Abstract
The atypical lipomatous tumor (ALT)/well-differentiated liposarcoma (WDLS) and the de-differentiated liposarcoma (DDLS) represent the most common category of liposarcomas. ALT/WDLSs and DDLSs are often difficult to distinguish from other tumors with similar morphological characteristics. In this study, we investigated whether the detection of amplified or overexpressed murine double-minute 2 (MDM2) can be a useful diagnostic ancillary aid. We used fluorescent in situ hybridization (FISH) and immunohistochemistry (IHC) to detect MDM2 amplification and protein overexpression, respectively, in 49 WDLSs, 5 DDLSs, 23 myxoid liposarcomas, 25 benign lipomatous tumors, and 75 spindle and pleomorphic sarcomas. MDM2 amplification was detected in 48 of 49 WDLSs, 5 of 5 DDLSs, 2 of 9 malignant peripheral nerve sheath tumors, and 2 of 10 myxofibrosarcomas. We did not detect MDM2 amplification in any of the benign lipomatous tumors. FISH-mediated detection of MDM2 amplification was the most valuable diagnostic aid for ALT/WDLS, as determined by using the Fisher exact test to compare two different diagnoses of 19 biopsies. On the contrary, unequivocal nuclear overexpression of MDM2 was found in only 10 of 50 ALT/WDLSs. The sensitivity and specificity of MDM2 amplification in distinguishing a DDLS from spindle and pleomorphic sarcomas were 100% and 95%, respectively, while those of MDM2 overexpression were 100% and 87%, respectively. In conclusion, our results indicate that FISH-mediated detection of MDM2 amplification is the most useful adjunct in the diagnosis of both ALT/WDLS and DDLS. However, IHC-mediated detection of MDM2 protein is useful only for the diagnosis of DDLS.
Keywords: Liposarcoma, MDM2, FISH, IHC
Introduction
Liposarcoma is the most frequent soft tissue sarcoma in adults. The World Health Organization (WHO) divides liposarcomas into 4 subtypes: atypical lipomatous tumor (ALT)/well-differentiated liposarcoma (WDLS), myxoid liposarcoma (MXLS), de-differentiated liposarcoma (DDLS), and pleomorphic liposarcoma. Among them, ALT/WDLS and MXLS are the most common liposarcomas, while pleomorphic liposarcomas are rare. DDLS is a high-grade nonlipogenic sarcoma that arises from a pre-existing WDLS [1].
During the last several years, there have been great strides in our understanding of liposarcomas, largely as a result of cytogenetic studies [2]. We now know that the genomic profiles of ALT/WDLS and DDLS are characterized by amplified sequences of chromosomal region 12q14-15, which contains the murine double minute-2 (MDM2) and cyclin dependent kinase-4 (CDK4) genes. MDM2 is consistently amplified in cases of ALT/WDLS and could be considered as a target gene of this amplicon. MDM2, which was originally cloned from a spontaneously transformed BALB/C mouse 3T3 cell line [3,4], is an oncogene whose expression controls both tumorigenesis and the cell cycle by promoting degradation of tumor suppressor protein p53 (TP53). CDK4 is located at 12q14.1 and is frequently amplified in tumors that have amplified MDM2. CDK4 phosphorylates retinoblastoma 1 (RB); this phosphorylation disrupts RB’s interactions with E2F transcription factors and allows the cell cycle to proceed through the G1-S checkpoint [5]. Cytogenetic studies have also determined that MXLSs are characterized by a specific translocation, t(12;16), that in almost all cases results in a fusion of fused in sarcoma (FUS, also known as TLS) with DNA-damage-inducible transcript 3 (DDIT3); in a small number of cases t(12;22) results in fusion of DDIT3 with Ewing sarcoma breakpoint region 1 (EWSR1, also known as EWS). Fluorescence in situ hybridization (FISH) is the best method for detecting these types of cytogenetic changes in individual cells. MDM2 amplification can be detected by comparing the number of signals for MDM2 to that of signals for centromere 12. Rearrangement of the DDIT3 locus is detected as separated signals of centromeric and telomeric DDIT3 probes that have been differentially labeled [6,7].
Both ALT/WDLSs and DDLSs are often difficult to identify: ALT/WDLSs are difficult to distinguish from benign lipomatous tumors by using morphological criteria; while distinguishing DDLSs from other high-grade sarcomas may be challenging, especially in needle biopsy specimens that lack areas of well-differentiated liposarcoma [8]. Amplification of MDM2 is known to result in overexpression of MDM2 protein [9]; thus in biopsy samples, immunohistochemistry (IHC) may be a useful method to indirectly detect gene amplification by detecting protein expression [10,11]. However, it has not yet been fully clarified if amplified copies of MDM2 are consistently transcribed or if IHC can successfully detect the overexpressed protein. In fact, recent reports show considerable discordance between the results of IHC analyses and those of molecular analyses [8,12].
In this study, we examined the utility of FISH-mediated detection of MDM2 amplification in the differential diagnosis of ALT/WDLS/DDLS from other morphologically similar sarcomas and from benign lipomatous tumors by reviewing two cohorts of tumors.
Materials and methods
Archival cases
We searched the 2003-2007 case files of the Bone and Soft-Tissue Tumor Study Group of Kanazawa University for cases of soft tissue sarcomas. We retrieved 45 liposarcoma cases (25 ALT/WDLSs, 17 MXLSs, and 3 DDLSs) and 69 cases of various spindle cell- and pleomorphic-cell sarcomas. The tumor distribution is shown in Table 1A. MDM2 overexpression and MDM2 amplification were examined using IHC and FISH, respectively. All cases of this study were reviewed by pathologists who specialize in soft-tissue sarcomas (TN and YD), who had knowledge of the results of the IHC and FISH analyses.
