Abstract
It has been suggested that ubiquitin-conjugating enzyme E2C (UBE2C, also known as UBCH10) represents a promising cancer biomarker. However, the clinicopathological or prognostic significance as well as the functions of UBE2C in bladder cancer are largely unknown. To investigate the significance of UBE2C expression in bladder cancer, immunohistochemical analysis was performed using a tissue microarray. UBE2C positivity was observed in 51 of 82 (62%) bladder urothelial carcinoma cases treated with radical cystectomy. In contrast, UBE2C was negative in all of the non-neoplastic urothelium examined. UBE2C positivity was significantly associated with higher tumor stage (p=0.0061) and presence of lymphovascular invasion (p=0.0045). In addition, UBE2C positivity was significantly associated with shorter cancer-specific survival after cystectomy (log rank p=0.0017; multivariate hazard ratio, 2.49; 95% confidence interval, 1.09-5.71). Small interfering RNA-mediated suppression of UBE2C in UM-UC-3 bladder cancer cells inhibited cell proliferation in vitro. Taken together, our results suggest that UBE2C is a novel prognostic biomarker as well as a potential therapeutic target in bladder cancer.
Keywords: Cell cycle, immunohistochemistry, pathology, prognosis, urinary tract
Introduction
Bladder cancer is the second most common cancer in the genitourinary system [1]. Despite considerable progress in its treatment, the prognosis of patients with locally advanced or muscle-invasive bladder cancer remains poor [2]. Although many molecular markers have been studied for their potential use in assessing the prognosis of bladder cancer, few molecular biomarkers have been repeatedly shown to predict outcome, especially for muscle-invasive bladder cancer treated with radical cystectomy [3-7]. Molecular biomarkers that are predictive of outcomes would help clinicians provide individualized prognostications and allow risk-stratified clinical decision making regarding adjuvant therapy [4,8,9].
Ubiquitin-conjugating enzyme E2C (UBE2C, also known as UBCH10), one of the components of the ubiquitin/proteasome pathway, plays a pivotal role in the regulation of mitotic progression [10-13]. UBE2C is required for the destruction of mitotic cyclins, thereby assisting in the regulation of cell cycle progression through the M phase. Overexpression of UBE2C at the mRNA level has been reported in a number of cancer cell lines and primary tumors, whereas only low levels have been found in normal tissues [14]. In addition, it has been shown that overexpression of UBE2C is associated with worse patient outcomes in a number of cancers [15-21]. Consequently, it has been proposed that UBE2C represents a promising cancer biomarker [13]. Nonetheless, little is known about UBE2C expression in bladder cancer. Whereas a recent study has shown that high expression of UBE2C is associated with shorter progression-free survival in superficial bladder cancer patients who were treated with transurethral resection [22], no study has examined the clinicopathological or prognostic significance of UBE2C in bladder cancer treated with radical cystectomy. Furthermore, functions of UBE2C in bladder cancer cells are largely unknown.
We therefore examined the clinicopathological and prognostic associations of UBE2C expression in bladder cancer patients who underwent radical cystectomy. In addition, the effects of UBE2C knockdown in bladder cancer cells were evaluated to determine if UBE2C inhibition may be a potential therapeutic strategy for bladder cancer.
Materials and methods
Study population
Eighty-two consecutive bladder urothelial carcinoma (UC) patients who were treated with radical cystectomy at the University of Tokyo Hospital from 1990 to 2005 were included in this study. All pT0 (no remaining cancer in cystectomy specimen) cases were excluded. All research protocols in the present study were approved by our institutional review board.
Histopathological evaluation
Hematoxylin and eosin-stained sections of all cases were examined by a pathologist (T.M.) unaware of clinical outcome data. Tumor histology and grade were defined by the World Health Organization/International Society of Urologic Pathology consensus classification [23,24]. All the tumors in this study were high-grade tumors. Staging of the tumors was performed according to the TNM classification [23]. Lymphovascular invasion was examined by hematoxylin and eosin staining and Elastica van Gieson staining.
