Abstract
Targeting of lysosomes is a novel therapeutic anti-cancer strategy for killing the otherwise apoptosis-resistant cancer cells. Such strategies are urgently needed for treatment of brain tumors, especially the glioblastoma, which is the most frequent and most malignant type. The aim of the present study was to investigate the presence of lysosomes in astrocytic brain tumors focussing also on the therapy resistant tumor stem cells. Expression of the lysosomal marker LAMP-1 (lysosomal-associated membrane protein-1) was investigated by immunohistochemistry in 112 formalin fixed paraffin embedded astrocytomas and compared with tumor grade and overall patient survival. Moreover, double immunofluorescence stainings were performed with LAMP-1 and the astrocytic marker GFAP and the putative stem cell marker CD133 on ten glioblastomas. Most tumors expressed the LAMP-1 protein in the cytoplasm of the tumor cells, while the blood vessels were positive in all tumors. The percentage of LAMP-1 positive tumor cells and staining intensities increased with tumor grade but variations in tumors of the same grade were also found. No association was found between LAMP-1 expression and patient overall survival in the individual tumor grades. LAMP-1/GFAP showed pronounced co-expression and LAMP-1/CD133 was co-expressed as well suggesting that tumor cells including the proposed tumor stem cells contain lysosomes. The results suggest that high amounts of lysosomes are present in glioblastomas and in the proposed tumor stem cells. Targeting of lysosomes may be a promising novel therapeutic strategy against this highly malignant neoplasm.
Keywords: LAMP-1, lysosomes, glioblastomas, immunohistochemistry, tumor stem cells
Introduction
Glioblastomas are the most frequent and malignant type of brain tumors. Although the overall survival increased with the introduction of the Stupp regimen in 2005 [1] including radiation and concomitant temozolomide followed by adjuvant temozolomide, the median overall patient survival is less than 15 months [1]. This short survival of the patients is a consequence of limited effects of chemotherapy and radiation combined with a very infiltrative behaviour of these tumors preventing radical surgery. In the last decade, a population of tumor stem cells has been proposed to be present in glioblastomas, being responsible for treatment failure and tumor recurrence [2,3].
The lysosomal cell death pathway is a novel concept in which lysosomal membrane permeabilization is involved in induction of cell death, using death pathways still functional in the cancer cells [4-7]. Because of a high lysosomal content of hydrolytic enzymes including in particular the cathepsins, rupture of the lysosomal membrane could be potentially damaging to the cell. The fate of the cell has been shown to be determined by the extent of lysosomal damage. A limited lysosomal release thus results in cell death by apoptosis, whereas a massive lysosomal breakdown results in cytosolic acidification and necrosis [8]. This necrotic type of cell death is of major interest since cancer cells often acquire mutations in the apoptotic machinery protecting them from cell death by the classical apoptotic pathways [5,9]. However, since both cancer cells and healthy cells contain lysosomes, distinctions between the cells are important. Fortunately, cancer cells undergo profound changes in their lysosomes upon transformation, affecting size, intracellular localization, and enzymatic activity [8]. Moreover, studies have shown that lysosomes in cancer cells often are larger [10] and hence more fragile than normal lysosomes [11], thereby being more sensitive towards lysosomal membrane permeabilization.
Lysosomal-associated membrane protein-1 (LAMP-1) is among the most abundant lysosomal membrane proteins [4,12,13] being a heavily glycosylated protein creating a sugar coat or glycocalyx on the inner side of the lysosomal membrane aiding to the protection of the membrane from the hydrolytic enzymes and degradation [12,13]. Lamp-1 is expressed mainly in the endosome-lysosomal membrane of cells but has also been found in the plasma membrane (1-2% of total Lamp-1) [14]. Lamp-1 has been found on the cell surface of highly metastatic tumor cells [15-17] and especially metastatic colon cancer cells showed a high expression of Lamp-1 compared to cancer cells with low metastatic potential, suggesting a role for Lamp-1 in cell-cell adhesion and migration [14,15,18].
The aim of this study was to investigate the distribution of lysosomes in astrocytomas including the putative tumor stem cells thereby investigating the possible role of the lysosomal cell-death pathway in killing of both tumor cells and tumor stem cells. Based on the high abundance of LAMP-1 in lysosomes, we used LAMP-1 as a marker associated with the amount and distribution of the lysosomes. The expression pattern of LAMP-1 was investigated in astrocytic brain tumors of increasing grade and compared with the overall survival hypothesizing that LAMP-1 was associated with tumor grade and survival. A high expression of LAMP-1 mRNA has previously been correlated with longer survival in patients with pancreatic carcinoma [15], whereas a high expression of another lysosomal membrane protein, LAPTM4B-35, investigated immunohistochemically, indicated a bad prognosis in patients with hepatocellular carcinoma [19], colorectal carcinoma [20] and pancreatic carcinoma [21]. The present study is a retrospective immunohistochemical study including 112 astrocytomas WHO grade II-IV. Double immunostaining with glial fibrillary acidic protein (GFAP) was performed to ensure the glial identity of the cells expressing LAMP-1. To identify possible LAMP-1 expression in the proposed tumor stem cells co-staining with the marker CD133 was performed. Glioblastoma stem cells were identified in 2003 [22,23] by the expression of the transmembrane marker CD133.
Materials and methods
Patients
Archive tumor material was obtained from 112 patients who underwent initial surgery of diffuse astrocytoma (23 patients) and anaplastic astrocytoma (17 patients) between 1995 and 2005, and glioblastoma (72 patients) between 2001 and 2005 at Odense University hospital, Denmark. For further details about tissue selection, exclusion criteria’s and tissue preparation see Aaberg-Jessen et al. 2009 and Christensen et al. 2008 [24,25].
