Abstract
Objective: To detect vascular endothelial growth factor (VEGF) expression and micro-vessel density (MVD) in patients with severe intrauterine adhesion before and after therapy, and to preliminarily explore the role of angiogenesis in the therapy of severe intrauterine adhesion. Methods: A total of 36 patients with severe intrauterine adhesion were prospectively recruited into the treatment group. In the control group, 20 patients with normal uterine were recruited. Patients with severe intrauterine adhesion received transcervical resection of adhesions under hysteroscope and then received artificial hormone therapy for 3 months. Methods: The changes in the organelles of endometrial cells were evaluated under an electric microscope; Immunohistochemistry was done to detect the VEGF expression and MVD in patients with severe intrauterine adhesion, which was compared with that in the control group; VEGF expression and MVD were compared among patients with different prognoses. Results: Electric microscopy showed vascular closure and hypoxic changes in the endometrial tissues of patients with intrauterine adhesion. After treatment, angiogenesis was observed, and the hypoxic changes in the endometrial glands and interstitium were also improved. Moreover, the VEGF expression and score of MVD also increased significantly when compared with those before treatment and in the control group. The VEGF expression and MVD score in intrauterine adhesion patients recovering from treatment were significantly higher than those in patients non-responding to treatment. Conclusion: In patients with intrauterine adhesion, the endometrial tissues present with vascular closure, and angiogenesis will be present in the endometrial tissues after treatment. The angiogenesis in the endometrial tissues may affect the endometrial repair.
Keywords: Intrauterine adhesion, angiogenesis, vascular endothelial growth factor, micro-vessel density
Introduction
Intrauterine adhesion (IUA) is also known as Asherman syndrome and was first described in 1894 by Fritsch [1]. In 1894, Asherman first defined IUA as menstrual abnormalities, infertility and recurrent miscarriage due to partial or complete uterine occlusion secondary to endometrial injury. In recent years, the incidence of IUA is increasing [3], and the etiology of IUA has been thought to be related to trauma, pregnancy, infection, and others. It was reported that 90% of IUA was secondary to the damage to the uterine due to the intrauterine surgery [3]. The manifestations of IUA include decrease in menstrual flow, infertility, recurrent miscarriage, placenta accreta, amenorrhea, etc, which significantly influence the reproductive health. Patients with IUA have high risk for complications when they receive intrauterine surgery, and the recurrence of IUA is at a high level after intrauterine surgery, especially for patients with severe IUA. Recently, transcervical resection of adhesions (TCRA) has been a standardized method for the treatment of IUA with the development of hysteroscopic technique [4]. However, the post-operative endometrial repair and prevention of recurrent IUA are two challenges in the clinical practice. Currently, placement of intrauterine device (IUD) and application of artificial hormone therapy with high dose estrogen are used to prevent the post-operative recurrence of IUA and promote the endometrial repair [5,6]. Of note, the estrogen therapy alone has poor efficacy and is usually accompanied by some adverse effects, which significantly limits its wide application. In the present study, on the basis of changes in the organelles of endometrial cells and the structure of microvessels in the endometrial interstitium, we further detect the vascular endothelial growth factor (VEGF) and microvessel density (MVD) were detected in endometrium of patients with severe IUA, aiming to explore the role of angiogenesis in the endometrial repair. Our findings may provide evidence for the clinical treatment of IUA.
Materials and methods
Subjects
IUA patients
patients who visited our hospital due to amenorrhea, decrease in menstrual flow, infertility and repeated miscarriage were recruited from June 2011 to June 2012. They were diagnosed as severe IUA by hysteroscopy, and following conditions were excluded: 1) a history of irregular menstrual cycle, abnormal sex hormone level, and amenorrhea, decrease in menstrual flow, infertility of other causes; 2) other pathological conditions causing abnormal vaginal bleeding, such as endometrial polyps, submucosal uterine fibroids, ovarian tumors, etc; 3) other contradictions for estrogen and progesterone therapy, such as cardiovascular diseases, liver diseases and thrombotic diseases. A total of 36 patients were recruited with a mean age of 25.4±2.7 years (range: 21-36 years). The number of intrauterine surgery was 1-6 times (mean: 3±2.3 times). The mean time from the last intrauterine surgery to symptoms was 2.7±1.3 years (range: 3 months-4 years).