Table 1.
IHC analysis of MDM2 overexpression in archived tumors (A) and newly diagnosed tumors (B)
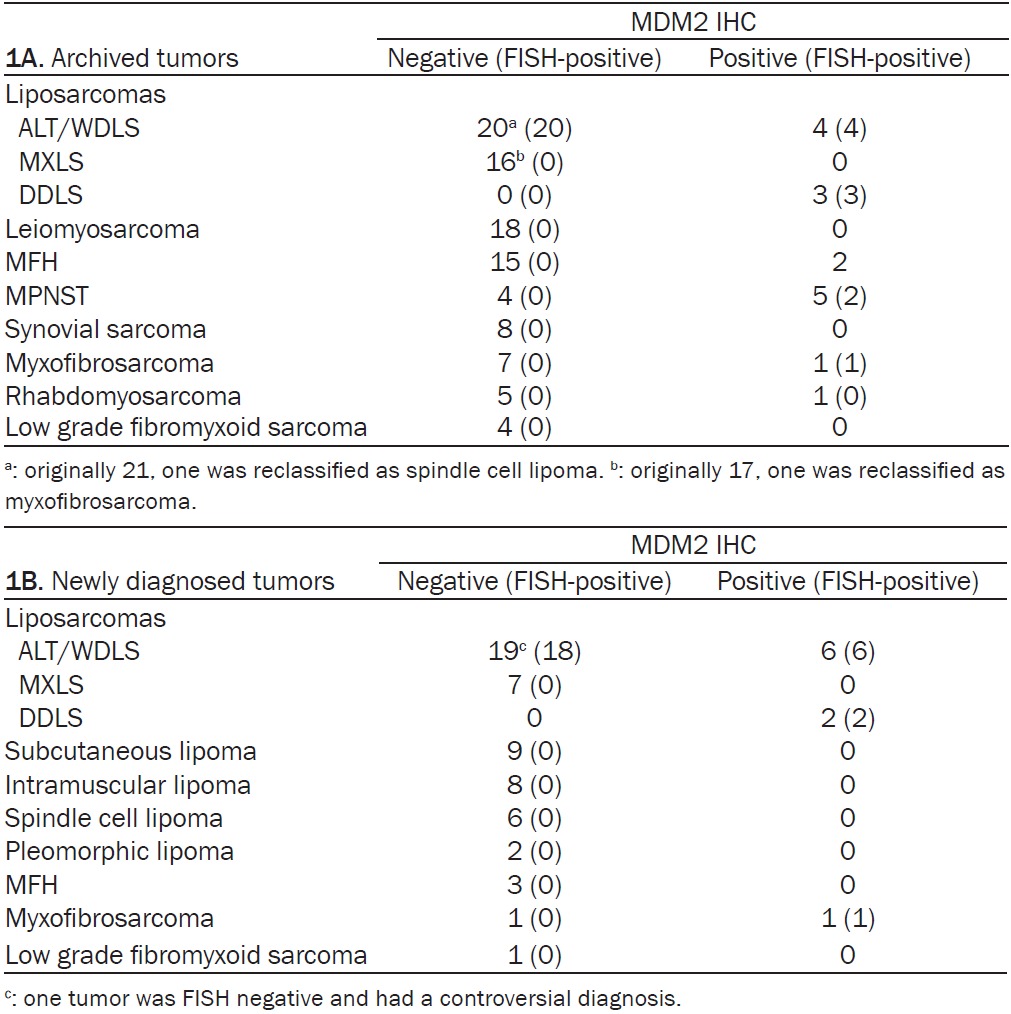
|
Newly diagnosed cases
In 2008, our study group introduced the use of IHC- and FISH-mediated detection of protein overexpression and amplification of MDM2 and CDK4, respectively, as diagnostic adjuncts for the diagnoses of liposarcomas and morphologically similar tumors. Tumors subjected to these analyses are summarized in Table 1B. A preoperative biopsy was performed in 19 of 25 surgically resected ALT/WDLSs.
In order to assess the utility of FISH-mediated detection of MDM2 amplification as a diagnostic adjunct, the 19 ALT/WDLS biopsies were reviewed by 3 board-certified pathologists (OT, S-KS, HI) who are not soft-tissue tumor specialists. The diagnoses of these pathologists, who were blinded to the results of the FISH analyses, were compared to the final diagnoses of the resected tumors. Final diagnoses were made by 2 experts (TN and YD) who had knowledge of the results of the FISH analyses.
IHC
Hematoxylin-eosin staining, IHC, and FISH analyses were conducted on serially cut (4 μm) biopsy specimens and representative whole tissue sections. All sections were mounted on MAS-coatedTM glass slides (Matsunami, Tokyo, Japan). Prior to IHC, the antigens were activated by immersing the slides in citrate buffer (pH 6.0) and autoclaving (121°C) for 15 minutes. The following primary antibodies were used: Anti-human MDM2 antibody (mouse monoclonal IF2; working dilution, 1:40) (Calbiochem, La Jolla CA, USA) and anti-human CDK4 antibody (rabbit polyclonal H-303; working dilution, 1:100) (Santa Cruz Biotechnology, Santa Cruz CA, USA). Proteins were visualized by avidin-biotin binding to peroxidase-conjugated secondary antibodies (DAKO A/S, Glostrup, Denmark). In each analysis, one section of a liposarcoma that had been previously confirmed to overexpress MDM2 and CDK4 was included as a positive control.