LAMP-1 immunohistochemistry
Paraffin sections of all tumors were stained using the LAMP-1 monoclonal antibody, clone H4A3 (Developmental Studies, Hybridoma Bank, the University of Iowa, USA). The immunostainings were performed using a Dako Autostainer Universal Staining System (Dako, Glostrup, Denmark). All reagents were obtained from Dako A/S Denmark and used as described by manufacturer’s instructions. Paraffin sections were deparaffinised and endogenous peroxidase activity was quenched by immersion in 1.5% hydrogen peroxide followed by heat-induced epitope retrieval in T-EG buffer. The sections were subsequently incubated for 60 min with the LAMP-1 antibody (1+2000). The detection of the antigen-antibody complex was performed using anti-mouse PowerVision followed by visualization with diaminobenzidine (DAB) as chromogen. Finally, the sections were counterstained with Mayer’s haematoxylin. Paraffin sections of tissue microarrays with 28 normal tissues and 12 cancers were used as positive control. LAMP-1 was particularly found in the thyroid gland, placenta and cervix uterus. Primary antibody omission was used as a negative control.
Scoring of the LAMP-1 staining
The immunohistochemical score was based on the average percentage of LAMP-1 positive cells and blood vessels and their average staining intensity for the whole tissue section. Regarding the percentage of positive tumor cells, the score 0 corresponds to 0%-2% positive cells, score 1 to 2%<-20% positive cells, score 2 to 20%<-70% positive cells and score 3 to 70%<-100% positive cells. Regarding the tumor cell staining intensity, the score 0 corresponds to no staining, score 1 to faint staining, score 2 to moderate staining and score 3 to intense immunostaining. The percentage of positive tumor blood vessels and the blood vessel staining intensity were assessed in the same way. The necrotic areas of the glioblastomas were not included when the tumors were scored. Moreover, the invasion zones were not included. In order to include all four scores per tumor in one total value, the four scores for each tumor were summed. Thus a maximum score of 12 points could be obtained for each tumor. For comparison with overall patient survival, the tumors were divided into three groups according to increasing total scores. Group one: total scores 0-9, group two: total scores 10, and group three: total scores 11-12. This division was chosen to ensure an equal distribution of the glioblastoma patients in the three groups.
LAMP-1 double immunofluorescence staining
Double immunofluorescence staining was analyzed on 10 selected paraffin embedded glioblastomas cut in 3 μm slices on a microtome and mounted on glass slides. The paraffin sections were dewaxed and the endogenous peroxidase activity was quenched. The stainings were performed using a Dako Autostainer Universal Staining System (Dako, Glostrup, Denmark). Performing LAMP-1/GFAP double stainings, LAMP-1 antibody was applied after HIER with T-EG, and detected with Catalyzed Signal Amplification II kit with FITC as fluorochrome. After another round of HIER with T-EG, GFAP antibody was applied using Alexa 555 (Invitrogen, Denmark) as fluorochrome. The coverslips were mounted with mounting medium containing 4,6-diamidino-2-phenylindole (DAPI) (VWR International Aps, Denmark) to identify the nuclei. Co-expression was visualized using confocal microscopy (Nikon, Inverted Microscope, ECLIPSE TE2000-E). When performing the LAMP-1/CD133 double immunofluorescence staining, HIER was performed in T-EG buffer followed by incubation of the tissue sections with CD133 antibody. The Catalyzed Signal Amplification II kit with FITC was used as detection system (Dako, Glostrup, Denmark). Then a second round of HIER with T-EG buffer was performed and the tissue sections were incubated with LAMP-1 followed by detection with the Tyramide Signal Amplification system with tetramethylrhodamine as fluochrome (PerkinElmer, Denmark). The coverslips were mounted as above.
Statistical analysis
For comparison of LAMP-1 scores with increasing tumor grades, ANOVA with Bonferroni correction was used. Statistical significance was defined as *P<0.05, **P<0.01, ***P<0.001. Overall patient survival was defined from the day of initial surgery until death of the patient. For each tumor grade, the LAMP-1 immunohistochemical scores corresponding to the percentage of LAMP-1 positive tumor cells and blood vessels and their staining intensity (0-3) were compared with overall patient survival in a Kaplan-Meier plot using the log rank test. Furthermore, the glioblastoma patients were stratified into three groups based on the total LAMP-1 score (0-9; 10; 11-12) for comparison with overall patient survival in the same way. When performing the analysis, nine patients with diffuse astrocytoma, one patient with anaplastic astrocytoma and one patient with glioblastoma were still alive. These patients were therefore censored. The multivariate Cox regression model was used to adjust for age and gender. P<0.05 was considered significant. The statistical analyses were performed using SPSS 16.0 software (Statistical Package for the Social Sciences, SPSS, Denmark).
Results
LAMP-1 expression in normal brain tissue
LAMP-1 expression was investigated in normal brain tissue and a wide distribution was found in the endothelial cells in blood vessels. A faint staining was observed in cells with microglial and astroglial morphology, whereas neurons in general were negative. Some staining, however, was found in the purkinje cells. In the ventricular zone, the ependymal layer and the ependymal islands had a distinct LAMP-1 expression.
LAMP-1 expression in astrocytomas
Astrocytomas had a widespread expression pattern of LAMP-1 in the tumor cells. The staining was very granular with a distribution throughout most of the cell cytoplasm. In the blood vessels, LAMP-1 expression was found at the luminal side corresponding to the endothelial cells (Figure 1B, 1J).
