Abstract
Wnt5a, a member of the Wnt gene family, encodes a cysteine-rich growth factor involved in signal transduction during growth and differentiation. The Fzd2 gene codes for a cell membrane receptor called Frizzled-2 have a structure similar to G protein coupled receptors. The extracellular N-terminal of the Fzd2 receptor has a cysteine-rich domain (CRD) that binds Wnt ligands and thus primes the Wnt signal pathway. Downregulation of the Wnt signal pathway occurs in neurodegenerative diseases including Amyotrophic Lateral Sclerosis (ALS). However, little is known about Wnt5a/Fzd2 signaling in mammalian nerve cells, and it is not clear whether Wnt5a or Fzd2 functioning are changed in ALS. The influence of Wnt5a and Fzd2 signal transduction pathway on ALS was investigated in adult SOD1G93A transgenic mice. Changes in Wnt5a and Fzd2 expression in the spinal cord of SOD1G93A transgenic mice (ALS), SOD1G93A transfected NSC-34 cells, and primary cultures of astrocytes from SOD1G93A transgenic mice were detected by immunofluorescent staining, Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Western blotting. The results provide further insight into the role of Wnt5a and Fzd2 in the pathogenesis of ALS transgenic mice, which provides evidence that should help in the search for treatments of ALS.
Keywords: ALS, Wnt5a, Fzd2, NSC34, astrocyte, Wnt signaling pathway
Introduction
Amyotrophic Lateral Sclerosis (ALS) is a fatal, adult-onset, neurodegenerative disease characterized by the selective death of motor neurons in the motor cortex, brain stem, and spinal cord [1]. The clinical manifestations of ALS include progressive asthenia with development of paralysis and generalized atrophy. Death occurs at average three to five years after onset of the first symptoms. In 90% of cases, ALS is a sporadic disease. The remaining 10% of patients have a history of familial ALS, approximately 20% of those cases are caused by mutation in the gene encoding superoxide dismutase 1 (SOD1) [2,3]. Rosen and Marx [4] constructed ALS transgenic mice by introducing a G93A mutation. Transgenic mice over-expressing mutant human SOD1 develop an age-dependent degeneration of motor neurons leading to paralysis and death. This transgenic mouse model is a valuable tool for ALS research.
Wnt signaling pathways are involved not only in formation of the embryonic dorsoventral axis, but also in a number of other developmental events that depend on establishment of cell polarity or determination of cell fate. There are at least three different Wnt pathways: the classical pathway, the planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway [5,6]. The protein encoded by the Wnt gene is a secretory glycoprotein that contains a signal peptide and a conserved cysteine residue at position 23 or 24. It thus has the characteristics shared by growth factors having both autocrine and paracrine functions [7]. As little is known about Wnt5a/Fzd2 signaling in mammalian nerve cells, and it is clear whether normal Wnt5a or Fzd2 functioning are changed in ALS. The influence of Fzd2 and the Wnt5a signal transduction pathway on ALS were investigated in adult SOD1G93A transgenic mice.
Materials and methods
Animals and tissue preparation
We obtained ALS transgenic mice from Jackson Laboratories (Bar Harbor, ME, USA). All the animal experiments were carried out in accordance with the National Institute’s Health guidelines for the Care and Use of Laboratory Animals and the regulations of Weifang Medical University for animal experimentation. The transgenic mice carrying the mutated human SOD1G93A gene and mice with the wild-type human SOD1 gene were crossbred. Genotyping was performed using Polymerase Chain Reaction (PCR) amplification of genomic DNA extracted from tail tissue. Mice were killed at the early stage (95 days), the middle stage (108 days), and the end stage (122 days) of the neurodegenerative disease. The mice were perfused intracardially with 4% paraformaldehyde, and frozen sections (10 μm) were prepared for immunohistological analysis. Some mice were killed, and the spinal cord was removed and frozen in liquid nitrogen for immediate molecular analysis.
Primary culture of astrocytes
Astrocytes isolated from spinal cord tissue of 1-day old ALS transgenic and wild-type mice by mincing the spinal cord tissues with scissors in DMEM. The tissue was triturated after trypsinization (0.5% trypsin, 10 min, 37°C/5% CO2). The cell suspension was washed in culture medium for astrocytes (DMEM supplemented with 10% FBS (Gibco Inc., Carlsbad, CA, USA), 100 mg/ml streptomycin and 100 U/ml penicillin) and cultured at 37°C/5% CO2 in 75 cm2 BD Falcon culture flasks in borate buffer (2.37 g borax and 1.55 g boric acid dissolved in 500 ml of sterile water, pH 8.4) for 1 h and then rinsed thoroughly with sterile phosphate buffered solution. The medium was changed after 24 h in culture and twice a week thereafter, for a total culture time of 10-14 d. After removal of the primary microglial culture, the remaining cells were predominately astrocytes. Cultured cells were collected for Western blotting to investigate the changes in Wnt5a and Fzd2 protein expression.
Transfection of NSC34 mouse motor neuron-like hybrid cells
One day before transfection, 105 cells/well were seeded in 6-well plates, in culture medium (DMEM supplemented with 10% FBS) without antibiotics and cultured at 37°C/5% CO2 in 75 cm2 BD Falcon culture flasks. Transfection was carried out when the cells reached 50–60% confluences using Lipofectamine 2000 (Invitrogen Life Technologies) according to the manufacturer’s protocol. The pEGFP-WT-SOD1 (wild type) and pEGFP-G93A-SOD1 (G93A mutant) plasmids (Poletti et al. 2012) were a gift from Professor Angelo Poletti (University of Milan, Italy). Mouse motor neuron-like hybrid (NSC34) cells were incubated in Opti-MEM® with pEGFP-WT-SOD1 or pEGFP-G93A-SOD1 plasmid-Lipofectamine 2000 complexes (2 μg/mL) for 6 h, and then replaced with fresh Dulbecco’s Modified Eagle Medium (DMEM). Total RNA or proteins were extracted from transfected NSC-34 cells at 24 h and 48 h for RT-PCR and Western blot analysis. Wnt5a and Fzd2 were also assayed in cultured transfected NSC-34 by double immunofluorescence staining. Three times replicates and repetitions were performed for the transfections.