Immunohistochemical analysis
A tissue microarray was constructed as previously described [25]. Briefly, two pieces of 2-mm tissue cores were selected from representative tumor areas and transferred to each tissue microarray block. Preparations of sections, antigen retrieval and immunostaining were carried out essentially as described previously [26]. A mouse monoclonal antibody against UBE2C (clone 9D3; 1:100 dilution; Abnova, Taipei, Taiwan) was applied, and slides were incubated for 16 h at 4°C. Visualization was achieved using EnVisionTM+/HRP, Mouse (Dako, Carpinteria, CA, USA), diaminobenzidine (Dako) and hematoxylin counterstain. Human placenta was used as a positive control. For a negative control, the primary antibody was omitted. Immunoreactivity was evaluated by a pathologist (H.A.) blinded to other data. UBE2C is localized in both the nucleus and the cytoplasm [13]. Staining in either the nucleus or the cytoplasm was scored as absent, weak, moderate, or strong. Absent or weak staining was categorized as negative, while moderate or strong staining was categorized as positive for subsequent analyses.
Cell line and small interfering RNA
A bladder cancer cell line UM-UC-3 was maintained in minimum essential medium (Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% fetal calf serum (MP Biomedicals, Costa Mesa, CA, USA), at 37°C in a 5% CO2 incubator. Transfection of cells with small interfering RNAs (siRNAs) targeting UBE2C was performed using HiPerFect (Qiagen, Düsseldorf, Germany) at a final siRNA concentration of 20 nmol/L, according to the manufacturer’s instructions. The target sequences for siRNAs were as follows: 5’-GAAGTACCTGCAAGAAACCTACTCA-3’ for UBE2C_#1, and 5’-CAGCAGGAGCTGATGACCCTCATG-3’ for UBE2C_#2. siRNAs targeting UBE2C and a negative control siRNA (StealthTM RNAi Negative Control Medium GC Duplex; catalog #12935-300) were purchased from Invitrogen (Carlsbad, CA, USA).
Western blot analysis
Western blot analysis was performed as described previously [26]. Anti-UBE2C antibody (1:500 dilution) or goat polyclonal anti-b-actin antibody (0.2 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used as primary antibodies.
Cell viability assay
Cells were seeded at 4×103 cells per well in 96-well plates and cultured for 72 h. Cell viability was determined using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer’s instructions. Experiments were conducted in triplicate and the results were averaged. At least three independent experiments were performed for each condition, and similar results were obtained.
Statistical analysis
All statistical analyses were performed using SAS software (Version 9.3, SAS Institute, Cary, NC, USA). All p values were two-sided. Differences were considered significant at p<0.05. For categorical data, the chi-square test was performed. Kaplan-Meier method and log-rank test were used for survival analysis. To control for confounding, multivariate Cox proportional hazards regression models was used. Student’s t-test was used to test differences in cell viability assays.
Results
UBE2C status in bladder cancer and normal urothelium
Among the 82 bladder UC cases treated with radical cystectomy, 51 cases (62%) showed UBE2C positivity (Figure 1). In contrast, UBE2C was negative in all of the 14 non-neoplastic urothelium examined (Figure 1). UBE2C positivity was also observed in eight of the 13 specimens of lymph node metastasis of UC (Figure 1).
Figure 1.
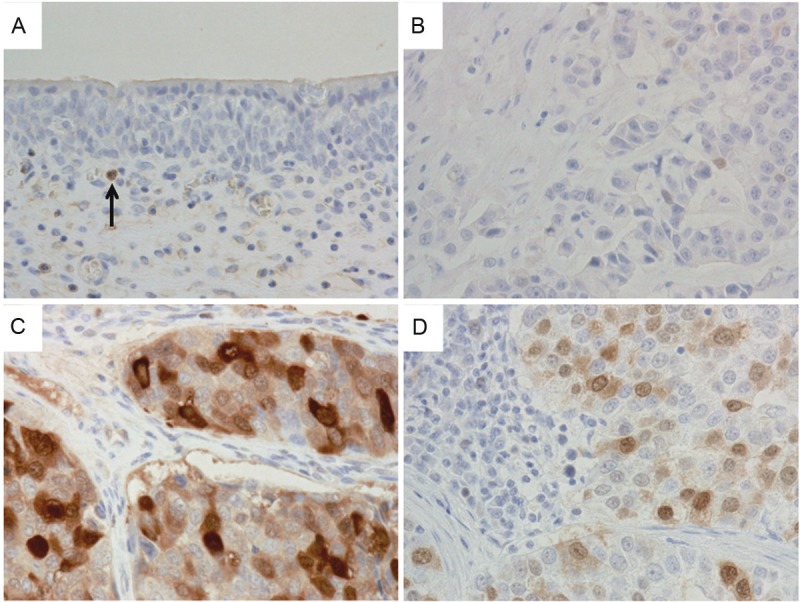
UBE2C expression in non-neoplastic bladder mucosa and bladder urothelial carcinoma (UC). A: Normal bladder mucosa is negative for UBE2C. Arrow indicates UBE2C-positive inflammatory cell. B: High-grade invasive UC with weak nuclear UBE2C staining. Weak staining was categorized as negative in this study. C: UBE2C-positive high-grade invasive UC. D: UBE2C-positive lymph node metastasis of UC. A-D: Magnification, ×400.