Figure 1.
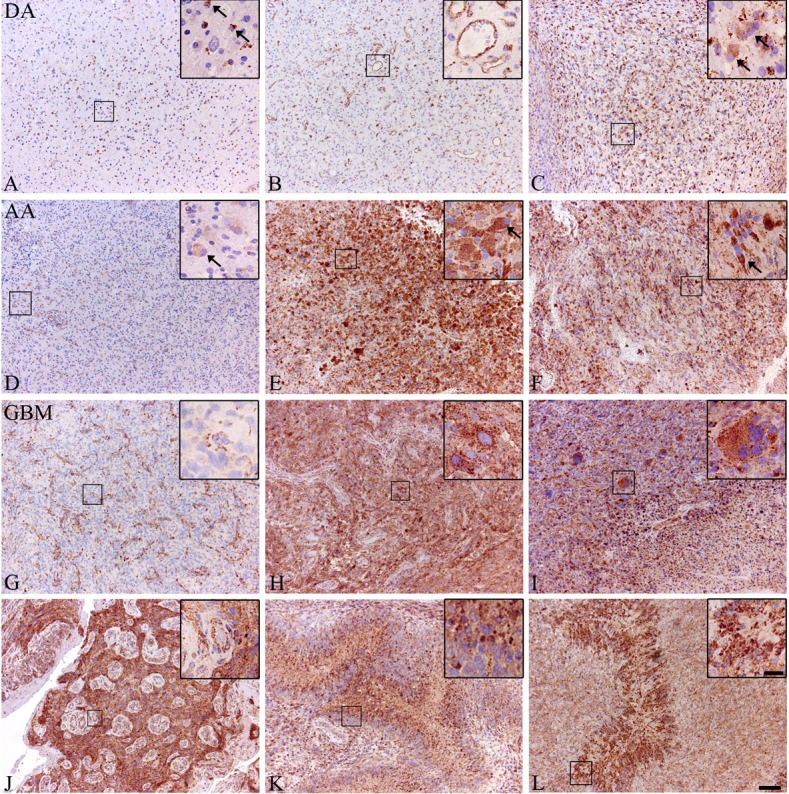
LAMP-1 immunohistochemical expression in astrocytomas. The expression of LAMP-1 was investigated immunohistochemically in diffuse and anaplastic astrocytomas as well as in glioblastomas. The expression in diffuse astrocytomas was primarily found in cells with microglial morphology (A), in blood vessels (B) and in cells with gemistocytic morphology (C). In the anaplastic astrocytomas, cells with a gemistocytic morphology also showed expression of LAMP-1 with both low (D) and high (E) expression levels. Positive cells with other morphologies were also found (F). In glioblastomas the majority of the tumors showed high expression of LAMP-1 in the tumor cells, but also tumors with large negative areas were found. In these areas the highest LAMP-1 expression was found in the blood vessels (G). LAMP-1 expression was found in tumor cells with different morphologies with different degrees of pleomorphism. Both gemistocytic (H) tumor cells as well as multinucleated giant cells (I) were found. Besides being found in tumor cells, LAMP-1 expression was also found in blood vessels (J) and in and around necrotic palisades (K, L). Scalebar A-L 100 μm, inserts 20 μm.
In diffuse astrocytomas, LAMP-1 expression was found predominantly in cells with microglial morphology (Figure 1A) and in the blood vessels (Figure 1B). Expression of LAMP-1 in tumor cells was also found, particularly in tumor cells with gemistocytic morphology (Figure 1C). LAMP-1 expression was found in 21 out of 23 tumors.
Anaplastic astrocytomas resembled the diffuse astrocytomas in expression pattern but had a more intense staining, both including tumor cells with a gemistocytic morphology (Figure 1D and 1E) and tumor cells with other morphologies (Figure 1F). LAMP-1 expression was found in 15 out of 17 tumors.
In glioblastomas, a few tumors with low LAMP-1 expression but positive blood vessels were found (Figure 1G), however, most tumors had a high LAMP-1 expression (Figure 1H). Both tumor cells with pleomorphic and gemistocytic morphologies (Figure 1H) expressed LAMP-1 as well as multinuclear giant cells (Figure 1I). LAMP-1 expression was also found in tumor cells around blood vessels and around necroses (Figure 1J-L). Positive staining was found in all 72 tumors investigated.
Distribution of immunohistochemical score
Glioblastomas had the highest percentage of positive tumor cells compared to diffuse and anaplastic astrocytomas, thus most often receiving the score 3 (Figure 2A). Both anaplastic and diffuse astrocytomas received the score 1 in approximately half of the investigated tumors (Figure 2A). The highest scores for tumor cell staining intensity were also most often given to the glioblastomas (Figure 2B), and again the diffuse and anaplastic astrocytomas received the score 1 in most tumors (Figure 2B). LAMP-1 expression was found in all blood vessels (endothelial cells) in all tumors investigated (Figure 2C). All tumors therefore received the score 3. The blood vessel staining intensity was also investigated and in this case the majority of glioblastomas and both diffuse and anaplastic astrocytomas received the scores 1 and 2 (Figure 2D). The total score corresponding to the sum of scores for positive tumor cells, tumor cell intensity, positive blood vessels and intensity of the blood vessels was calculated for each tumor. Scores from 0-9 were mostly observed in diffuse and anaplastic astrocytomas, whereas score 10, and 11-12 were most often seen in the glioblastomas (Figure 2E).