RNA extraction and RT-PCR
Isolation of total RNA from spinal cord tissue and transfected NSC-34 cells was carried out using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocols. First-strand cDNA was synthesized from 2 μg of total RNA using Superscript II reverse transcriptase (Invitrogen), oligo (dT), 10 mM dNTP Mix, 5x RT buffer, and RNase inhibitor. The PCR was performed using a thermal cycler and specific Wnt5a, Fzd2 and beta-actin primers. The following primers were used: Wnt5a (sense, 5′- ATT TCC ACG CTA TAC CAA CTC CT-3′; antisense, 5′-ATT CCT TGA TGC CTG TCT TCG-3′); Fzd2 (sense, 5′ - ATT TAG TGG ACA TGC AGC GAT TTC-3′; antisense, 5′-AGC AGG AAG GAT GTA CCG ATG AA-3′); and beta-actin (sense, 5′-AAC AGT CCG CCT AGA AGC AC-3′; antisense, 5′-CGT TGA CAT CCG TAA AGA CC-3′). Amplification products were visualized in 2% agarose/TAE gels and stained with ethidium bromide. All analysis was done in duplicate and repeated at least three times. Band intensity was evaluated using UVI soft Image Acquisition and Analysis software (UVItec, Cambridge, UK). The expression of endogenous beta-actin was used for normalization.
Western blotting
The spinal cords of ALS transgenic mice were removed at 95 days, 108 days, and 122 days. Transfected NSC-34 cells and astrocytes from primary culture were collected at 24 h and 48 h. The spinal cord tissue and the cells were lysed in a buffer solution containing 50 mM Tris/HCl (pH 7.4), 50 mM NaCl, 1% Triton X-100, 1 mM EDTA, 20 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. Samples were clarified by centrifugation at 14,000 g for 15 min at 4°C, and total protein was extracted from the supernatant. Protein concentrations were measured using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Equivalent amounts of protein (120 μg) from each sample were resolved electrophoretically on 15% SDS-polyacrylamide gels, separated by electrophoresis, and transferred to nitrocellulose membranes (Biorad, Hercules, CA, USA). The membranes were blocked with 5% dry fat-free milk in phosphate buffered saline (PBS) for 1 h at room temperature and then incubated overnight at 4°C with the following primary antibodies: Wnt5a mouse monoclonal antibody (1:800; Santa Cruz Biotechnology, Inc., USA), Fzd2 rabbit polyclonal antibody (1:750; Sigma-Aldrich, Inc., MO, USA), and GAPDH mouse monoclonal antibody (1:10000; Sigma, St Louis, MO, USA). The membranes were treated with horseradish peroxidase-conjugated sheep anti-mouse (1:10000; Jackson ImmunoResearch, West Grove, PA, USA) or sheep anti-rabbit secondary antibodies (1:10000; Jackson ImmunoResearch) for 2 h at room temperature, followed by an enhanced chemiluminescence assay (Thermo, Rockford, IL, USA). All analysis was done in duplicate and repeated at least three times. The optical density of blots was analyzed using Labworks Software (Ultra-Violet Products, Cambridge, UK).
Immunofluorescence labeling
Frozen sections were rinsed three times with 0.01 M PBS (pH 7.4), blocked with 10% goat serum for 30 min at 37°C. The sections were incubated simultaneously with two compatible primary antibodies overnight at 4°C. The following primary antibodies were applied: mouse anti-Wnt5a IgG (1:50; Santa Cruz Biotechnology, Inc., USA), rabbit anti-Fzd2 IgG (1:100; Sigma-Aldrich, Inc., MO, USA), chicken anti-glial fibrillary acidic protein (GFAP) IgY (1:1000; Abcam, Cambridge, CA, USA), and chicken anti-beta-tubulin III IgY (1:600; Abcam). The samples were soaked for 2 h in 0.01 M PBS (pH 7.4), with 0.1% Triton X-100 containing the following secondary antibodies: goat anti-mouse IgG conjugated to FITC (1:100; Jackson ImmunoResearch), goat anti-mouse IgG conjugated to TRITC (1:100; ZSGB-BIO), goat anti-rabbit IgG conjugated to Cy3 (1:100; Jackson ImmunoResearch), or goat anti-chicken IgY-H&L (DyLight 488) (1:500; Abcam). Add hoechst33258 (1:1000; Sigma-Aldrich, Inc., MO, USA) to redyeing nuclear. Images were obtained using a laser scanning confocal microscope. The primary antibody was replaced with PBS as a negative control. No specific staining was identified in these controls.
Statistical analysis
Statistical comparisons of data were made with one-way ANOVA using SPSS13.0 software. Data were presented as the mean ± standard error of the mean (SEM); P < 0.05 or < 0.01 were considered statistically significant.
Results
Upregulation of Wnt5a and Fzd2 in the spinal cord of ALS transgenic mice
Both Wnt5a and Fzd2 mRNA and protein were detected in spinal cord tissues from ALS transgenic and wild-type mice at 95, 108, and 122 days (Figure 1A, 1B, 1E, 1F). The mRNA and protein levels were significantly higher in the ALS mice than those in wild-type littermates at three time point (P < 0.05). The greatest differences occurred at 122 days (Figure 1C, 1D, 1G, 1H).
Figure 1.