The results of the IHC analyses were reviewed by 3 pathologists (HI, SSK, TO), who were unaware of the results of the FISH analyses. The intensities of MDM2 and CDK4 expression in 25 ALT/WDLSs and 25 benign lipomatous tumors (Table 1B) were scored using a three-tiered system: negative, no discernible staining or background staining; 1+, equivocal nuclear staining; 2+, unequivocal intense nuclear staining. A tumor was classified as either positive or negative according to its summed score for the 2 antigens: positive tumors had scores of 4-6; negative tumors had scores of 0-3.
FISH
The probes used for the FISH analyses were generated using the following bacterial artificial chromosomes (BACPAC Resources, Oakland CA, USA): RP11-775J10, which is specific for the MDM2 locus (12q15; AC026121); and RP11-571M6, which is specific for the CDK4 locus (12q14.1; ENSG00000257921). The probes were labeled with SpectrumOrangeTM or SpectrumGreenTM using a nick translation kit (Abbott, Abbott Park IL, USA). A SpectrumGreenTM-labeled probe specific to a centromeric region of chromosome 12 (CEP12TM, Abbott) was included in the hybridization reaction to standardize the chromosome number. When necessary, rearrangement of the DDIT3 gene was examined using FISH with the DDIT dual-color break-apart rearrangement probeTM (Abbott) [6,7]. FISH was performed using standard methods and included a RNase A treatment [13]. The tissue sections were counterstained in phosphate-buffered saline containing 4’,6-diamidine-2’-phenylindole dihydrochloride (DAPI II), p-phenylenediamine, and glycerol (Abbott), then examined with a fluorescence microscope (Olympus, Tokyo, Japan) equipped with a Triple Bandpass FilterTM set (Abbott) for detecting DAPI II, SpectrumOrangeTM, and SpectrumGreenTM.
When scoring the FISH results, only nuclei with at least one CEP12TM signal were evaluated. The ratio of the number of signals for either MDM2 or CDK4 to the number of CEP12TM signals was calculated for each nucleus. If more than 10 nuclei had a ratio > 2, the tumor was considered as positive for gene amplification. The amplification was categorized as high-level when one nucleus had 10 or more MDM2 signals, and as low-level when it had less than 10 signals. Nuclei that had increased numbers of signals for both the gene and centromere 12 (ratio of ≤ 2) were categorized as polysomic for CEP12. After FISH, images of the sections were captured using a cooled charged coupled device camera and recorded on a personal computer.
This study was approved by the Institutional Review Board of the Kanazawa University Hospital (Approval No. 226), and written informed consent was obtained from all patients.
Results
Archived tumors
The results of FISH and IHC analyses of MDM2 amplification and protein expression are shown in Table 1A. Overexpression of MDM2 was detected in 4 of 25 ALT/WDLSs and in 3 of 3 DDLSs. The results of FISH analysis of MDM2 amplification could be interpreted in 107 of 114 archived tumors. Among the 25 tumors diagnosed as ALT/WDLS, 24 were positive for MDM2 amplification. Twelve of the 24 positive tumors were classified as a high-level amplification. After review, the diagnosis of the ALT/WDLS tumor that was negative for MDM2 amplification was corrected to spindle cell lipoma. The 3 tumors diagnosed as DDLS were all positive for MDM2 amplification and were classified as cases of high-level amplification. The 17 MXLSs were negative for MDM2 amplification. The consultants consented to a diagnosis of MXLS in 16 of the 17 tumors. The diagnosis of the remaining tumor was changed to myxofibrosarcoma because FISH analysis did not detect any rearrangements at the DDIT3 locus.
MDM2 overexpression was found in 9 of the 70 non-lipomatous sarcomas, and among these 9 samples, MDM2 amplification was detected in 3:1 myxofibrosarcoma and 2 malignant peripheral nerve sheath tumors (MPNST) (Figure 1). One of the MPNSTs occurred in a case of neurofibromatosis type I and showed rhabdomyoblastic differentiation. We detected polysomy of chromosome 12 in 76% of the non-lipomatous sarcomas.
Figure 1.
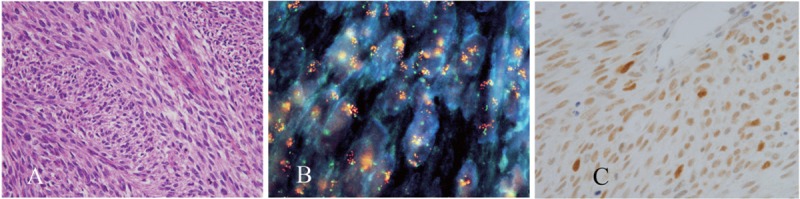
A malignant peripheral nerve sheath tumor with amplified MDM2. Representative histology (A). The results of dual-color FISH with probes directed against MDM2 (orange fluorescence) and centromere 12 (green fluorescence) (B). The results of IHC for MDM2 (C).
Newly diagnosed tumors
The FISH analyses were successful in all 65 tumors examined.