Figure 2.
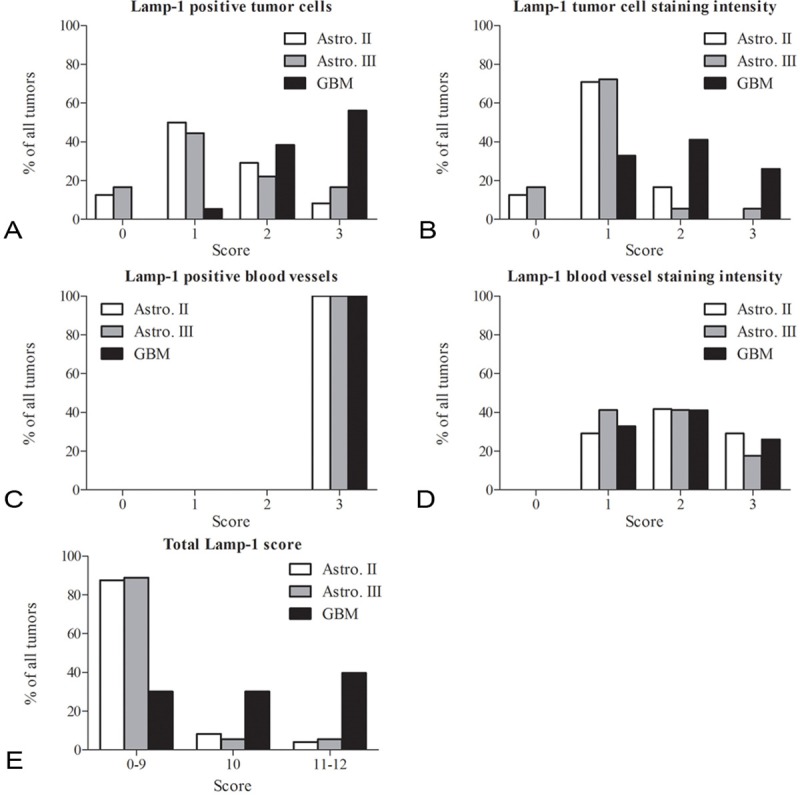
LAMP-1 distribution in astrocytomas. The distribution of LAMP-1 scores was compared for the diffuse and anaplastic astrocytomas and the glioblastomas in four categories. The four categories were percentage LAMP-1 positive tumor cells (A), LAMP-1 tumor cell staining intensity (B), percentage LAMP-1 positive blood vessels (C) and LAMP-1 blood vessel staining intensity (D). A fifth category included was the total sum of LAMP-1 scores (E) divided into three groups with scores from 0-9, 10 and 11-12. In the category percentage positive tumor cells, glioblastomas most often received the highest score of 2 and 3, whereas both the diffuse and anaplastic astrocytomas most often received the score 1 and 2 (A). In the tumor cell staining intensity category, the scores for glioblastomas were evenly distributed between the scores 1-3, whereas the most frequent staining intensity for the diffuse and anaplastic astrocytomas was 1 (B). In the category positive blood vessels, all tumors independently of tumor grade received the score 3 (C). In the category blood vessel staining intensity, there was a small preference for the score 2 (D). For total LAMP-1 score, glioblastomas were almost evenly distributed in the three groups, however, with a small preference for the score 11-12. The diffuse and anaplastic astrocytomas primarily received total scores between 0 and 9 (E).
The mean score calculated for each tumor grade showed that glioblastomas had a significantly higher percentage tumor cell score (Figure 3A), tumor cell staining intensity (Figure 3B), blood vessel staining intensity (Figure 3D) and total LAMP-1 score (Figure 3E). Since all tumors displayed LAMP-1 positive blood vessels, the mean score for all groups was 3 (Figure 3C).
Figure 3.
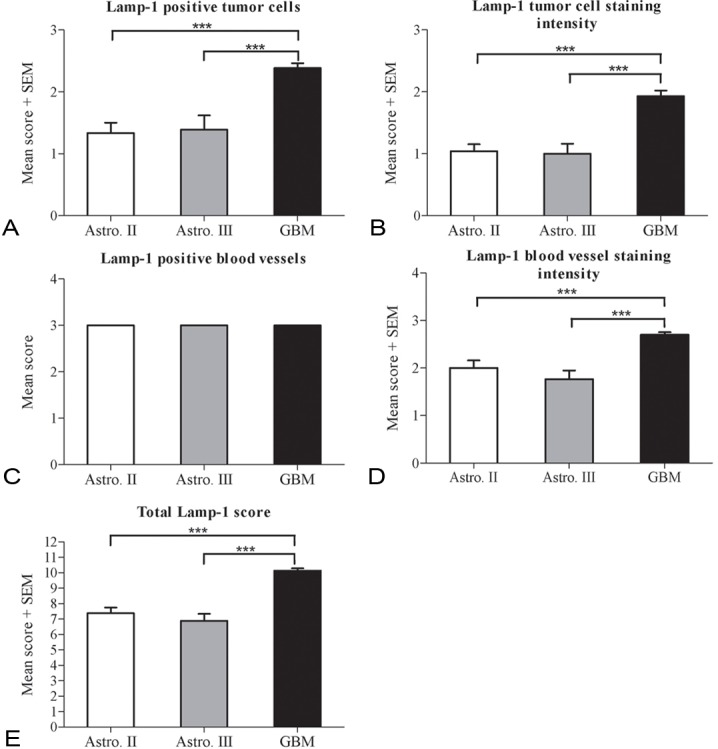
Mean LAMP-1 score in the astrocytomas. The mean LAMP-1 score was compared for diffuse and anaplastic astrocytomas and glioblastomas in the five categories (A-E). The glioblastomas showed the highest mean score in the categories LAMP-1 positive tumor cells (A), LAMP-1 tumor cell staining intensity (B), LAMP-1 blood vessel staining intensity (D) and total LAMP-1 score (E). The same mean score was found for LAMP-1 positive blood vessels (C) in the three tumor grades. Data are analyzed using ANOVA with Bonferroni correction for comparison with control and statistical significance *P<0.05, **P<0.01, ***P<0.001.