The mRNA and protein of Wnt5a and Fzd2 receptor in the spinal cord of adult amyotrophic lateral sclerosis (ALS) mice and wild-type (WT) littermates at 95, 108, and 122 days of age. A. The gene expression of Wnt5a was higher in ALS mice than that in WT mice at each age. Beta-actin was used for normalization. B. Representative immunoblot showing that Wnt5a expression was greater in ALS mice than that in WT mice at each age. GAPDH was used for normalization. C. Densitometric analysis of the RT-PCR results shown in panel (A). Relative amounts of Wnt5a were calculated as the ratio of Wnt5a to beta-actin densities (n = 6). D. Densitometric analysis of the immunoblot shown in panel (B). Relative amounts of Wnt5a were calculated as the ratio of Wnt5a to GAPDH densities (n = 6). E. The gene expression of Fzd2 was greater in ALS mice at each age than that in WT mice. Beta-actin was used for normalization. F. Representative immunoblot showing that Fzd2 expression was greater in ALS mice at each age than that in WT mice. GAPDH was used for normalization. G. Densitometric analysis of RT-PCR shown in panel (E). Relative amounts of Fzd2 were calculated as the ratio of Wnt5a to beta-actin densities (n = 6). H. Densitometric analysis of the Western blot shown in panel (F). Relative amounts of Fzd2 were calculated as the ratio of Fzd2 to GAPDH densities (n = 6).
The distribution and localization of Wnt5a and Fzd2 positive cells were determined by immunofluorescence microscopy. The majority of positive cells were neurons and astrocytes with cytoplasmic staining. Wnt5a and Fzd2 positive cells were detected in the gray matter, white matter, and central canal in both transgenic and wild-type mice. Within the gray matter, most positive cells were located in the ventral horn. In the white matter, the positive cells were located in the neuron axons and nerve fibers. Weakly positive cells were detected in the central canal.
Co-localization of Wnt5a and Fzd2 with beta-tubulin III for neurons or GFAP for mature astrocytes was detected by double labeling. In ALS mice, there were significantly more Wnt5a/GFAP and Fzd2/GFAP double-labeled cells than that in wild-type mice (Figures 2 and 4). However, Wnt5a/beta-tubulin III and Fzd2/beta-tubulin III double-labeled cells were significantly reduced in the ALS mice (Figures 3 and 5). These differences could be accounted for by motor neuron degeneration, necrosis and glial cell proliferation in the neurodegenerative disease that developed in the ALS mice.
Figure 2.
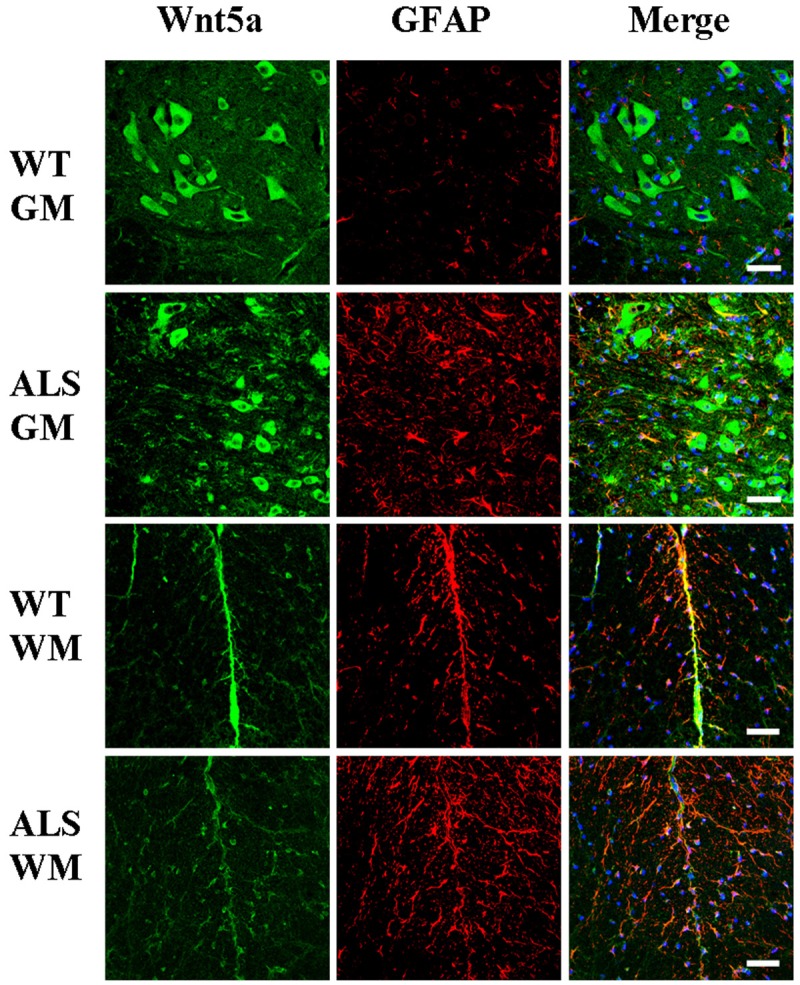
Wnt5a/glial fibrillary acidic protein (GFAP) co-localization in the gray matter (GM), and white matter (WM) of the spinal cords of adult amyotrophic lateral sclerosis (ALS) mice and wild-type (WT) mice at 122 days of age. Scale bar = 50 μm.
Figure 4.

Fzd2/glial fibrillary acidic protein (GFAP) co-localization in the gray matter (GM), and white matter (WM) of the spinal cords of adult amyotrophic lateral sclerosis (ALS) and wild-type (WT) mice at 122 days of age. Scale bar = 50 μm.
Figure 3.