Normal controls
Patients who received artificial insemination or hysteroscopy due to male infertility or tubal factor infertility were recruited from our department in the same period and severed as controls. They met following criteria: 1) The baseline endocrine was normal; 2) the menstrual cycle was normal; 3) They did not receive steroid therapy or intrauterine operation in the past 3 months; 4) They had no submucosal uterine fibroids, ovarian tumors and other diseases. A total of 20 controls were recruited with a mean age of 25.6±3.2 years (range: 22-36 years). Hysteroscopy was done in these subjected to exclude intrauterine lesions.
Diagnostic criteria for IUA
IUA was diagnosed according to the criteria for IUA developed by the European Society for Gynecological Endoscopy (ESGE) [7].
Surgical procedures
Surgery was performed in the early follicular phase and under the general anesthesia. STORZ hysteroscope (GERMANY) was used, and 5% mannitol served as perfusion solution. The uterine direction and depth were explored, and the cervix was expanded to 10 mm in diameter. The needle like electrode was used to separate the adherent lesions with the inflation pressure of 100-120 mmHg and flow rate of 100-150 ml/min. The separation was done until the outlets of bilateral oviduct were opened and the size and shape of uterine returned to normal. During the surgery, endometrial tissues were collected for electric microscopy and immunohistochemistry. Postoperatively, an IUD was placed in the uterine.
Post-operative artificial menstrual cycle therapy and follow up
Estradiol valerate was administered at (3 mg, three times daily) to patients in the IUA group for 21 days. In the later 10 days, medroxyprogesterone acetate (10 mg/d) was administered for artificial menstrual cycle therapy (a total of 3 cycles). At 1 and 3 months after surgery, hysteroscopy was done to observe the recurrent adhesion.
Sample collection and processing
Informed consent was obtained from each patient. During the surgery and at 3 months after surgery, the endometrial tissues were collected from the posterior wall of the uterine. A fraction of tissues (1-1.5 cm) were fixed in 10% formaldehyde and embedded in paraffin for immunohistochemistry. The remaining tissues (0.5-1.0 mm) were fixed and stored at 4°C for transmission electron microscopy. For the controls, sample collection was done during the hysteroscopy. Sample collection was done in the early follicular phase aiming to avoid the influence of estrogen and progesterone.
Transmission electron microscopy
After fixation, dehydration, transparentization, embedding and polymerization, ultrathin sections were obtained for transmission electron microscopy (JEM2100SX, Japan).
Immunohistochemistry
Mouse anti-human polyclonal CD34 antibody (QBEnd/IO; R&D, USA; 1:100) and rabbit anti-human VEGF monoclonal antibody (SP28; 1:100; R&D, USA) were used to detect the MVD and VEGF. Cells positive for CD34 had brown granules in the membrane and cytoplasm. The MVD was determined according to the previously described [3]: at a magnification of 100×, region of interest was identified, and then the blood vessels were counted at a higher magnification (400×). Two pathologists determined the MVD of same sections, and a mean was obtained as the MVD. VEGF expression was semi-quantitatively determined according to previously described [8]: the staining intensity was determined according to the proportion of positive cells and staining and classified into 5 grades (0-4): 0%, 25%, 50%, 75% and 100%. The score in immunohistochemistry was calculated as follow: H-scor=Σpi (i+1), where i is staining intensity, and p is percentage of positive cells to total cells counted. Two clinicians who were blind to the study detected the VEGF expression and a mean was obtained.