Comparison of LAMP-1 protein expression and overall patient survival
The Kaplan-Meier plot (Figure 4) compares the total Lamp-1 expression scores in the 72 glioblastomas patients with overall patient survival in months. The multivariate Cox regression test adjusting for age and gender revealed that the positive Lamp-1 tumor cells and blood vessels did not influence the overall patient survival (p=0.820). This was also true when correlating the individual score groups or different combination of score groups corresponding to percentage LAMP-1 positive tumor cells and blood vessels as well as staining intensity in the tumor cells and blood vessel staining intensity with patient overall survival (data not shown). Similar results were found for diffuse and anaplastic astrocytomas and for all grades together (not shown).
Figure 4.
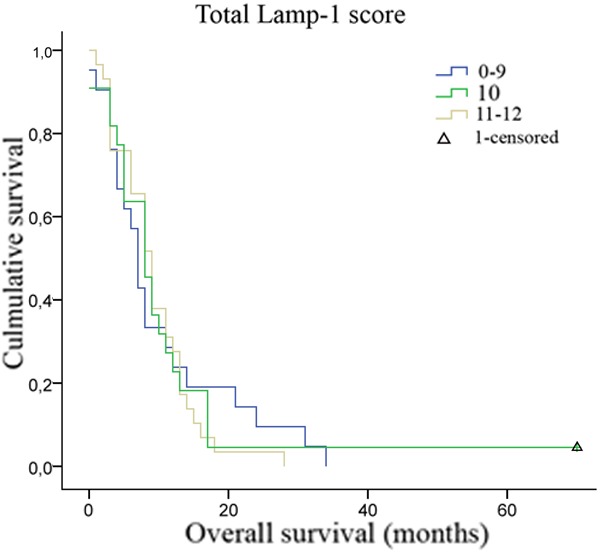
Correlation between LAMP-1 scores and overall patient survival. A Kaplan-Meier survival plot for the 72 glioblastoma patients grouped according to the total LAMP-1 immunohistochemical score showed no correlation between LAMP-1 expression and patient overall survival (p=0.820). Group 0-9, n=22, group 10, n=21, group 11-12, n=29.
Co-expression of LAMP-1/GFAP and LAMP-1/CD133
To confirm the presence of LAMP-1 in tumor cells a LAMP-1/GFAP double immunofluorescence staining was performed. Co-expression was found in all tumors investigated and in tumor cells with different morphologies. Co-expression illustrated as yellow staining with a dot-like pattern was found in gemistocytic tumor cells (Figure 5A) and in tumor cells with other morphologies (Figure 5B). Many multinuclear giant cells were also found to express both LAMP-1 and GFAP (Figure 5C). However, not all cells expressing GFAP also expressed LAMP-1.
Figure 5.
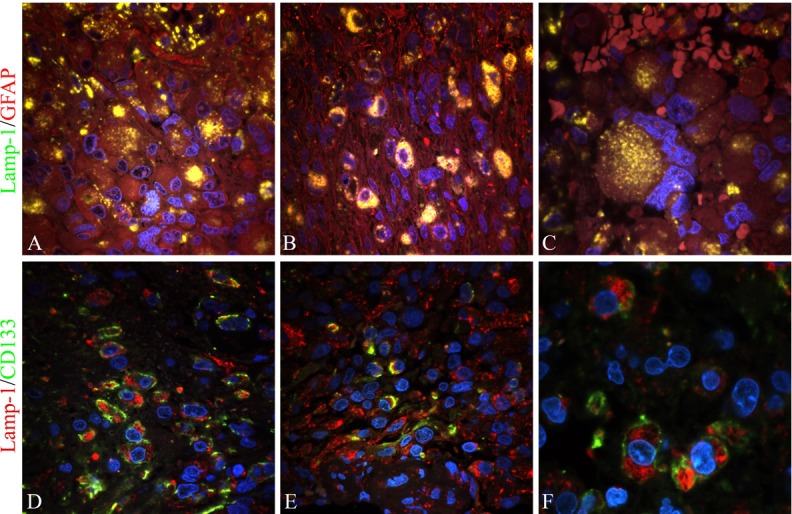
Double immunofluorescence staining with LAMP-1/GFAP and LAMP-1/CD133. Expression of LAMP-1 in tumor cells and tumor stem cells of 10 glioblastomas were investigated by confocal microscopy and double immunofluorescence using the astrocytic marker GFAP and the tumor stem cell marker CD133, respectively. Co-expression of LAMP-1 and GFAP was identified as a yellow dot-like staining in the cell cytoplasm. It was found in cells with a gemistocytic appearance (A), in tumor cells with other morphologies (B) as well as in multinuclear giant cells (C). Co-expression of LAMP-1 and CD133 was identified as cells with a red cytoplasm representing LAMP-1 and a green membrane staining being CD133. Co-expression was found in CD133 niches consisting of a cluster of several cells expressing CD133 (D) as well as in dispersed CD133 single cells (E and F). The CD133 single cells were surrounded by LAMP-1 positive but CD133 negative cells. The images were recorded by a 60x lens (A, B, D, E) using an additional zoom in some areas (C and F).