The relationship between UBE2C positivity and clinicopathological features in bladder UC patients who underwent radical cystectomy is summarized in Table 1. UBE2C positivity was significantly associated with higher tumor stage (p=0.0061) and presence of lymphovascular invasion (p=0.0045). There was no significant association between UBE2C positivity and the other parameters including gender, age and lymph node metastasis.
Table 1.
Correlation between UBE2C positivity and clinicopathological features in bladder urothelial carcinoma patients who underwent radical cystectomy (N=82)
| UBE2C | ||||
|---|---|---|---|---|
|
|
||||
| Clinicopathological feature | Total N | negative | positive | p value |
| All cases | 82 | 31 (38%) | 51 (62%) | |
| Gender | 0.86 | |||
| Male | 68 | 26 (38%) | 42 (62%) | |
| Female | 14 | 5 (36%) | 9 (64%) | |
| Age | 0.25 | |||
| ≤64 | 41 | 18 (44%) | 23 (56%) | |
| ≥65 | 41 | 13 (32%) | 28 (68%) | |
| Tumor stage | 0.0061a | |||
| pTis/pT1 | 25 | 15 (60%) | 10 (40%) | |
| pT2 | 22 | 6 (27%) | 16 (73%) | |
| pT3-pT4 | 35 | 10 (29%) | 25 (71%) | |
| Lymph node metastasis | 0.92 | |||
| Absent | 63 | 24 (38%) | 39 (62%) | |
| Present | 19 | 7 (37%) | 12 (63%) | |
| Lymphovascular invasion | 0.0045 | |||
| Absent | 34 | 19 (56%) | 15 (44%) | |
| Present | 48 | 12 (25%) | 36 (75%) | |
pTis/pT1 vs. pT2-pT4.
UBE2C positivity and survival after radical cystectomy
Among the 82 bladder UC patients who underwent radical cystectomy, there were 39 cancer-specific deaths during a median follow-up of 68 months (interquartile range, 54-106 months) for censored cases. In Kaplan-Meier analysis, UBE2C positivity was significantly associated with shorter cancer-specific survival (log rank p=0.0017) (Figure 2A). UBE2C positivity was also significantly associated with shorter cancer-specific survival in univariate and multivariate Cox models (Table 2). Univariate and multivariate hazard ratios (HRs) for UBE2C positivity were 3.29 [95% confidence interval (CI), 1.50-7.22] and 2.49 (95% CI, 1.09-5.71), respectively.
Figure 2.
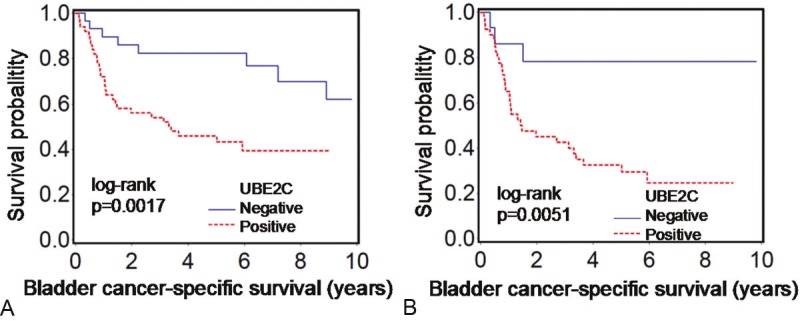
Kaplan-Meier curves for cancer-specific survival after radical cystectomy according to UBE2C status in bladder urothelial carcinoma. A: All cases (N=82). B: Cases with muscle-invasive bladder urothelial carcinoma (N=57).