ALT/WDLSs and benign lipomatous tumors
Twenty-four of the 25 surgically resected ALT/WDLSs received a consensus diagnosis. The consultants did not agree upon the diagnosis of the remaining tumor because of controversy over the features of WDLS compared to MXLS. Among the ALT/WDLSs, we observed a wide range of cellular morphologies, from unequivocal lipoblasts to slightly atypical lipocytes (Figure 2A-C). Without the use of adjunct FISH analysis, we found it difficult to distinguish between a benign lipoma and an ALT/WDLS in tumors that had cells with slightly atypical morphologies. Two such tumors, which contained a few hyperchromatic lipocytes, were recurrences of tumors that had been resected 8 and 5 years previously and diagnosed as benign lipomas (Figure 2C). Excluding the case that did not receive a consensus diagnosis, the remaining 24 ALT/WDLSs were all positive for MDM2 amplification. In a single nucleus, the ratio of the number of MDM2 signals to the number of signals for centromere 12 varied from 2 to 5:1 to more than 20:1 (Figure 2D-F). Occasionally, we observed large clusters of MDM2 signals (Figure 2D). Twelve tumors were classified as cases of high-level amplification and 12 as cases of low-level amplification. Generally, the levels of MDM2 amplification were higher in tumors with a greater degree of nuclear atypism. Sixteen of the 25 tumors were positive for both MDM2 and CDK4 amplification (Figure 2G). Clinicopathologically, no other distinction was found between the 2 groups of tumors (data not shown). All 25 benign lipomatous tumors were negative for MDM2 amplification. No nucleus had more than 2 signals for centromere 12; therefore, all tumors were considered to be disomic for chromosome 12, and were probably euosmic. Occasionally, 2 nuclei of a lipomatous tumor were closely apposed and appeared as a single nucleus, as shown in Figure 2G. If we were aware of the overlap of the 2 nuclei, there were no cases in which we had difficulty distinguishing between chromosome 12 disomy and a case of low-level MDM2 amplification. Six of 25 ALT/WDLSs contained cells that were immunopositive for MDM2. Three tumors had a maximum score according to our tiered system and were classified as positive tumors with a high level of overexpression. The strength of the immunostaining varied both among the cells of a single tumor sample and among the different tumor samples. The percentage of cells that were immunopositive for MDM2 within one tumor also showed variation among the samples (Figure 2H, 2I). The remaining 19 WDLSs and the 25 benign lipomatous tumors did not display unequivocal nuclear staining and were classified as negative tumors (Figure 2J). All 19 ALT/WDLS biopsy specimens were positive for MDM2 amplification. We wished to determine if using FISH to detect MDM2 amplification is a diagnostically valid method to differentiate between ALT/WDLSs and benign lipomatous tumors. To do this, we compared two different diagnoses for each of the 19 ALT/WDLSs that had a preoperative biopsy. One diagnosis was made by a committee of 3 general pathologists (procedure and personnel are described in Methods), who classified the tumor as either ‘malignant’ or ‘benign’ after review of the biopsy specimen. After the tumor was resected and the results of the FISH analysis were known, the second diagnosis was made by two specialists. The comparison revealed that using FISH to detect MDM2 amplification is statistically a more valuable diagnostic aid for liposarcomas (Table 2). We next determined if using IHC to detect MDM2 overexpression was a helpful adjunct in the differential diagnosis of ALT/WDLSs from benign lipomatous tumors. This was accomplished by comparing the results of IHC analysis of the 25 ALT/WDLSs to those of the 25 lipomatous tumors as shown in Table 3. The results of McNemar’s chi-square test indicate that predictions of the appropriate diagnosis that were based on the results of IHC analysis of MDM2 overexpression were significantly incorrect.
Figure 2.
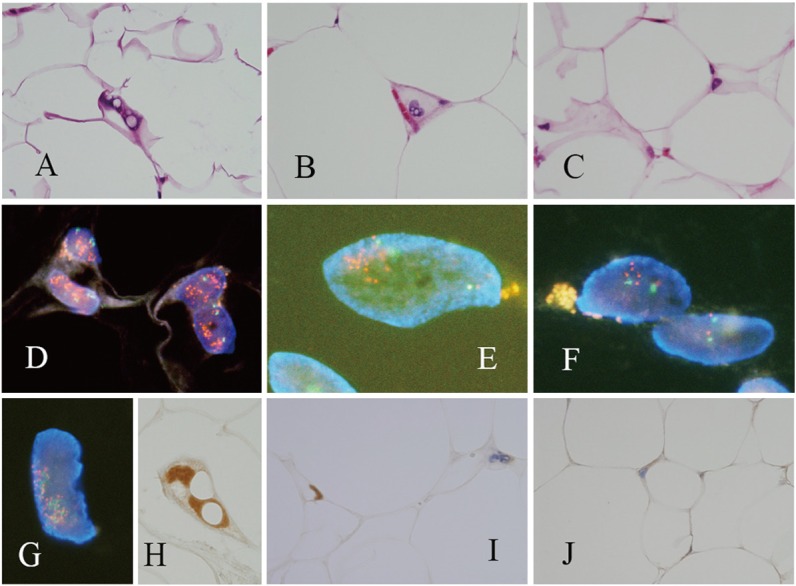
An ALT/WDLS with typical lipoblasts (case 1, A) and atypical lipocytes (case 2, B), and one tumor that cannot be distinguished from a benign lipoma without the use of FISH (case 3, C). FISH with a probe directed against MDM2 demonstrated that MDM2 is amplified (D-F: orange fluorescence, MDM2; green fluorescence, centromere 12). Dual color FISH with probes directed against MDM2 (orange fluorescence) and CDK4 (green fluorescence) demonstrated that both genes are amplified (G: the 2 nuclei overlap). Analysis of MDM2 expression by IHC revealed unequivocal nuclear staining (H), heterogenous staining (I: positive and negative nuclei), and negative staining (J). (Case 1: A, D, G, and H; Case 2: B, E, and I; Case 3: C, F, and J).