Evaluation of clinical prognosis
Cure: the menstrual flow returned to normal or clinical symptoms improved, hysteroscopy at 1-3 months after surgery showed normal shape of the uterine, normal outlets of bilateral oviduct, and normal uterine horns. The endometrium was significantly thickened when compared with that before surgery; 2) Improvement: the menstrual flow increased when compared with that before surgery, but was lower than the normal menstrual flow; the uterine was larger than that before surgery, but adhesion was partially observed; The endometrium was significantly thickened when compared with that before surgery; 3) Failure: the menstrual flow and clinical symptoms remained unchanged and massive IUA was present; the uterine presented with cylinder-like adhesion.
Statistical analysis
Statistical analysis was done with SPSS version 15.0 for Windows. Quantitative data were compared with analysis of variance and t test. A value of P<0.05 was considered statistically significant.
Results
Microstructure of endometrial cells of IUA patients
Transmission electric microscopy showed the swelling of glandular epithelial cells in the endometrium at a low magnification and the loose cytoplasmic matrix, reduction in electron density, expansion of endoplasmic reticulum, loss of ribosome, swelling of mitochondria, shortening and reduction in mitochondrial cristae and vacuolized mitochondria at a high magnification (Figure 1A). In the endometrial interstitium, the matrix was loose, a large number of fibroblasts aggregated, cells were rich in organelle, the number of rough endoplasmic reticulum increased, and the mitochondria slightly expanded. In the matrix, a large amount of collagen was diffuse or aggregated, the capillaries were closed and presented with stenosis, no blood cells were found in the capillaries, the tight junction between endothelial cells was evident, the cytoplasmic matrix was loose in the epithelial cells, the endoplasmic reticulum and mitochondria expanded, and vacuolar changes were noted in the endoplasmic reticulum and mitochondria. In addition, a large amount of collagens aggregated and surrounded the blood vessels (Figure 1B, 1C).
Figure 1.
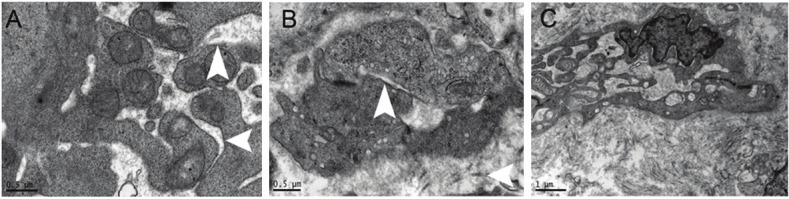
A: Evident expansion of endoplasmic reticulum, loss of ribosome, swelling of mitochondria, shortening and reduction in mitochondrial cristae, and vacuolized mitochondria; B: In the matrix, the capillaries were closed and presented with stenosis, no blood cells were found in the capillaries, the tight junction between endothelial cells was evident, and a large amount of collagens surrounded the blood vessels; C: The endoplasmic reticulum in the fibroblasts expanded and a large amount of collages surrounded these fibroblasts.
After TCRA and artificial menstrual cycle therapy, there were following changes in the ultrastructure of endometrial cells: at 3 months after surgery, the swelling of glandular epithelial cells was improved; at a high magnification, the cytoplasmic matrix of glandular epithelial cells was nearly normal, the morphology of endoplasmic reticulum and mitochondria was nearly normal and had no swelling (Figure 2A). The interstitium was still loose, but the number of fibroblasts reduced in the interstitium, elastic fibers were diffused and the collagens reduced; in the interstitium, newly generated capillaries were found, the tight junction between endothelial cells was evident, the nucleus of endothelial cells was large, these cells were rich in euchromatin, these capillaries presented with slight stenosis and the basement membrane was no complete (Figure 2B).
Figure 2.
