjuvant chemotherapy and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2002;183:274–279. doi: 10.1016/S0002-9610(02)00795-X. [DOI] [PubMed] [Google Scholar]
- 17.Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 18.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 19.Boonstra JJ, Kok T, Wijnhoven BPL, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: longterm results of a randomized controlled trial. BMC Cancer. 2011;11:181. doi: 10.1186/1471-2407-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ychou M, Boige V, Pignon J-P, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 21.Barnett SA, Rizk NP. Randomized clinical trials in esophageal carcinoma. Surg Oncol Clin N Am. 2010;19:59–80. doi: 10.1016/j.soc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Hagen PV, Hulshof MCCM, Lanschot JJBV, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 23.Staal EFWC, Aleman BMP, Boot H, et al. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg. 2010;97:1482–1496. doi: 10.1002/bjs.7175. [DOI] [PubMed] [Google Scholar]
- 24.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–399. doi: 10.1016/j.athoracsur.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brucher BL, Stein HJ, Zimmermann F, et al. Responders benefit from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase-II trial. Eur J Surg Oncol. 2004;30:963–971. doi: 10.1016/j.ejso.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds JV, Muldoon C, Hollywood D, et al. Long-term outcomes following neoadjuvant chemoradiotherapy for esophageal cancer. Ann Surg. 2007;245:707–716. doi: 10.1097/01.sla.0000254367.15810.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 29.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 30.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 31.Kaklamanos IG, Walker GR, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10(7):754–761. doi: 10.1245/ASO.2003.03.078. [DOI] [PubMed] [Google Scholar]
- 32.Malthaner RA, Wong RKS, Rumble RB, et al. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med. 2004;2:35. doi: 10.1186/1741-7015-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery. 2005;137:172–177. doi: 10.1016/j.surg.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 34.Kranzfelder M, Schuster T, Geinitz H, et al. Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg. 2011;98:768–783. doi: 10.1002/bjs.7455. [DOI] [PubMed] [Google Scholar]