Table 2.
UBE2C positivity in bladder urothelial carcinoma and patient mortality after radical cystectomy
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| All cases (N=82) | ||||
| UBE2C (positive vs. negative) | 3.29 (1.50-7.22) | 0.003 | 2.49 (1.09-5.71) | 0.031 |
| Gender (female vs. male) | 1.73 (0.78-3.83) | 0.18 | 1.33 (0.58-3.04) | 0.50 |
| Age (≥65 vs. ≤64) | 1.68 (0.89-3.17) | 0.11 | 1.80 (0.93-3.47) | 0.081 |
| Tumor stage (pT2-pT4 vs. pTis/pT1) | 4.52 (1.87-10.9) | 0.0008 | 2.16 (0.83-5.66) | 0.12 |
| Lymph node metastasis (present vs. absent) | 4.02 (2.00-8.08) | <0.0001 | 2.50 (1.13-5.53) | 0.024 |
| Lymphovascular invasion (present vs. absent) | 3.99 (1.84-8.65) | 0.0004 | 1.74 (0.70-4.33) | 0.23 |
| ≥pT2 cases (N=57) | ||||
| UBE2C (positive vs. negative) | 4.69 (1.43-15.4) | 0.011 | 5.32 (1.59-17.8) | 0.0067 |
| Gender (female vs. male) | 1.27 (0.55-2.95) | 0.58 | 1.04 (0.42-2.57) | 0.94 |
| Age (≥65 vs. ≤64) | 1.60 (0.79-3.24) | 0.20 | 2.18 (1.03-4.60) | 0.041 |
| Tumor stage (pT3/pT4 vs. pT2) | 1.60 (0.76-3.38) | 0.22 | 1.46 (0.65-3.27) | 0.36 |
| Lymph node metastasis (present vs. absent) | 2.13 (1.04-4.34) | 0.038 | 1.96 (0.88-4.39) | 0.10 |
| Lymphovascular invasion (present vs. absent) | 2.56 (0.98-6.67) | 0.056 | 1.69 (0.59-4.87) | 0.33 |
CI, confidence interval; HR, hazard ratio.
We also examined the prognostic significance of UBE2C in muscle-invasive bladder UC patients. UBE2C positivity was significantly associated with shorter cancer-specific survival in Kaplan-Meier analysis (Figure 2B) and in univariate and multivariate Cox models (Table 2). Univariate and multivariate HRs for UBE2C positivity in muscle-invasive bladder UC patients were 4.69 [95% CI, 1.43-15.4] and 5.32 (95% CI, 1.59-17.8), respectively. Older age was also significantly associated with shorter cancer-specific survival in multivariate model, consistent with prior reports [27,28].
Suppression of UBE2C inhibits bladder cancer cell growth
To examine the role of UBE2C in bladder cancer, UM-UC-3 cells were transfected with UBE2C siRNAs and cell growth was evaluated. Western blot analysis (Figure 3A) demonstrated an efficient knockdown of UBE2C protein levels at 48 h after transfection, concurrently demonstrating the specificity of the antibody against UBE2C used in this study. Analysis of cell growth in the presence or absence of the UBE2C siRNAs revealed that blocking UBE2C protein synthesis significantly inhibited UM-UC-3 cell growth (Figure 3B).
Figure 3.