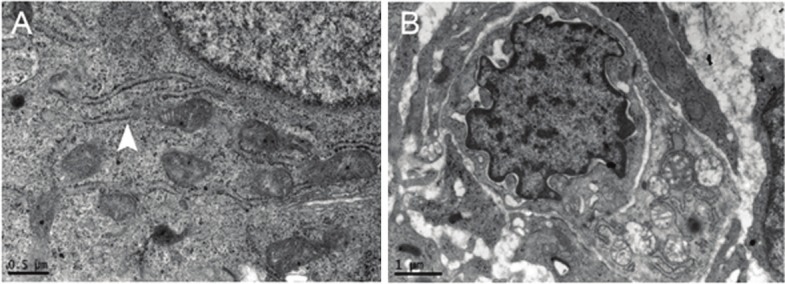
A: the morphology of endoplasmic reticulum and mitochondria was nearly normal in the glandular epithelial cells. B: There were newly generated capillaries, the tight junction between endothelial cells was evident, the nucleus of endothelial cells was large, and these cells were rich in euchromatin.
Immunohistochemistry for VEGF and MVD
In the normal endometrium and the endometrium of IUA patients with and without treatment, VEGF expression was present, mainly in the cytoplasm of glandular epithelial cells, but VEGF had a low expression in the interstitium (Figure 3A, 3B).
Figure 3.
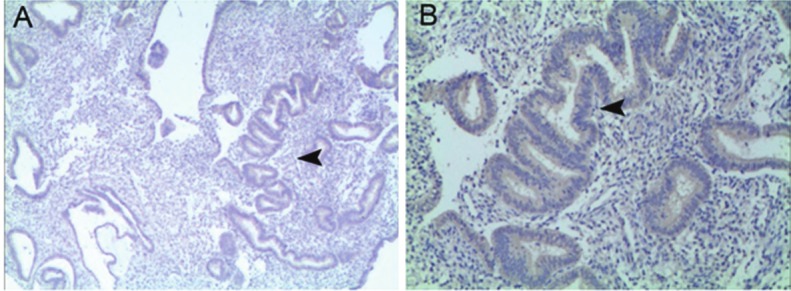
A: VEGF expression in the endometrium of IUA patients (IHC; ×40, scare bar: 100 μm); B: VEGF expression in the endometrium of IUA patients (IHC; ×100, scare bar: 30 μm).
Analysis showed the VEGF expression had a normal distribution, and then analysis of variance was performed to compare the VEGF expression among three groups. Results showed there was marked difference in the VEGF expression among three groups (Table 1).
Table 1.
VEGF expression in the endometrium of patients in three groups (X̅±S)
| Group | n | VEGF expression score | F | P |
|---|---|---|---|---|
| Normal control | 20 | 3.19±0.58 | 3.621 | <0.01 |
| IUA patients before surgery | 36 | 3.49±0.63 | ||
| IUA patients after surgery | 36 | 5.37±0.87 |
Detection of MVD by immunohistochemistry
CD34 expression was found in the endometrium of normal endometrium and the endometrium of IUA patients before and after treatment. The CD34 expression was mainly noted on the endothelial cells (Figure 4A, 4B).
Figure 4.
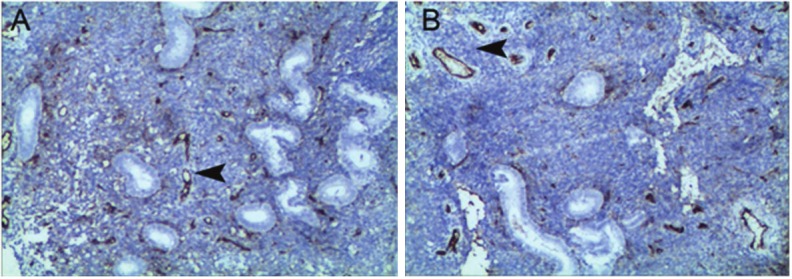
A: VEGF expression in the normal endometrium (IHC; ×40, scare bar: 100 μm); B: VEGF expression in the normal endometrium (IHC; ×100, scare bar: 30 μm).
At a low magnificent, the region of interest was identified and then observed at a high magnification. The blood vessels in 10 randomly selected fields were counted. The number of blood vessels presented with normal distribution, and then analysis of variance was performed to compare the number of blood vessels (MVD) in the endometrium among three groups. Results revealed that there was significant difference in the MVD of the endometrium among three groups (Table 2).