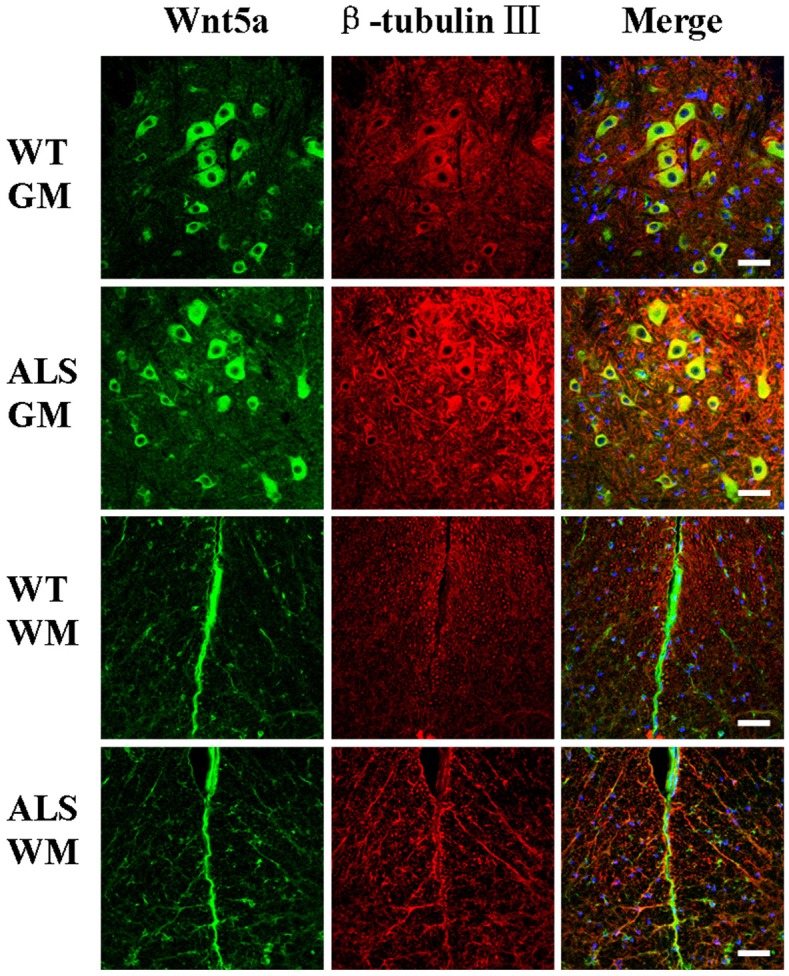
Wnt5a/beta-tubulin III co-localization in the gray matter (GM), and white matter (WM) of the spinal cords of adult amyotrophic lateral sclerosis (ALS) mice and wild-type (WT) mice at 122 days of age. Scale bar = 50 μm.
Figure 5.
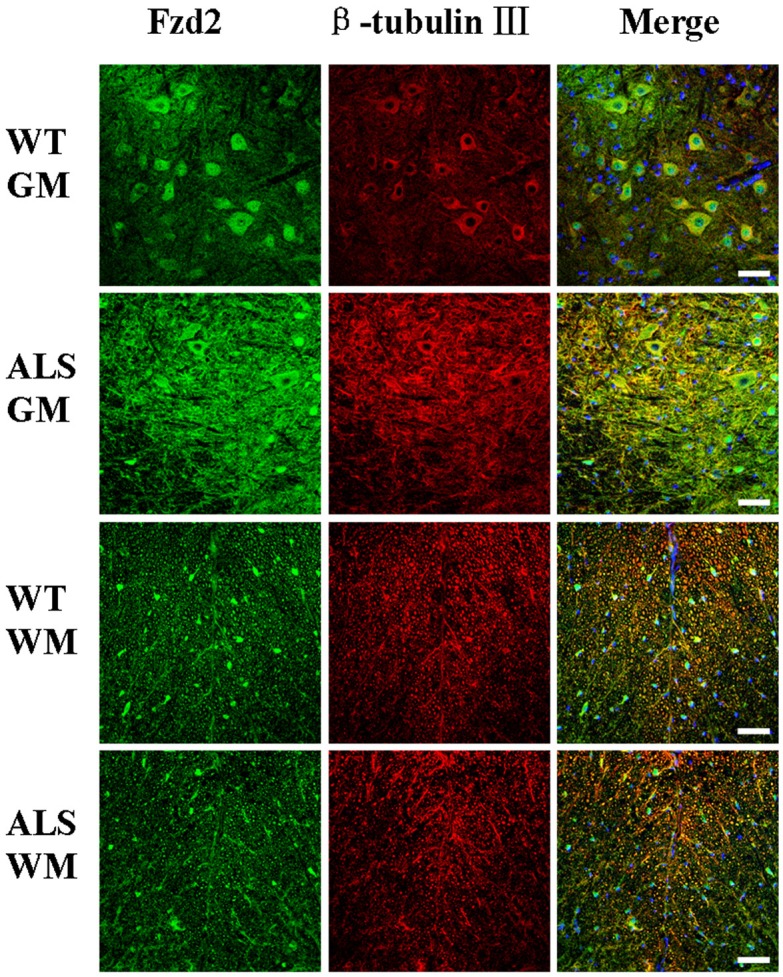
Fzd2/beta-tubulin III co-localization in the gray matter (GM), and white matter (WM) of the spinal cords of adult amyotrophic lateral sclerosis (ALS) and wild-type (WT) mice at 122 days of age. Scale bar = 50 μm.
Upregulation of Wnt5a and Fzd2 in cultured astrocytes
Western blots of proteins isolated from primary astrocyte cultures of newborn mouse spinal cord tissue showed that Wnt5a and Fzd2 protein was higher in the astrocytes from ALS transgenic mice than those from wild-type controls (Figure 6A and 6B).
Figure 6.

Wnt5a and Fzd2 protein expression in primary astrocytes from the spinal cords of adult amyotrophic lateral sclerosis (ALS) and wild-type (WT) mice at 1 day of age. Representative immunoblots showed that both (A) Wnt5a expression and (B) Fzd2 expression were greater in ALS mice than that in WT mice. GAPDH was used for normalization.
Downregulation of Wnt5a and Fzd2 in transfected NSC-34 cells
Wnt5a and Fzd2 mRNA and proteins were detected in pEGFP-WT-SOD1 and pEGFP-G93A-SOD1 transfected NSC-34 cells by RT-PCR and Western blot (Figure 7A, 7B, 7E, 7F). The NSC-34 cells that carried the pEGFP-G93A-SOD1 plasmid had lower levels of Wnt5a or Fzd2 mRNA and proteins at both 24 and 48 h than that in NSC-34 cells transfected with the wild-type plasmid (P < 0.05, Figure 7C, 7D, 7G, 7H).
Figure 7.