Double immunofluorescence with LAMP-1 and CD133 was performed to identify proposed tumor stem cells expressing LAMP-1. Co-expression was identified in cells with a green CD133 membrane staining and a red LAMP-1 dot-like staining in the cytoplasm. Co-expression was found in both CD133 niches (Figure 5D) and in CD133 single cells (Figure 5E and 5F). The majority of CD133 positive cells co-expressed LAMP-1.
Discussion
In the present study the immunohistochemical expression and distribution of the lysosomal-membrane associated protein LAMP-1 in astrocytomas was investigated. The highest expression was found in glioblastomas but different degrees of staining were also found in these tumors. However, no correlation was found between LAMP-1 scores and overall patient survival. Most interestingly, both LAMP-1/GFAP and LAMP-1/CD133 co-expression was found.
LAMP-1 expression in astrocytomas
A significantly higher LAMP-1 expression was found in glioblastomas compared with diffuse and anaplastic astrocytomas suggesting an increase in the amount of lysosomes present. To support this, co-expression of LAMP-1 and the astrocytic marker GFAP was found suggesting that the tumor cells indeed expressed LAMP-1. The lysosomal content in a cell has previously been shown to be directly related to the LAMP expression [26] suggesting glioblastomas to have an increased number of lysosomes compared to diffuse and anaplastic astrocytomas. Previous findings demonstrated an increase in the amount of lysosomes in the ras-transformed version of a mouse mammary epithelial cell line compared to the non-transformed cells [26] and in a study investigating the expression of LAMP-1 in colorectal neoplasms a significant increase in expression was found in adenomas and cancers compared to normal colorectal epithelial cells [18]. These studies support the hypothesis that transformed cells and cancer cells have a higher amount of lysosomes compared to non-cancerous cells.
LAMP-1 expression in tumor stem cells
In the present study co-expression of LAMP-1 and the stem cell marker CD133 was found. This is in line with a previous study in our group [27], where an increase in both CD133 and LAMP-1 expression was found in glioblastoma tumor stem cell containing spheroids in hypoxia compared to normoxia. Tumors and especially glioblastomas are often very hypoxic supporting a higher amount of lysosomes in the glioblastomas. A study investigating the ultrastructures of glioma stem cells/progenitor cells by transmission electron microscopy found only rarely lysosomes in a glioma cell line and in patient tissue from a mixed glioma [28]. In this study both isolated tumor stem cells from a human glioma cell line and tumor spheroids were used for analysis. The result contradict with the findings in the present study, however, the results should be interpreted with caution since only one cell line and tumor tissue from one patient was used.
Expression of lysosomal proteins and patient survival
No association between LAMP-1 expression and overall survival was found in the present study suggesting that the presence of LAMP-1 protein itself does not contribute to survival of the patients. Surprisingly, LAMP-1 mRNA has previously been correlated to prognosis in patients with pancreatic carcinoma [15], where high expression of LAMP-1 mRNA was found to be associated with longer survival. Moreover, LAMP-1 expression was in this study suggested to correlate to local tumor progression instead of metastasis indicating a role of LAMP-1 in intercellular adhesion processes. Another lysosomal membrane protein, LAPTM4B-35 has been extensively investigated in pancreatic, colorectal and hepatocellular carcinomas [19-21]. A high expression of LAPTM4B-35 was in all cases associated with a poor prognosis. LAPTM4B-35 has been suggested to play a role in tumor initiation/progression and overexpression of the protein has furthermore been shown to promote proliferation, migration, and invasion [20]. Interestingly the protein has also been associated with lysosomal cell death [29] rendering tumor cells resistant to lysosomal-mediated cell death triggered by environmental and genotoxic stress.
The lysosomal proteases, the cathepsins, have also been correlated with patient survival. Studies have investigated the association of expression of the different cathepsins with prognosis in glioblastomas [30,31]. A high expression of cathepsin B [31] and cathepsin D [30] was found to be associated with poor overall survival. However, this is believed to be related to the role of cathepsins released extracellularly contributing to migration and angiogenesis and not to the lysosomal-cell death pathway. The cathepsins are believed to play a dual role being involved in both tumor progression and in the lysosomal cell death pathway. Upon release of the cathepsins to the extracellular matrix they are believed to be involved in ECM degradation, migration, and angiogenesis, whereas the cathepsins upon release from the lysosomes into the cytosol play an important role in inducing caspase-independent cell death [5,32].
Lysosomal cell death in glioblastoma
The high expression of LAMP-1 in glioblastomas found in the present study suggests the presence of a high amount of lysosomes in these tumors thereby proposing a role for induction of cell death through the lysosomal cell death pathway. Interestingly, killing of glioma cells surpassing both the intrinsic and extrinsic apoptotic pathways has previously been described [33] in a study using parvovirus H-1. The results showed that parvovirus induced cell death through a non-apoptotic pathway mediated by cathepsins and most likely through lysosomal membrane permabilization leading to release of cathepsins. The use of viruses may be difficult in a clinical setting but instead the use of the lysosome-destabilizing drug siramesine could prove efficient by inducing lysosomal cell death in the same manner as the parvovirus. Siramesine has earlier been found to directly destabilize the lysosomal membrane followed by lysosomal dysfunction leading to permabilization of the membrane and release of cathepsins to the cytosol resulting in cathepsin-mediated cell death [34,35]. Accordingly, exposure to siramesine leads to extensive cell death in a variety of immortalized and transformed cancer cells in vitro [34-39].