Table 2.
MVD of the endoemtrium in three groups (X̅±S)
| Group | n | MVD score | F | P |
|---|---|---|---|---|
| Normal control | 20 | 23.83±1.43 | 291.28 | <0.01 |
| IUA patients before surgery | 36 | 25.64±1.84 | ||
| IUA patients after surgery | 36 | 37.19±2.96 |
Correlation of prognosis with VEGF expression and MVD in IUA patients receiving surgery
Among 36 patients receiving surgery, IUA was cured in 16 patients with the cure rate of 44.4% (16/36); improvement was found in 15 patients with the improvement rate of 41.7% (15/36); surgical failure was noted in 5 patients with the failure rate of 13.9% (5/36). Among these patients, 2 had pregnancy after surgery (1 had 8+ weeks of gestational age but the pregnancy was discontinued due to absence of embryo; 1 had 26 weeks of gestational age but received odynopoeia due to fetal multiple malformations demonstrated by ultrasonography, and placenta adhesion and implantation were absent).
Statistical analysis showed the VEGF expression and MVD after surgery remained unchanged when compared with those before surgery. However, the VEGF expression in patients responding to surgery was significantly higher than that in patients non-responding to surgery; the MVD in patients responding to surgery was markedly increased when compared with patients non-responding to surgery (Table 3).
Table 3.
VEGF expression and MVD in IUA patients before and after surgery (X̅±S)
| Group | n | Before surgery | After surgery | ||
|---|---|---|---|---|---|
|
| |||||
| VEGF | MVD | VEGF | MVD | ||
| responder | 16 | 3.55±0.48 | 25.71±1.24 | 5.89±0.33 | 38.23±2.05 |
| non-responder | 5 | 3.24±0.73 | 22.68±1.93 | 4.11±0.56 | 27.23±2.11 |
| P | 0.012 | 0.021 | 0.006 | 0.014 | |
Discussion
Pathological basis of IUA and endometrial repair
After endometrial injury, the impairment of endometrial repair is a major cause of IUA. It has been reported that more than 90% of IUA is caused by intrauterine operation. In patients with severe IUA, the basal layer of endometrium is severely damaged, which makes the regeneration of endometrial cells and glands be inactive and causes a poor endometrial receptivity [10]. In clinical practice, intrauterine surgery or infection may cause damage to the basal layer of endometrium, leading to the impaired regeneration of epithelial cells and injured angiogenesis, which may cause difficulty in endometrial repair [11]. Under this condition, the absence of endometrium in the uterine may cause formation of fibrous scar and adhesion between anterior and posterior walls. In the present study, electric microscopy showed the glandular epithelial cells in the endometrium presented with swelling, and the endoplasmic reticulum in these cells also expanded, the ribosome was lost, the mitochondria were swelling, the mitochondrial cristae shortened and reduced and vacuolization of mitochondria was also found. The interstitium was loose, the capillaries in the interstitium were closed or presented with stenosis, and no blood cells were found in these capillaries. In addition, the tight junction between endothelial cells was obvious, and a large amount of fibroblasts aggregated in the interstitium.
Angiogenesis in endometrial repair in IUA patients
In the normal menstrual cycle, the growth of spiral arteries in the endometrial functional layer is cyclical; the spiral arterioles in the interstitium interstitium develop in the proliferative phase, their wall thickens and their lumen enlarges; in the secretory phase, these spiral arteries increased and curled, the blood vessel network remodels and becomes mature, which changes the vascular resistance and prepares for the embryo implantation [7]. It has been reported that the growth of spiral arteries and subepithelial capillaries occur in the whole functional layer during the proliferative phase [8]. In the present study, transmission electric microscopy showed focal ischemic/hypoxic changes in the endometrium. After TCRA and artificial hormone therapy for 3 months, electric microscopy revealed the angiogenesis in the endometrium. In addition, the morphology of endoplasmic reticulum and mitochondria in the glandular epithelial cells was nearly normal and had no evident swelling; the fibroblasts in the intestinum reduced significantly and a small amount of elastic fibers was diffused in the interstitium and the collagens reduced. These findings suggest that the impaired revascularization and focal ischemia in the endometrium are the main causes of impaired endometrial repair and formation of endometrial scar and IUA; the revascularization and increase in blood supply provide support for the endometrial repair.