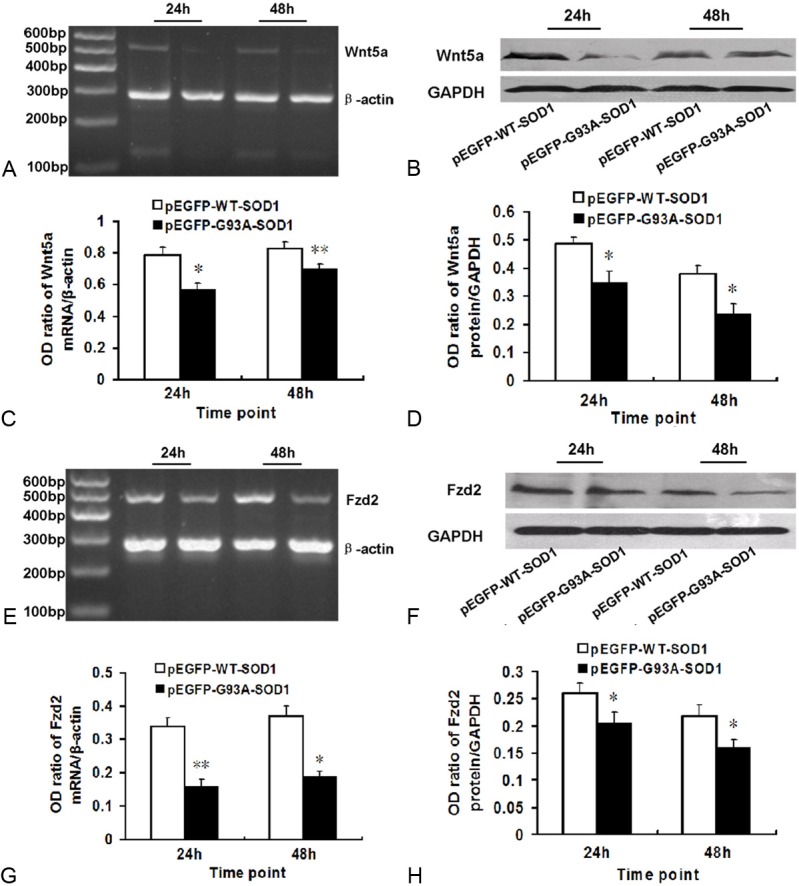
Downregulation of Wnt5a and its receptor Fzd2 in pEGFP-G93A-SOD1 transfected NSC-34 cells, compared with pEGFP-WT-SOD1 transfected NSC-34 cells at 24 and 48 h incubation. A. Gene expression of Wnt5a was lower in pEGFP-G93A-SOD1 transfected cells at both time point than that in pEGFP-WT-SOD1 transfected cells. Beta-actin was used for normalization. B. Representative immunoblot showing that Wnt5a expression was lower in the pEGFP-G93A-SOD1 transfected NSC-34 cells at both time point than that in pEGFP-WT-SOD1 transfected cells. GAPDH was used for normalization. C. Densitometric analysis of RT-PCR results shown in panel (A). Relative amounts of Wnt5a were calculated as the ratio Wnt5a to beta-actin densities (n = 6). D. Densitometric analysis of blot shown in panel (B). Relative amounts of Wnt5a were calculated as the ratio of Wnt5a to GAPDH densities (n = 6). E. Gene expression of Fzd2 was lower in the pEGFP-G93A-SOD1 transfected cells at both time points than that in pEGFP-WT-SOD1 transfected cells. Beta-actin was used for normalization. F. Representative immunoblot showing that Fzd2 expression was lower in the pEGFP-G93A-SOD1 transfected cells at both time point than that in pEGFP-WT-SOD1 transfected cells. GAPDH was used for normalization. G. Densitometric analysis of the RT-PCR results shown in panel (E). Relative amounts of Fzd2 were calculated as the ratio of Wnt5a to beta-actin densities (n = 6). H. Densitometric analysis of the immunoblot shown in panel (F). Relative amounts of Fzd2 were calculated as the ratio Fzd2 to GAPDH densities (n = 6).
Immunofluorescence results showed that Wnt5a and Fzd2 expression at 24 h and 48 h was decreased in cells transfected with the mutant pEGFP-G93A-SOD1 plasmid compared to the wild type (Figures 8 and 9). Otherwise, the nuclei of pEGFP-G93A-SOD1transfected NSC-34 cells appeared pyknotic.
Figure 8.
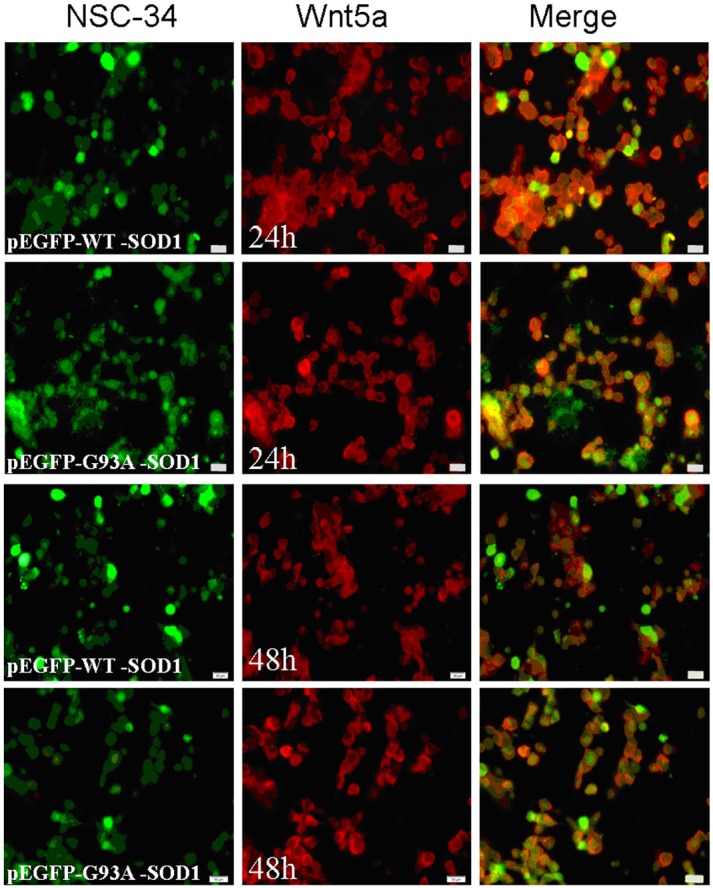
Wnt5a positive staining detected at 24 and 48 h incubation in NSC-34 cells transfected with pEGFP-WT-SOD1 and pEGFP-G93A-SOD1 plasmids. Scale bar = 50 μm.