Even though the glioblastomas received the highest LAMP-1 scores several of these tumors also showed low expression of LAMP-1. Thus if an effective treatment targeting the lysosomes is developed it might prove useful in only a subset of the glioblastomas providing the need of a lysosomal biomarker to predict treatment response.
Interestingly, co-expression of LAMP-1 and the tumor stem cell marker CD133 in the present study suggested that a part of the proposed tumor stem cells also had a high content of lysosomes. A therapeutic strategy targeting lysosomes may thereby be speculated to be efficient also in terms of eradicating the tumor stem cells.
In conclusion, we found a high LAMP-1 protein expression in glioblastomas compared to diffuse and anaplastic astrocytomas. There was no association between expression level in glioblastomas and survival. The supposed high amount of lysosomes in glioblastomas may suggest these tumors to be very sensitive to drugs targeting lysosomes. Using double-immunofluorescence we found co-expression of LAMP-1 and the stem cell marker CD133 suggesting that tumor stem cells may be efficiently eradicated by therapeutic strategies targeting lysosomes. Such targeting of tumor stem cells may be crucial for improving glioblastoma treatment since these cells are believed to escape conventional therapies thereby being responsible for tumor recurrence.
Acknowledgement
We acknowledge the excellent laboratory work done by technicians Helle Wohlleben and Tanja Dreehsen Højgaard. This work was supported by the Danish Cancer Society, Danish Medical Research Council, Kathrine and Vigo Skovgaard’s Foundation, The Cancer Foundation, Johs. Clemmesen’s Research Foundation, Memorial Foundation for Alice Brenaa, Merchant M. Kristian Kjær and wife Margrethe Kjær born la Cour-Holmen’s Foundation, Eva and Henry Frænkel’s Memorial Foundation, and Merchant M. Brogaard and wife’s Memorial Foundation.
Declaration of conflict of interest
There is no conflict of interest.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Groth-Pedersen L, Jaattela M. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer Lett. 2013 May 28;332:265–74. doi: 10.1016/j.canlet.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Jaattela M. Multiple cell death pathways as regulators of tumour initiation and progression. Oncogene. 2004;23:2746–2756. doi: 10.1038/sj.onc.1207513. [DOI] [PubMed] [Google Scholar]
- 6.Fehrenbacher N, Jaattela M. Lysosomes as targets for cancer therapy. Cancer Res. 2005;65:2993–2995. doi: 10.1158/0008-5472.CAN-05-0476. [DOI] [PubMed] [Google Scholar]
- 7.Kirkegaard T, Jaattela M. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta. 2009;1793:746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003;5:533–545. doi: 10.1016/s1476-5586(03)80037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ono K, Kim SO, Han J. Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor alpha-induced cell death. Mol Cell Biol. 2003;23:665–676. doi: 10.1128/MCB.23.2.665-676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskelinen EL, Tanaka Y, Saftig P. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 2003;13:137–145. doi: 10.1016/s0962-8924(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 13.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson-Lawrence EJ, Dean CJ, Chang M, Hopwood JJ, Meikle PJ, Brooks DA. Immunochemical analysis of CD107a (LAMP-1) Cell Immunol. 2005;236:161–166. doi: 10.1016/j.cellimm.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Kunzli BM, Berberat PO, Zhu ZW, Martignoni M, Kleeff J, Tempia-Caliera AA, Fukuda M, Zimmermann A, Friess H, Buchler MW. Influences of the lysosomal associated membrane proteins (Lamp-1, Lamp-2) and Mac-2 binding protein (Mac-2-BP) on the prognosis of pancreatic carcinoma. Cancer. 2002;94:228–239. doi: 10.1002/cncr.10162. [DOI] [PubMed] [Google Scholar]
- 16.Andrejewski N, Punnonen EL, Guhde G, Tanaka Y, Lullmann-Rauch R, Hartmann D, von FK, Saftig P. Normal lysosomal morphology and function in LAMP-1-deficient mice. J Biol Chem. 1999;274:12692–12701. doi: 10.1074/jbc.274.18.12692. [DOI] [PubMed] [Google Scholar]
- 17.Sarafian V, Jadot M, Foidart JM, Letesson JJ, Van den BF, Castronovo V, Wattiaux R, Coninck SW. Expression of Lamp-1 and Lamp-2 and their interactions with galectin-3 in human tumor cells. Int J Cancer. 1998;75:105–111. doi: 10.1002/(sici)1097-0215(19980105)75:1<105::aid-ijc16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Furuta K, Ikeda M, Nakayama Y, Nakamura K, Tanaka M, Hamasaki N, Himeno M, Hamilton SR, August JT. Expression of lysosome-associated membrane proteins in human colorectal neoplasms and inflammatory diseases. Am J Pathol. 2001;159:449–455. doi: 10.1016/S0002-9440(10)61716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Xiong FX, Lin M, Yang Y, Nie X, Zhou RL. LAPTM4B-35 overexpression is a risk factor for tumor recurrence and poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:275–281. doi: 10.1007/s00432-009-0659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang Y, Yin M, Jiang W, Zhang H, Xia B, Xue Y, Huang Y. Overexpression of LAPTM4B-35 is associated with poor prognosis in colorectal carcinoma. Am J Surg. 2012;204:677–83. doi: 10.1016/j.amjsurg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Liang Y, Huang Y, Chen Y, Zhou R. Elevated Lysosome-associated Protein Transmembrane-4beta-35 is an Independent Prognostic Marker in Pancreatic Carcinoma. J Int Med Res. 2012;40:1275–1283. doi: 10.1177/147323001204000406. [DOI] [PubMed] [Google Scholar]