MVD is an objective indicator for the evaluation of angiogenesis and has been widely applied in the assessment of the growth of blood vessels. The CD34 is specific and has been regarded to be a most sensitive marker of endothelial cells. In the present study, immunohistochemistry for CD34 was done to detect the microvessels. The microvessels were counted under a microscopy, and the MVD was compared in IUA patients before and after therapy. VEGF is an important pro-angiogenic factor and promote the division of endothelial cells and maintain the vascular permeability. Our findings revealed that the VEGF expression and MVD in the endometrium of IUA patients after therapy were markedly increased when compared with those in IUA patients before therapy and in normal endometrium. These findings suggest that the post-operative therapy may promote the revascularization and angiogenesis in the endometrium. In the follow up, our results showed the VEGF expression and MVD in patients responding to therapy were significantly higher than those in patients non-responding to therapy, which indicates that the revascularization and angiogenesis in the endometrium may influence the endometrial repair.
Relationship between angiogenesis and estrogen, and role of angiogenesis in future treatment of IUA
A variety of studies have shown that VEGF and its receptors are expressed in human endometrium, and their expressions change periodically [12]. In the in vivo experiment, estrogen can significantly stimulate the VEGF expression in the endometrium [13]. In the ovariectomized rats, estradiol (100 ng) treatment for 24 h may significantly increase the proliferation of vascular endothelial cells in the endometrium, accompanied by increase in ratio of vascular density to interstitial cell density [14]. Our findings also revealed that, after TCRA and artificial menstrual cycle therapy for 3 months, the VEGF expression and MVD increased significantly when compared with the IUA patients before therapy and the controls. These findings indicate that estrogen may promote the angiogenesis in the endometrium, which is helpful for the endometrial repair.
In clinical practice, TCRA has been a standard strategy for the treatment of IUA [5]. Postoperatively, estrogen is often used at a high dose aiming to promote the endometrial repair, and the dose of estrogen is increasing in recent years. The estrogen at high dose may increase the risk for thrombosis and malignancies. In addition, the current therapeutic efficacy of IUA is still unsatisfactory, and recurrence of IUA is often found after TCRA. There is evidence showing that the incidence of IUA recurrence is as high as 20-62% after surgery [3]. Thus, the application of estrogen at high dose is now questioned by some clinicians.
In mice with IUA [15], estrogen was administered at different doses. Results showed the mice treated with estrogen at physiological dose had the lowest score of endometrial fibrosis. The uterine is a special environment. Although the pro-adhesion and pro-fibrotic factors (such as TGF-β and bFGF) are at a low level in the presence of estrogen at low dose, this condition is not beneficial for the endometrial repair. When the endometrium is mildly damaged and the majority of endometrium is still intact and has normal function, estrogen at appropriate dose may promote the differentiation of basal layer of the endometrium into functional layer which may cover the injured endometrium and is helpful for the functional and morphological recovery of endometrium. When the basal layer of the endometrium is severely damaged, the basal layer loses the ability to regenerate. Under this condition, a large number of neutrophils, mononuclear macrophages, lymphocytes and fibroblasts aggregate in the injured endometrium to induce acute and chronic inflammation and secrete a variety of inflammatory mediators. The estrogen at high dose under this condition may induce the endometrial fibrosis by interacting with other cytokines including TGF-β. However, estrogen at a low dose may cause the endometrial atrophy due to absence of support by hormone, which may cause the proliferation of fibroblasts and finally result in endometrial fibrosis. Thus, the estrogen at high dose is not beneficial for prevention of IUA recurrence after surgery.