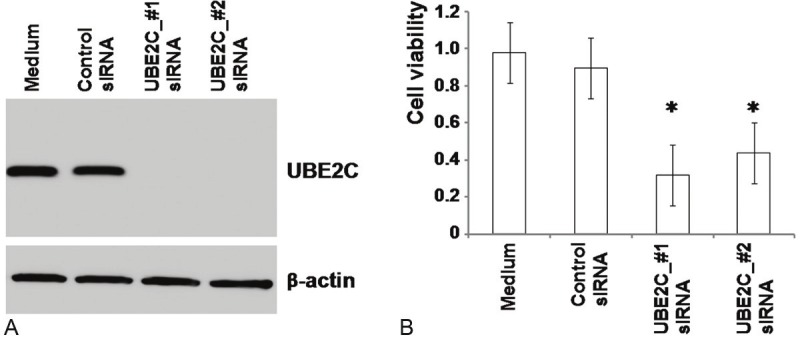
Expression of UBE2C protein and the effect of UBE2C suppression in UM-UC-3 bladder cancer cell line. A: UM-UC-3 cells were transfected with control siRNA or two sequences of UBE2C siRNA and cultured for 48 h. Cells were lysed and UBE2C protein levels were detected by western blotting. β-actin expression was used to control equal protein loading. B: At 72 h after siRNA transfection, the viability of UM-UC-3 cells was determined, as described in Materials and Methods. Columns, absorbance; bars, standard deviation. *: p<0.01 compared with control siRNA.
Discussion
Here, we report our findings on UBE2C expression in bladder cancer treated with radical cystectomy. We found that invasive bladder cancers were frequently positive for UBE2C, whereas all of the non-neoplastic urothelium examined was negative for UBE2C. UBE2C positivity was independently associated with shorter cancer-specific survival after radical cystectomy. Moreover, suppression of UBE2C synthesis, using siRNA, inhibited the growth of UM-UC-3 bladder cancer cells. Our results suggest that UBE2C plays a critical role in the progression of bladder cancer, and that UBE2C positivity may define a bladder cancer phenotype with more aggressive biological behavior.
It has recently been shown that UBE2C expression is associated with a poorer prognosis in breast [17,20], lung [18,21], colon [19], ovarian [16] and hepatocellular carcinomas [15]. A recent study by Fristrup et al. has also shown that high expression of UBE2C is associated with shorter progression-free survival in superficial bladder cancer patients who were treated with transurethral resection [22], consistent with our results. We report the clinicopathological and prognostic significance of UBE2C expression in bladder cancer treated with radical cystectomy for the first time. We have found that UBE2C positivity was significantly associated with higher tumor stage, presence of lymphovascular invasion, and increased mortality. Multivariate analysis revealed that UBE2C positivity was an independent prognostic factor. A subset analysis also revealed that UBE2C was an independent indicator of poorer prognosis in muscle-invasive bladder UC patients, for whom biomarkers that are predictive of outcomes are required. Our data, together with the recent study by Fristrup et al. [22], suggest that UBE2C may be a promising biomarker to predict clinical outcome in bladder cancer.
Experimental data generally support a tumor-promoting role for UBE2C [14-16,19,29-32]. Okamoto and co-workers showed that UBE2C overexpression in NIH 3T3 cells accelerated cell growth, increased the efficiency of colony formation, and promoted anchorage-independent growth in soft agar [14]. van Ree and co-workers found that transgenic mice overexpressing UBE2C developed a wide variety of spontaneous tumors, including lung adenocarcinomas, hepatic adenocarcinomas and lymphomas [33]. In thyroid [34], esophageal [29], hepatocellular [15], ovarian [16], breast [30] and colon [19] cancer cell lines in vitro, siRNA-mediated knockdown of UBE2C reduces cell proliferation. It has also been shown that depletion of UBE2C suppresses anchorage-independent growth of breast cancer cells [30]. Wagner and co-workers showed that selective silencing of UBE2C by siRNA, in combination with TRAIL/DR5 agonistic antibodies, led to increased cell death in neoplastic but not nonmalignant human cells [31]. In the present study, we have shown that siRNA-mediated knockdown of UBE2C inhibited the growth of UM-UC-3 bladder cancer cells, suggesting that targeting UBE2C is a potential therapeutic strategy for bladder cancer.
Recent studies suggest that UBE2C may be involved in resistance to radiation [32] and chemotherapy [21]. Bose and co-workers demonstrated that repression of UBE2C by dominant negative-UBE2C sensitized cervical cancer cells to radiation [32]. Zhao and co-workers showed that suppression of UBE2C by siRNA increased chemosensitivity to gemcitabine or paclitaxel in SK-MES-1 lung cancer cells [21]. Considering that both radiation and gemcitabine chemotherapy are standard therapies for locally advanced or metastatic bladder cancer [2], future studies are warranted to examine the roles of UBE2C in resistance to those therapies in bladder cancer.