- 22.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 23.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaberg-Jessen C, Christensen K, Offenberg H, Bartels A, Dreehsen T, Hansen S, Schroder HD, Brunner N, Kristensen BW. Low expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in glioblastoma predicts longer patient survival. J Neurooncol. 2009;95:117–128. doi: 10.1007/s11060-009-9910-8. [DOI] [PubMed] [Google Scholar]
- 25.Christensen K, Schroder HD, Kristensen BW. CD133 identifies perivascular niches in grade II-IV astrocytomas. J Neurooncol. 2008;90:157–70. doi: 10.1007/s11060-008-9648-8. [DOI] [PubMed] [Google Scholar]
- 26.Cella N, Cornejo-Uribe RR, Montes GS, Hynes NE, Chammas R. The lysosomal-associated membrane protein LAMP-1 is a novel differentiation marker for HC11 mouse mammary epithelial cells. Differentiation. 1996;61:113–120. doi: 10.1046/j.1432-0436.1996.6120113.x. [DOI] [PubMed] [Google Scholar]
- 27.Kolenda J, Jensen SS, Aaberg-Jessen C, Christensen K, Andersen C, Brunner N, Kristensen BW. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J Neurooncol. 2011;103:43–58. doi: 10.1007/s11060-010-0357-8. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Huang Q, Zhang T, Dong J, Wang A, Lan Q, Gu X, Qin Z. Ultrastructural studies of glioma stem cells/progenitor cells. Ultrastruct Pathol. 2008;32:241–245. doi: 10.1080/01913120802289165. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Zhang Q, Tian R, Wang Q, Zhao JJ, Iglehart JD, Wang ZC, Richardson AL. Lysosomal transmembrane protein LAPTM4B promotes autophagy and tolerance to metabolic stress in cancer cells. Cancer Res. 2011;71:7481–7489. doi: 10.1158/0008-5472.CAN-11-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuda ME, Iwadate Y, Machida T, Hiwasa T, Nimura Y, Nagai Y, Takiguchi M, Tanzawa H, Yamaura A, Seki N. Cathepsin D is a potential serum marker for poor prognosis in glioma patients. Cancer Res. 2005;65:5190–5194. doi: 10.1158/0008-5472.CAN-04-4134. [DOI] [PubMed] [Google Scholar]
- 31.Colin C, Voutsinos-Porche B, Nanni I, Fina F, Metellus P, Intagliata D, Baeza N, Bouvier C, Delfino C, Loundou A, Chinot O, Lah T, Kos J, Martin PM, Ouafik L, Figarella-Branger D. High expression of cathepsin B and plasminogen activator inhibitor type-1 are strong predictors of survival in glioblastomas. Acta Neuropathol. 2009;118:745–754. doi: 10.1007/s00401-009-0592-2. [DOI] [PubMed] [Google Scholar]
- 32.Kallunki T, Olsen OD, Jaattela M. Cancer-associated lysosomal changes: friends or foes? Oncogene. 2013 Apr 18;32:1995–2004. doi: 10.1038/onc.2012.292. [DOI] [PubMed] [Google Scholar]
- 33.Di PM, Mader C, Geletneky K, Herrero YC, Weber E, Schlehofer J, Deleu L, Rommelaere J. Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRAIL-resistant glioma cells. J Virol. 2007;81:4186–4198. doi: 10.1128/JVI.02601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostenfeld MS, Fehrenbacher N, Hoyer-Hansen M, Thomsen C, Farkas T, Jaattela M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 2005;65:8975–8983. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- 35.Ostenfeld MS, Hoyer-Hansen M, Bastholm L, Fehrenbacher N, Olsen OD, Groth-Pedersen L, Puustinen P, Kirkegaard-Sorensen T, Nylandsted J, Farkas T, Jaattela M. Anti-cancer agent siramesine is a lysosomotropic detergent that induces cytoprotective autophagosome accumulation. Autophagy. 2008;4:487–499. doi: 10.4161/auto.5774. [DOI] [PubMed] [Google Scholar]
- 36.Groth-Pedersen L, Ostenfeld MS, Hoyer-Hansen M, Nylandsted J, Jaattela M. Vincristine induces dramatic lysosomal changes and sensitizes cancer cells to lysosome-destabilizing siramesine. Cancer Res. 2007;67:2217–2225. doi: 10.1158/0008-5472.CAN-06-3520. [DOI] [PubMed] [Google Scholar]
- 37.Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, Ojcius DM, Jaattela M, Kroemer G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003;197:1323–1334. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fehrenbacher N, Bastholm L, Kirkegaard-Sorensen T, Rafn B, Bottzauw T, Nielsen C, Weber E, Shirasawa S, Kallunki T, Jaattela M. Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res. 2008;68:6623–6633. doi: 10.1158/0008-5472.CAN-08-0463. [DOI] [PubMed] [Google Scholar]
- 39.Rammer P, Groth-Pedersen L, Kirkegaard T, Daugaard M, Rytter A, Szyniarowski P, Hoyer-Hansen M, Povlsen LK, Nylandsted J, Larsen JE, Jaattela M. BAMLET activates a lysosomal cell death program in cancer cells. Mol Cancer Ther. 2010;9:24–32. doi: 10.1158/1535-7163.MCT-09-0559. [DOI] [PubMed] [Google Scholar]