Table 2.
Diagnostic status and use of FISH as an adjunct diagnostic aid in study biopsies
| FISH analysis of MDM2 amplification | ||
|---|---|---|
|
|
||
| Diagnoses (by a consensus of two examiners) | Not used | Used |
| Malignant | 36 | 57 |
| Benign | 21 | 0 |
Fischer exact probability test P ≤ .001.
Table 3.
IHC analysis of MDM2 overexpression in cases of ALT/WDLS and benign lipomatous tumors
| Negative | Positive | |
|---|---|---|
| ALT/WDLS | 19 | 6 |
| Benign lipomatous tumors | 25 | 0 |
McNemar’s chi-square test: P = .001.
Archived and newly diagnosed cases of DDLS and other sarcomas
In the 2 cohorts of DDLSs, all 5 overexpressed MDM2 and were cases of high-level amplification of MDM2. Four of the 5 cases were typical DDLS. The fifth had been erroneously diagnosed as ‘parosteal osteosarcoma with de-differentiated components’ by biopsy not only because it exhibited the characteristic histology of a spindle cell tumor, with an anastomosing osteoid-like fibrous stroma, (Figure 3A) but also because of the clinical and radiological findings. Furthermore, the findings that the tumor overexpressed MDM2 protein and was positive for MDM2 amplification (Figure 3B, 3C) are consistent with recent reports, which indicate that low-grade osteosarcomas have a high frequency of MDM2 amplification [14]. However, after the tumor was removed an adjacent lipomatous zone was detected that was also positive for MDM2 amplification (Figure 3D); accordingly, the diagnosis was corrected to DDLS. Among the tumors that are morphologically similar to DDLS, all 3 of the pleomorphic lipomas contained nuclei that had between 2 to 10 signals for MDM2. The signals were usually closely apposed to signals for the centromere of chromosome 12, indicating that the pleomorphic lipomas are negative for MDM2 amplification. The results also indicate that giant cell nuclei are polysomic for chromosome 12 (Figure 4A-C). In an exceptional case, one myxofibrosarcoma was positive for both MDM2 amplification and MDM2 protein overexpression. Because MDM2 overexpression and amplification were detected in the biopsy specimen of this tumor (Figure 5), our preoperative diagnosis was DDLS. However, upon closer examination of the resected tumor, no lipomatous component was found; therefore, the consensus-verified diagnosis was myxofibrosarcoma.
Figure 3.
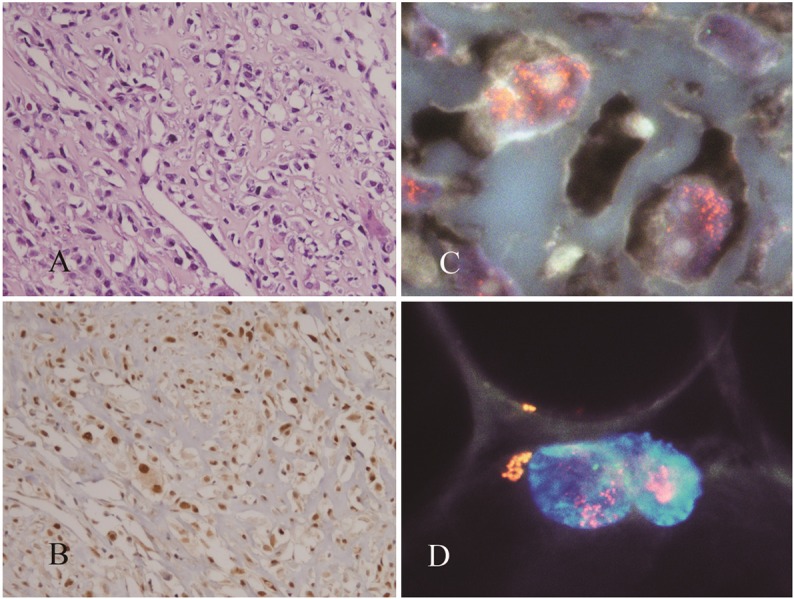
A DDLS with a histology that resembles osteosarcoma (A) contained nuclei with amplified MDM2 (B) MDM2 protein was also overexpressed (C). Nuclei of the adjacent lipomatous zone had amplified MDM2 (D).
Figure 4.
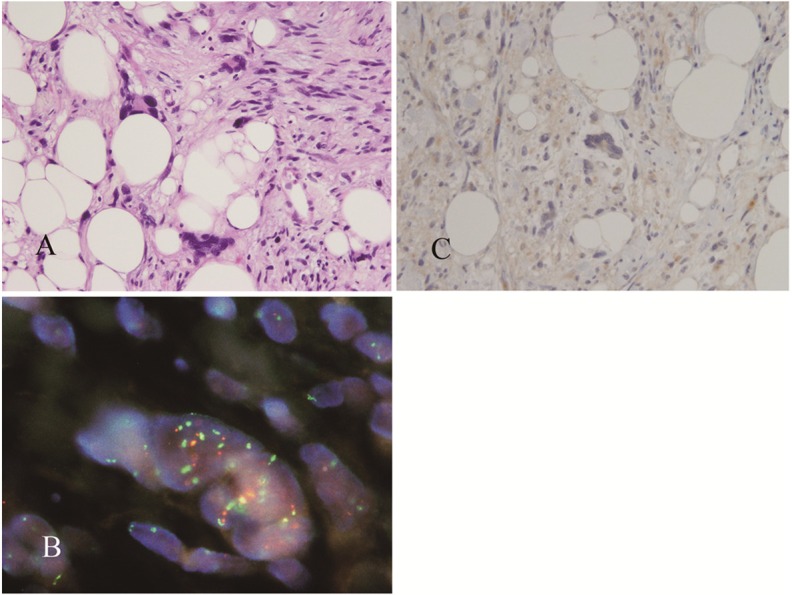
Pleomorphic lipoma showing marked nuclear atypia (A). FISH analysis with a probe directed against MDM2 (orange fluorescence) and centromere 12 (green fluorescence) detected nuclei with numerous MDM2 signals that were closely associated with centromere 12 signals (B). IHC analysis did not detect nuclear overexpression of MDM2 (C).