In summary, UBE2C positivity is independently associated with shorter cancer-specific survival in bladder UC patients who underwent radical cystectomy. In addition, UBE2C plays an important role in proliferation of bladder cancer cells. Although further studies are needed to validate our findings, our data suggest that UBE2C is a novel prognostic biomarker as well as a potential therapeutic target in bladder cancer.
Acknowledgement
This work was partly supported by a research grant from the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care to T.M. We are grateful to Kei Sakuma, Yoko Inoue and Yumiko Nagano for excellent technical support.
Disclosure of conflict of interest
The authors have declared no conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 3.Habuchi T, Marberger M, Droller MJ, Hemstreet GP 3rd, Grossman HB, Schalken JA, Schmitz-Drager BJ, Murphy WM, Bono AV, Goebell P, Getzenberg RH, Hautmann SH, Messing E, Fradet Y, Lokeshwar VB. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology. 2005;66:64–74. doi: 10.1016/j.urology.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, Cha EK, Matsumoto K, Baba S, Chromecki TF, Fajkovic H, Sun M, Karakiewicz PI, Scherr DS, Shariat SF. Immunohistochemical biomarkers for bladder cancer prognosis. Int J Urol. 2011;18:616–29. doi: 10.1111/j.1442-2042.2011.02809.x. [DOI] [PubMed] [Google Scholar]
- 5.Soini Y, Haapasaari KM, Vaarala MH, Turpeenniemi-Hujanen T, Karja V, Karihtala P. 8-hydroxydeguanosine and nitrotyrosine are prognostic factors in urinary bladder carcinoma. Int J Clin Exp Pathol. 2011;4:267–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Q, Shen WH, Chen ZW, Zhou ZS, Ji HX. High expression level of BLCA-4 correlates with poor prognosis in human bladder cancer. Int J Clin Exp Pathol. 2012;5:422–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Netto GJ. Molecular biomarkers in urothelial carcinoma of the bladder: are we there yet? Nat Rev Urol. 2011;9:41–51. doi: 10.1038/nrurol.2011.193. [DOI] [PubMed] [Google Scholar]
- 8.Shariat SF, Karakiewicz PI, Ashfaq R, Lerner SP, Palapattu GS, Cote RJ, Sagalowsky AI, Lotan Y. Multiple biomarkers improve prediction of bladder cancer recurrence and mortality in patients undergoing cystectomy. Cancer. 2008;112:315–25. doi: 10.1002/cncr.23162. [DOI] [PubMed] [Google Scholar]
- 9.Shariat SF, Tilki D. Bladder cancer: nomogram aids clinical decision making after radical cystectomy. Nat Rev Urol. 2010;7:182–4. doi: 10.1038/nrurol.2010.42. [DOI] [PubMed] [Google Scholar]
- 10.Townsley FM, Aristarkhov A, Beck S, Hershko A, Ruderman JV. Dominant-negative cyclin-selective ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase. Proc Natl Acad Sci U S A. 1997;94:2362–7. doi: 10.1073/pnas.94.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rape M, Kirschner MW. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature. 2004;432:588–95. doi: 10.1038/nature03023. [DOI] [PubMed] [Google Scholar]
- 12.Stegmeier F, Rape M, Draviam VM, Nalepa G, Sowa ME, Ang XL, McDonald ER 3rd, Li MZ, Hannon GJ, Sorger PK, Kirschner MW, Harper JW, Elledge SJ. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–81. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 13.Hao Z, Zhang H, Cowell J. Ubiquitin-conjugating enzyme UBE2C: molecular biology, role in tumorigenesis, and potential as a biomarker. Tumour Biol. 2012;33:723–30. doi: 10.1007/s13277-011-0291-1. [DOI] [PubMed] [Google Scholar]
- 14.Okamoto Y, Ozaki T, Miyazaki K, Aoyama M, Miyazaki M, Nakagawara A. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 2003;63:4167–73. [PubMed] [Google Scholar]