Figure 9.
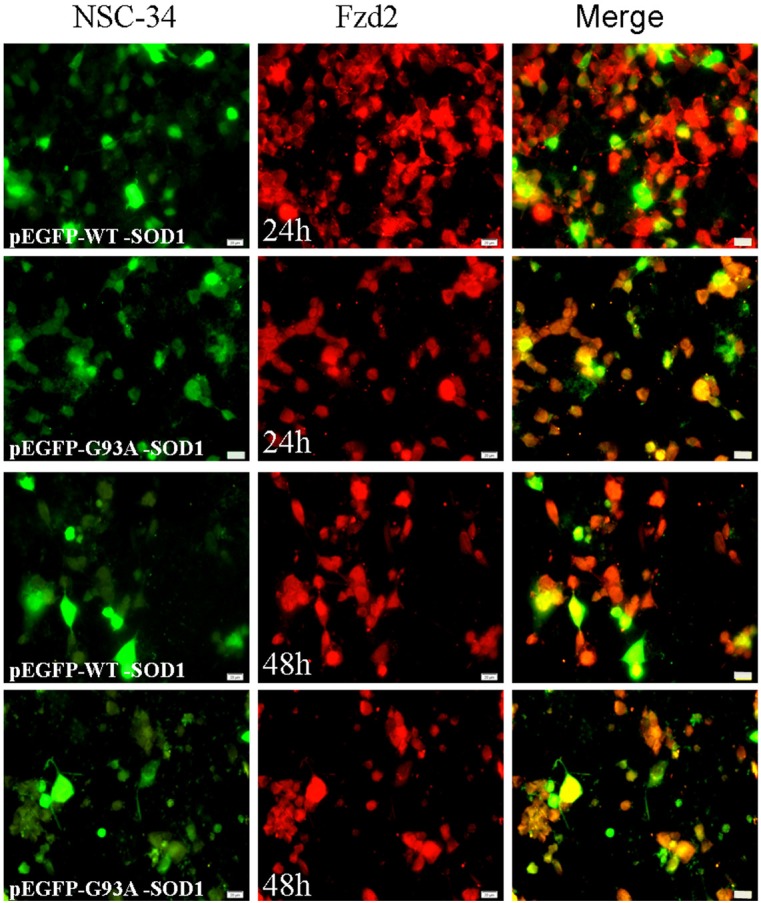
Fzd2 positive staining detected at 24 and 48 h incubation in NSC-34 cells transfected with pEGFP-WT-SOD1 and pEGFP-G93A-SOD1 plasmids. Scale bar = 50 μm.
Co-expression of Wnt5a and Fzd2
We used immunocytochemistry to investigate the relationship of Wnt5a with Fzd2 in the spinal cords of ALS transgenic mice, the results showed that a high concentration of Wnt5a-positive cells colocalized with Fzd2 in the ventral horns of ALS mice (Figure 10), suggesting that Wnt5a and Fzd2 interact in the spinal cords of ALS mice.
Figure 10.
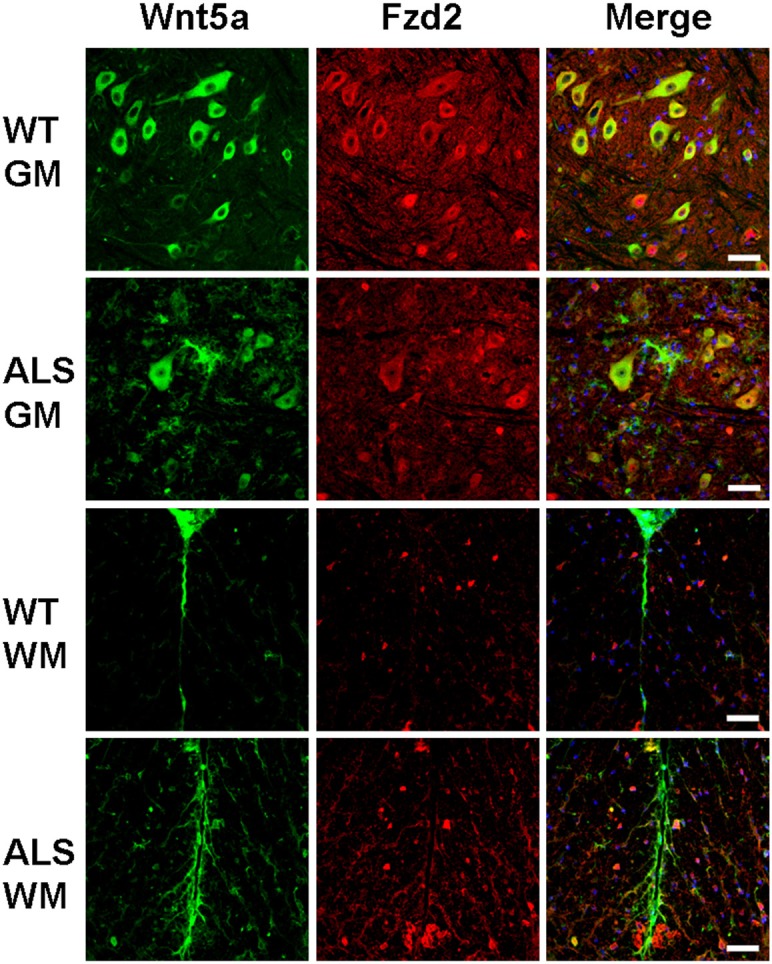
Co-localization of Wnt5a/Fzd2 in the gray matter (GM) and white matter (WM) of wild type (WT) and ALS mice. Scale bar = 50 μm.