Figure 5.
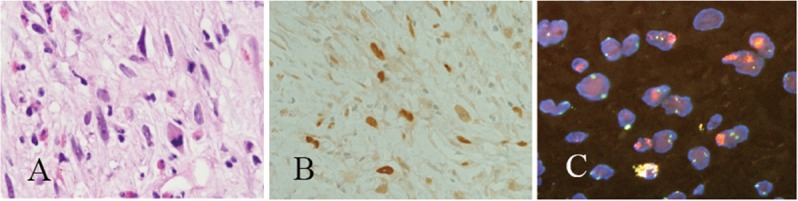
A biopsy obtained from a myxofibrosarcoma (A) contained nuclei that overexpressed MDM2 protein (B) and had amplified MDM2 (C).
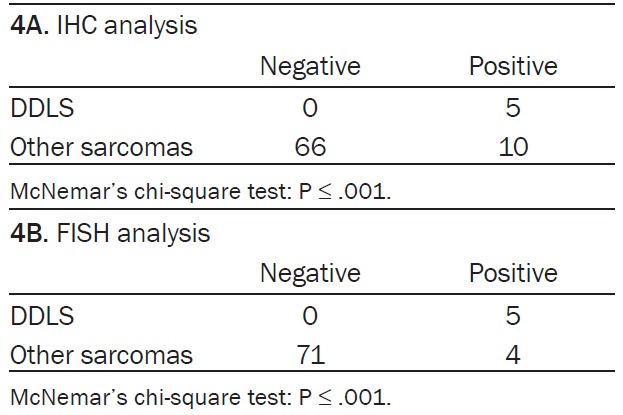
In distinguishing a DDLS from spindle and pleomorphic sarcomas, the sensitivity and specificity of MDM2 amplification were 100% and 95%, respectively, while MDM2 overexpression exhibited a sensitivity and specificity of 100% and 87%, respectively (Table 4).
Table 4.
IHC (A) and FISH (B) analysis of MDM2 amplification in cases of DDLS and other sarcomas
|
Discussion
Differentiating an ALT/WDLS from a benign lipomatous tumor by using histology alone was sometimes very difficult. This is reflected in the fact that 2 recurring ALT/WDLSs had eluded the scrutiny of our study group. In order to overcome this difficulty, we introduced IHC-mediated detection of MDM2 overexpression. Most surgical pathologists are familiar with IHC, which is a routine technique available in almost all pathology laboratories. Additionally, it is often the first ancillary method performed during diagnostic pathology to differentiate between tumors with similar morphologies. An early study reported that the sensitivity and the specificity of using IHC-mediated detection of MDM2 overexpression to distinguish between an ALT/WDLS and a benign adipocytic tumor were 100% and 96%, respectively [15]. A recent study also assessed the sensitivity at 100%; however, this study reported a much lower specificity of 59% [16]. In a study of needle biopsies that combined IHC and FISH analyses, Weaver et al [17] showed that diagnoses with adjunct IHC analysis to measure MDM2 expression had a false positive rate of 11%, compared to 0% for diagnoses with adjunct FISH analysis to measure MDM2 amplification. Thus, FISH had both a sensitivity and a specificity of 100%, while IHC had a sensitivity of 65% and a specificity of 89%. In a study that used both IHC and FISH, with or without quantitative polymerase chain reaction (Q-PCR), Sirvent et al reported that the concordance of MDM2 amplification and overexpression of MDM2 was only 48% (14 of 29) of cases of ALT/WDLS but 100% (8 of 8) of cases of DDLS [12]. In the present study, unequivocal nuclear overexpression of MDM2 was found in only 20% (10 of 49) of cases of ALT/WDLS. The 10 cases were classified as high-level amplifications and were morphologically unmistakable ALT/WDLSs, containing plain lipoblasts, atypical lipocytes, or both. Reflecting this fact, statistical analysis proved that predictions of the appropriate diagnosis that were based on the results of IHC analysis of MDM2 overexpression were significantly incorrect.
The results of our FISH analyses were interpretable in all of the newly diagnosed tumors and most of the archived tumors. MDM2 was amplified in almost all (48 of 49) of the ALT/WDLS tumors but in none of the benign lipomatous tumors. FISH analysis could detect an increased copy number of MDM2 on a nucleus-by-nucleus basis. We occasionally found large clusters of MDM2 signals, which may be copies of MDM2 in homogeneously staining regions [18].
In this study, gene amplification was defined as a MDM2:CEP12 signal ratio that was > 2 in at least 10 nuclei per sample. However, if the criterion for amplification had been 2 or 3 positive nuclei, instead of 10, the results would not have changed. Thus, FISH can even be used in small biopsy specimens. In addition to this objectivity and simplicity, FISH-mediated detection of MDM2 amplification was statistically proven to be the most valuable diagnostic aid for cases of ALT/WDLS.