On the basis of above findings, the post-operative application of estrogen is a “double-edged sword” in the prevention of IUA recurrence. Thus, whether we can promote the endometrial repair with other strategies in the presence of estrogen at appropriate dose? Our results showed the VEGF expression and MVD increased dramatically in IUA patients after surgery and artificial menstrual cycle therapy, when compared with IUA patients before therapy and controls. Moreover, the VEGF expression and MVD in patients responding to therapy were significantly higher than those in patients non-responding to therapy. This indicates that post-operative therapy may promote the angiogenesis in the endometrium, which may influence the endometrial repair.
Thus, whether we can take measures to promote the angiogenesis in the endometrium in the presence of estrogen at safe and effective dose to improve the prognosis of IUA patients? More studies are required to confirm it.
References
- 1.Fritsch H. Ein Fall von volligem Schwund der Gebormutterhohle nach Auskratzung. Zentralbl Gynaekol. 1894;18:1337–1342. [Google Scholar]
- 2.Asherman JG. Amenorrhoea Traumatica (Atretica) J Obstet Gynaecol Br Emp. 1948;55:23–30. doi: 10.1111/j.1471-0528.1948.tb07045.x. [DOI] [PubMed] [Google Scholar]
- 3.Dalton VK, Saunders NA, Harris LH, Williams JA, Lebovic DI. Intrauterine adhesions after manual vacuum aspiration for early pregnancy failure. Fertil Steril. 2006;85:1823. doi: 10.1016/j.fertnstert.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Salzani A, Yela DA, Gabiatti JR, Bedone AJ, Monteiro IM. Prevalence of uterine synechia after abortion evacuation curettage. Sao Paulo Med. 2007;125:261–4. doi: 10.1590/S1516-31802007000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romer T, Schmidt T, Foth D. Pre-and post-operative hormonal treatment in patients with hysteroscopic surgery. Contrib Gynecol Obstet. 2000;20:1–12. doi: 10.1159/000060283. [DOI] [PubMed] [Google Scholar]
- 6.Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome-one century later. Fertil Steril. 2008;89:759–79. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 7.Amer MI, Abd-El-Maeboud KH, Abdelfatah I, Salama FA, Abdallah AS. Human amnion as a temoprary biologic barrier after hysteroscopic lysis of severe intrauterine adhesions: pilot study. J Minim Invasive Gynecol. 2010;17:605–11. doi: 10.1016/j.jmig.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Deans R, Abbott J. Review of intrauterine adhesions. Minim Invasive Gynecol. 2010;17:555–569. doi: 10.1016/j.jmig.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Berman JM. Intrauterine adhesions. Semin Reprod Med. 2008;26:349–55. doi: 10.1055/s-0028-1082393. [DOI] [PubMed] [Google Scholar]
- 10.Roy KK, Baruah J, Sharma JB, Kumar S, Kachawa G, Singh N. Reproductive outcome following hysteroscopic adhesiolysis in patients with infertility due to Asherman’s syndrome. Arch Gynecol Obstet. 2010;281:55–361. doi: 10.1007/s00404-009-1117-x. [DOI] [PubMed] [Google Scholar]
- 11.Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman’s syndrome. Fertil Steril. 2008;89:715–722. doi: 10.1016/j.fertnstert.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 12.Nayak NR, Brenner RM. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J Clin Endocrinol Metab. 2002;87:1845–55. doi: 10.1210/jcem.87.4.8413. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. History of discovery: vascular endothelial growth factor. Arterioscler Thromb Vasc Bio. 2009;29:789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 14.Maybin J, Critchley H. Repair and regeneration of the human endometrium. Expert Rev Obstet Gynecol. 2009;4:283–298. [Google Scholar]
- 15.Nishi Y, Takeshita T. Ashennan syndrome. Nippon Rinsho. 2006;(suppl 2):418–421. [PubMed] [Google Scholar]