Discussion
ALS is a relatively common chronic progressive degenerative disease of the central nervous system. The clinical symptoms result from selective impingement of upper and lower motor neurons in the spinal anterior horn cells, the brain cortex pyramidal cells, corticobulbar tracts, and corticospinal tracts. The clinical manifestations of ALS are progressive myasthenia and general amyotrophy, eventually resulting in paralysis and death. The SOD1G93A transgenic mouse model was chosen for this study because its pathological characteristics and clinical manifestations are similar to human ALS patients.
The role of the Wnt signaling pathway
The Wnt gene plays a role in regulating cell proliferation and differentiation during development. The proteins encoded by Wnt are secretory glycoproteins composed of 350–400 amino acids [8], including a hydrophobic signal peptide, containing 23 or 24 conserved cysteine residues. The Wnt proteins can have a paracrine effect on the membrane receptors of adjacent cells or an autocrine effect on their own cell membrane receptors. Wnt proteins combine with membrane receptors to activate intracellular signaling pathways, regulating the expression of target genes. The Wnt signaling pathway is very important for cell proliferation, differentiation, polarization, migration, and apoptosis in embryonic development.
Wnt signaling pathways are involved in neurogenesis. In mammals, there are 19 Wnt family members [9-11], divided into two classes: Wnt1 and Wnt5a. Wnt1 class proteins, (e.g., Wnt1, Wnt2, Wnt3, Wnt3a, Wnt7a, Wnt8b, and Wnt10b) transmit signals through β-catenin by classical signal transduction. Wnt5a class proteins (e.g., wnt4, Wnt5a, and Wnt11) function via a nonclassical signal transduction pathway. The changes in expression of Wnt5a and its receptor Fzd2 that were observed in the ALS transgenic mice in this study suggested the relationship between the Wnt signaling pathway and the pathogenesis of ALS.
Expression of Wnt5a and Fzd2 is changed in ALS transgenic mice
Wnt5a regulates distinct signaling pathways by binding to Frizzled-2, the Fzd2 gene product [12]. Wnt5a is an important member of the Wnt gene family located on chromosome 3 (p14.2-p21.1). It is composed of 1172 adenine, 884 cytosine, 946 guanine, and 1172 thymine residues [13]. The growth factor encoded by this gene is rich in cysteine, and regulates intercellular signaling during growth and differentiation [14,15]. The Wnt5a proteins of mice and humans are 99% homologous [16,17]. As with other Wnt proteins, Wnt5a has autocrine and paracrine cell growth and differentiation signaling, i.e., binding to the Fzd (Frizzled) transmembrane receptor. Fzd2 is one of the Frizzled gene family members, and its structure is similar to G protein coupled receptors. Seven of the ten Frizzled receptors have been identified as Wnt receptors in humans and mice [18]. The Fzd2 protein extracellular N-terminal has a CRD that activates Wnt signal pathways by combining with Wnt ligands. Wnt5a can activate both the classical Wnt/beta-catenin signaling pathway and the nonclassical beta-catenin-independent pathway. The primary mediators of the nonclassical Wnt signal pathway are G protein coupled receptors. However, in the nonclassical Wnt pathway, Wnt5a can activate both Rac and Fzd2, but receptor-tyrosine-kinase-like orphan receptor 1 or 2 (Ror1 or Ror2) are required. Fzd2 is internalized through a clathrin-mediated route in response to Wnt5a, and inhibition of clathrin-dependent internalization suppresses the ability of Wnt5a to activate Rac. Wnt5a can also inhibit Wnt3a-dependent lipoprotein receptor-related protein 6 (LRP6) phosphorylation and beta-catenin accumulation. Fzd2 is also required for the Wnt3a-dependent accumulation of beta-catenin, and Wnt5a competes with Wnt3a for binding to Fzd2 in vitro and in intact cells, thereby inhibiting the beta-catenin pathway [19].
The immunofluorescence results showed Wnt5a and Fzd2 positive cells in the gray matter, white matter and central canal of the spinal cord in both adult ALS transgenic mice and wild-type mice. Within the gray matter, most positive cells were neurons and astrocytes in the ventral horn. In the white matter, the positive staining was seen in the neuron axon. Significantly more Wnt5a and Fzd2 positive cells were seen in the ALS transgenic mice than in the wild-type mice. RT-PCR and Western blotting results also found that Wnt5a and Fzd2 mRNA expression and protein synthesis were increased in the 95 day old, 108 day old, and 122 day old ALS mice, indicating that increased expression of Wnt5a and its receptor Fzd2 were closely related to development of ALS. Indeed, Wnt5a and Fzd2 expression increased in the course of ALS development.
Wnt5a binding to Fzd2 activates the Wnt/Ca2+ pathway. Fzd2 induces intracellular Ca2+ release mainly by way of G proteins. Signal transmission could activate downstream target genes to cause a series of expression changes, thereby promoting the development of ALS. Similar interactions of the Wnt/Ca2+ channel and classical Wnt pathway are known to play a role in cell differentiation, maturation, tumorigenesis, and development.
Expression of Wnt5a and Fzd2 is upregulated in astrocytes
Astrocytes are the most abundant neuroglial cells in the central nervous system (CNS). They provide the most nutritional support and protection to neurons, and have an active role in synaptic transmission [20,21]. In ALS pathogenesis, neurodegeneration and over-active gliocytes contribute to a micro-environment that increases neuronal damage. The most prominent pathological change in the process of ALS disease is abnormal proliferation of astrocytes [22]. At first, astrocyte proliferation was considered secondary in motor neuron disease. With further study, it was found that astrocyte dysfunction plays a decisive role in development of ALS disease. Functional abnormalities of astrocytes lead to decrease in release of neurotrophic factors, which directly or indirectly causes motor neuron damage [23]. Nagai [24] and DiGeorgio [25] found that astrocytes may be where the mutant SOD1 gene exerts its toxic effect on motor neurons. Reduced survival of motor neurons having normal SOD1 activity has been observed when they were co-cultured with astrocytes having mutant SOD1. Such mutated astrocytes may release soluble factors to trigger Bax-dependent neuronal apoptosis. Culturing activated astrocytes with motor neurons from mutant SOD1 mice in the presence of low nitric oxide concentrations can induce motor neuron degeneration, possibly by activating the P57NTR pathway [26].