Due to their marked nuclear polymorphism, some deep-seated spindle cell and pleomorphic lipomas are occasionally difficult to distinguish from liposarcomas with atypical lipoblastic cells [19]. However, as presented in our study and in previous reports, spindle cell lipomas and pleomorphic lipomas have no authentic gene amplification, and even display an increased copy number of MDM2 due to polysomy of chromosome 12 [20].
In contrast to the ALT/WDLSs, all 5 DDLSs showed protein overexpression and gene amplification of MDM2. This close association has also been reported in other studies [12], and may be explained as follows: the high-level amplification of MDM2 usually found in DDLS leads to higher overexpression of the protein, or more likely, posttranscriptional and posttranslational control of protein expression is functional in most cases of ALT/WDLS but not in cases of DDLS.
In distinguishing a DDLS from spindle and pleomorphic sarcomas, the sensitivity of both IHC-mediated detection of MDM2 and FISH-mediated detection of MDM2 was 100%. Although the specificity of IHC (87%) was lower than that of FISH (95%), both techniques can be useful diagnostic aids, because MDM2 amplification and overexpression occur frequently in low-grade osteosarcomas [14] but rarely in other sarcomas such as classical osteosarcomas [21,22], malignant fibrous histiocytomas (MFH), MPNSTs, and myxofibrosarcomas [6,8,23]. Considering that spindle and pleomorphic cell sarcomas rarely have amplified MDM2, which can be targeted by a molecular therapy (see below), it may be too time consuming and laborious to analyze these tumors by using FISH. Therefore, IHC-mediated detection of MDM2 overexpression may be useful for triage or sampling of specimens prior to FISH analysis, especially when the tumors are large and have a heterogeneous histology.
In addition to being a diagnostic utility, detecting amplification of MDM2 and CDK4 impacts cancer treatments that use selective inhibitors of MDM2 and CDK4 proteins [24,25]. In particular, the use of factors that interfere with MDM2-p53 interactions and reactivate p53 is an attractive therapeutic modality for treating cancers that h ave amplified MDM2 and wild-type p53. In fact, in an in vivo study, treatment with Nutlin, an MDM2 antagonist, induced p53-dependent transcription and apoptosis in liposarcoma cells that was positive for MDM2 amplification [26].
In conclusion, our results indicate that using FISH to assess MDM2 amplification is the most useful adjunct in the diagnostic approach for ALT/WDLS versus benign lipomatous tumors, and this role cannot be substituted for by the use of IHC.
Acknowledgement
This work was financially supported by the Japanese Ministry of Education, Sports, Science and Culture [Nos C22590310 (AO) and C23590409 (YD)] and the Smoking Research Foundation (YD).
Disclosure of conflict of interest
We have no personal or financial conflicts of interest.
References
- 1.Nascimento AG. Dedifferentiated liposarcoma. Semin Diagn Pathol. 2001 Nov;18:263–6. [PubMed] [Google Scholar]
- 2.Tanas MR, Goldblum JR. Fluorescence in situ hybridization in the diagnosis of soft tissue neoplasms: a review. Adv Anat Pathol. 2009 Nov;16:383–91. doi: 10.1097/PAP.0b013e3181bb6b86. [DOI] [PubMed] [Google Scholar]
- 3.Fakharzadeh SS, Trusko SP, George DL. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. Embo J. 1991 Jun;10:1565–9. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahilly-Snyder L, Yang-Feng T, Francke U, George DL. Molecular analysis and chromosomal mapping of amplified genes isolated from a transformed mouse 3T3 cell line. Somat Cell Mol Genet. 1987 May;13:235–44. doi: 10.1007/BF01535205. [DOI] [PubMed] [Google Scholar]
- 5.Brown VD, Phillips RA, Gallie BL. Cumulative effect of phosphorylation of pRB on regulation of E2F activity. Mol Cell Biol. 1999 May;19:3246–56. doi: 10.1128/mcb.19.5.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura Y, Keira Y, Ogino J, Nakanishi K, Noguchi H, Inoue T, Hasegawa T. Detection of specific genetic abnormalities by fluorescence in situ hybridization in soft tissue tumors. Pathol Int. 2012 Jan;62:16–27. doi: 10.1111/j.1440-1827.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 7.Downs-Kelly E, Goldblum JR, Patel RM, Weiss SW, Folpe AL, Mertens F, Hartke M, Tubbs RR, Skacel M. The utility of fluorescence in situ hybridization (FISH) in the diagnosis of myxoid soft tissue neoplasms. Am J Surg Pathol. 2008 Jan;32:8–13. doi: 10.1097/PAS.0b013e3181578d5a. [DOI] [PubMed] [Google Scholar]
- 8.Weaver J, Downs-Kelly E, Goldblum JR, Turner S, Kulkarni S, Tubbs RR, Rubin BP, Skacel M. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol. 2008 Aug;21:943–9. doi: 10.1038/modpathol.2008.84. [DOI] [PubMed] [Google Scholar]