The study results found that the expression of Wnt5a and Fzd2 protein were up-regulated in primary cultures of astrocytes from ALS transgenic mice. We presumed that overexpression of Wnt5a and Fzd2 could regulate Wnt signaling pathways and promote astrocyte proliferation so as to protect neurons.
Expression of Wnt5a and Fzd2 is down-regulated in transfected NSC-34 cells
Development of the NSC-34 cell line by hybridizing mouse neuroblastoma and embryonic mouse spinal cord cells to form a motor neuron-like cell line overcame the difficulties of culturing primary motor neurons while retaining the original features of primary motor neurons. The NSC-34 cell line is commonly used to study ALS.
The levels of Wnt5a and Fzd2 mRNA and protein in pEGFP-G93A-SOD1 transfected NSC-34 cells were lower at 24 h and 48 h than those observed in pEGFP-WT-SOD1 transfected cells. It is possible that mutant SOD1-induced neuronal degenerative diseases and accelerated neuron apoptosis are associated with reduced expression of Wnt5a and Fzd2 proteins. In the development of ALS disease, increased expression of Wnt5a and its receptor Fzd2 are related to astrocyte proliferation.
The cause of ALS and its pathogenesis are still unclear, and there is no effective treatment. Knowing the roles of Wnt5a and Fzd2 in ALS may increase the chances of finding effective treatments for ALS. Evidence is increasing that astrocytes are involved in the development of ALS disease. Further research on the biology, function, activation mechanisms, and regulation of astrocyte function is needed. Transplantation of astrocytes, improving motor neuron micro-environment, promoting the survival of motor neurons and regeneration of neurons may become starting points for ALS treatment.
Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China (81271413), the Shandong Province Science and Technology Development Program of China (2012GSF11827), the Shandong Province Natural Science Foundation of China (ZR2012HQ021), the Muscular Dystrophy Association, USA (254530 to X. W.), and the ALS Therapy Alliance (to X. W.). We are thankful to Dr. He Li’s lab in Tongji Medical College of Huazhong University of Science and Technology and Professor Angelo Poletti from the Inter-University Center on Neurodegenerative Diseases of the Universities of Milan, Florence, and Rome, Italy.
References
- 1.Corcia P, Gordon PH. Amyotrophic lateral sclerosis and the clinical potential of dexpramipexole. Ther Clin Risk Manag. 2012;8:359–366. doi: 10.2147/TCRM.S21981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakowski SA, Lunn JS, Busta AS, Oh SS, Zamora-Berridi G, Palmer M, Rosenberg AA, Philip SG, Dowling JJ, Feldman EL. Neuromuscular effects of G93A-SOD1 expression in zebrafish. Mol Neurodegener. 2012;7:44. doi: 10.1186/1750-1326-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borges-Alvarez M, Benavente F, Barbosa J, Sanz-Nebot V. Separation and characterization of superoxide dismutase 1 (SOD-1) from human erythrocytes by capillary electrophoresis time-of-flight mass spectrometry. Electrophoresis. 2012;33:2561–2569. doi: 10.1002/elps.201100672. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Guan Y, Zhang Z, Liu H, Wang S, Yu L, Wu X, Wang X. Wnt signaling pathway is involved in the pathogenesis of amyotrophic lateral sclerosis in adult transgenic mice. Neurol Res. 2012;34:390–399. doi: 10.1179/1743132812Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 5.Choi HJ, Park H, Lee HW, Kwon YG. The Wnt pathway and the roles for its antagonists, DKKS, in angiogenesis. IUBMB Life. 2012;64:724–731. doi: 10.1002/iub.1062. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Guan Y, Liu H, Wu X, Yu L, Wang S, Zhao C, Du H, Wang X. Activation of the Wnt/β-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun. 2012;20:397–403. doi: 10.1016/j.bbrc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 9.Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U S A. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signaling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- 12.Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signaling pathways by binding to Frizzled2. The EMBO Journal. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol. 2012;369:101–114. doi: 10.1016/j.ydbio.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen ED, Miller MF, Wang Z, Moon RT, Morrisey EE. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development. 2012;139:1931–1940. doi: 10.1242/dev.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varma RR, Hector SM, Clark K, Greco WR, Hawthorn L, Pendyala L. Gene expression profiling of a clonal isolate of oxaliplatin-resistant ovarian carcinoma cell line A2780/C10. Oncol Rep. 2005;14:925–932. [PubMed] [Google Scholar]
- 17.Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–8614. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- 18.Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934–941. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Poletti A, Cattaneo E, Taroni F. The neurotoxicity of mutant proteins 20 years after the discovery of the first mutant gene involved in neurodegeneration. Foreword. Prog Neurobiol. 2012;97:53. doi: 10.1016/j.pneurobio.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 21.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 22.Vargas MR, Johnson JA. Astrogliosis in amyotrophic lateral sclerosis: role and therapeutic potential of astrocytes. Neurotherapeutics. 2010;7:471–481. doi: 10.1016/j.nurt.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriques A, Pitzer C, Schneider A. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand? Front Neurosci. 2010;4:32. doi: 10.3389/fnins.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupuis L, Pehar M, Cassina P, Rene F, Castellanos R, Rouaux C, Gandelman M, Dimou L, Schwab ME, Loeffler JP, Barbeito L, Gonzalez de Aguilar JL. Nogo receptor antagonizes p75NTR-dependent motor neuron death. Proc Natl Acad Sci U S A. 2008;105:740–745. doi: 10.1073/pnas.0703842105. [DOI] [PMC free article] [PubMed] [Google Scholar]