- 9.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–41. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binh MB, Garau XS, Guillou L, Aurias A, Coindre JM. Reproducibility of MDM2 and CDK4 staining in soft tissue tumors. Am J Clin Pathol. 2006 May;125:693–7. doi: 10.1309/VMBP-67QU-NN6Q-3J0E. [DOI] [PubMed] [Google Scholar]
- 11.Pilotti S, Della Torre G, Mezzelani A, Tamborini E, Azzarelli A, Sozzi G, Pierotti MA. The expression of MDM2/CDK4 gene product in the differential diagnosis of well differentiated liposarcoma and large deep-seated lipoma. Br J Cancer. 2000 Apr;82:1271–5. doi: 10.1054/bjoc.1999.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sirvent N, Coindre JM, Maire G, Hostein I, Keslair F, Guillou L, Ranchere-Vince D, Terrier P, Pedeutour F. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol. 2007 Oct;31:1476–89. doi: 10.1097/PAS.0b013e3180581fff. [DOI] [PubMed] [Google Scholar]
- 13.Ooi A, Inokuchi M, Harada S, Inazawa J, Tajiri R, Kitamura SS, Ikeda H, Kawashima H, Dobashi Y. Gene amplification of ESR1 in breast cancers--fact or fiction? A fluorescence in situ hybridization and multiplex ligation-dependent probe amplification study. J Pathol. 2012 May;227:8–16. doi: 10.1002/path.3974. [DOI] [PubMed] [Google Scholar]
- 14.Dujardin F, Binh MB, Bouvier C, Gomez-Brouchet A, Larousserie F, Muret Ad, Louis-Brennetot C, Aurias A, Coindre JM, Guillou L, Pedeutour F, Duval H, Collin C, de Pinieux G. MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone. Mod Pathol. 2011 May;24:624–37. doi: 10.1038/modpathol.2010.229. [DOI] [PubMed] [Google Scholar]
- 15.Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R, Aurias A, Hostein I, Coindre JM. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005 Oct;29:1340–7. doi: 10.1097/01.pas.0000170343.09562.39. [DOI] [PubMed] [Google Scholar]
- 16.Aleixo PB, Hartmann AA, Menezes IC, Meurer RT, Oliveira AM. Can MDM2 and CDK4 make the diagnosis of well differentiated/dedifferentiated liposarcoma? An immunohistochemical study on 129 soft tissue tumours. J Clin Pathol. 2009 Dec;62:1127–35. doi: 10.1136/jcp.2009.070201. [DOI] [PubMed] [Google Scholar]
- 17.Weaver J, Rao P, Goldblum JR, Joyce MJ, Turner SL, Lazar AJ, López-Terada D, Tubbs RR, Rubin BP. Can MDM2 analytical tests performed on core needle biopsy be relied upon to diagnose well-differentiated liposarcoma? Mod Pathol. 2010 Oct;23:1301–6. doi: 10.1038/modpathol.2010.106. [DOI] [PubMed] [Google Scholar]
- 18.Shimada S, Ishizawa T, Ishizawa K, Matsumura T, Hasegawa T, Hirose T. The value of MDM2 and CDK4 amplification levels using real-time polymerase chain reaction for the differential diagnosis of liposarcomas and their histologic mimickers. Hum Pathol. 2006 Sep;37:1123–9. doi: 10.1016/j.humpath.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Dumollard JM, Ranchere-Vince D, Burel F, Coindre JM, Tallini G, Ligon AH, Mayaud R, Turc-Carel C, Martin C, Mosnier JF, Pedeutour F. [Spindle cell lipoma and 13q deletion: diagnostic utility of cytogenetic analysis] . Ann Pathol. 2001 Aug;21:303–10. [PubMed] [Google Scholar]
- 20.Weaver J, Goldblum JR, Turner S, Tubbs RR, Wang WL, Lazar AJ, Rubin BP. Detection of MDM2 gene amplification or protein expression distinguishes sclerosing mesenteritis and retroperitoneal fibrosis from inflammatory well-differentiated liposarcoma. Mod Pathol. 2009 Jan;22:66–70. doi: 10.1038/modpathol.2008.153. [DOI] [PubMed] [Google Scholar]
- 21.Gisselsson D, Palsson E, Hoglund M, Domanski H, Mertens F, Pandis N, Sciot R, Dal Cin P, Bridge JA, Mandahl N. Differentially amplified chromosome 12 sequences in low- and high-grade osteosarcoma. Genes Chromosomes Cancer. 2002 Feb;33:133–40. doi: 10.1002/gcc.1219. [DOI] [PubMed] [Google Scholar]
- 22.Mejia-Guerrero S, Quejada M, Gokgoz N, Gill M, Parkes RK, Wunder JS, Andrulis IL. Characterization of the 12q15 MDM2 and 12q13-14 CDK4 amplicons and clinical correlations in osteosarcoma. Genes Chromosomes Cancer. 2010 Jun;49:518–25. doi: 10.1002/gcc.20761. [DOI] [PubMed] [Google Scholar]
- 23.Coindre JM, Hostein I, Maire G, Derré J, Guillou L, Leroux A, Ghnassia JP, Collin F, Pedeutour F, Aurias A. Inflammatory malignant fibrous histiocytomas and dedifferentiated liposarcomas: histological review, genomic profile, and MDM2 and CDK4 status favour a single entity. J Pathol. 2004 Jul;203:822–30. doi: 10.1002/path.1579. [DOI] [PubMed] [Google Scholar]
- 24.McInnes C, Wang S, Anderson S, O’Boyle J, Jackson W, Kontopidis G, Meades C, Mezna M, Thomas M, Wood G, Lane DP, Fischer PM. Structural determinants of CDK4 inhibition and design of selective ATP competitive inhibitors. Chem Biol. 2004 Apr;11:525–34. doi: 10.1016/j.chembiol.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004 Feb 6;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 26.Muller CR, Paulsen EB, Noordhuis P, Pedeutour F, Saeter G, Myklebost O. Potential for treatment of liposarcomas with the MDM2 antagonist Nutlin-3A. Int J Cancer. 2007 Jul 1;121:199–205. doi: 10.1002/ijc.22643. [DOI] [PubMed] [Google Scholar]