- 15.Ieta K, Ojima E, Tanaka F, Nakamura Y, Haraguchi N, Mimori K, Inoue H, Kuwano H, Mori M. Identification of overexpressed genes in hepatocellular carcinoma, with special reference to ubiquitin-conjugating enzyme E2C gene expression. Int J Cancer. 2007;121:33–8. doi: 10.1002/ijc.22605. [DOI] [PubMed] [Google Scholar]
- 16.Berlingieri MT, Pallante P, Guida M, Nappi C, Masciullo V, Scambia G, Ferraro A, Leone V, Sboner A, Barbareschi M, Ferro A, Troncone G, Fusco A. UbcH10 expression may be a useful tool in the prognosis of ovarian carcinomas. Oncogene. 2007;26:2136–40. doi: 10.1038/sj.onc.1210010. [DOI] [PubMed] [Google Scholar]
- 17.Loussouarn D, Campion L, Leclair F, Campone M, Charbonnel C, Ricolleau G, Gouraud W, Bataille R, Jezequel P. Validation of UBE2C protein as a prognostic marker in node-positive breast cancer. Br J Cancer. 2009;101:166–73. doi: 10.1038/sj.bjc.6605122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadara H, Lacroix L, Behrens C, Solis L, Gu X, Lee JJ, Tahara E, Lotan D, Hong WK, Wistuba II, Lotan R. Identification of gene signatures and molecular markers for human lung cancer prognosis using an in vitro lung carcinogenesis system. Cancer Prev Res (Phila) 2009;2:702–11. doi: 10.1158/1940-6207.CAPR-09-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bavi P, Uddin S, Ahmed M, Jehan Z, Bu R, Abubaker J, Sultana M, Al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Prabhakaran S, Hussain AR, Al-Kuraya KS. Bortezomib stabilizes mitotic cyclins and prevents cell cycle progression via inhibition of UBE2C in colorectal carcinoma. Am J Pathol. 2011;178:2109–20. doi: 10.1016/j.ajpath.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Psyrri A, Kalogeras KT, Kronenwett R, Wirtz RM, Batistatou A, Bournakis E, Timotheadou E, Gogas H, Aravantinos G, Christodoulou C, Makatsoris T, Linardou H, Pectasides D, Pavlidis N, Economopoulos T, Fountzilas G. Prognostic significance of UBE2C mRNA expression in high-risk early breast cancer. A Hellenic Cooperative Oncology Group (HeCOG) Study. Ann Oncol. 2012;23:1422–7. doi: 10.1093/annonc/mdr527. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Jiang L, Wang L, He J, Yu H, Sun G, Chen J, Xiu Q, Li B. UbcH10 expression provides a useful tool for the prognosis and treatment of non-small cell lung cancer. J Cancer Res Clin Oncol. 2012;138:1951–61. doi: 10.1007/s00432-012-1275-2. [DOI] [PubMed] [Google Scholar]
- 22.Fristrup N, Birkenkamp-Demtroder K, Reinert T, Sanchez-Carbayo M, Segersten U, Malmstrom PU, Palou J, Alvarez-Mugica M, Pan CC, Ulhoi BP, Borre M, Orntoft TF, Dyrskjot L. Multicenter Validation of Cyclin D1, MCM7, TRIM29, and UBE2C as Prognostic Protein Markers in Non-Muscle-Invasive Bladder Cancer. Am J Pathol. 2013;182:339–49. doi: 10.1016/j.ajpath.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Cheng L, Montironi R, Davidson DD, Lopez-Beltran A. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod Pathol. 2009;22(Suppl 2):S70–95. doi: 10.1038/modpathol.2009.1. [DOI] [PubMed] [Google Scholar]
- 24.Grignon DJ. The current classification of urothelial neoplasms. Mod Pathol. 2009;22(Suppl 2):S60–9. doi: 10.1038/modpathol.2008.235. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa T, Hino R, Uozaki H, Maeda D, Ushiku T, Shinozaki A, Sakatani T, Fukayama M. Expression of ribonucleotide reductase M2 subunit in gastric cancer and effects of RRM2 inhibition in vitro. Hum Pathol. 2010;41:1742–8. doi: 10.1016/j.humpath.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa T, Sugiyama A, Kume H, Ota S, Kashima T, Tomita K, Kitamura T, Kodama T, Fukayama M, Aburatani H. Identification of Toll-like receptor 3 as a potential therapeutic target in clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:5703–9. doi: 10.1158/1078-0432.CCR-07-0603. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen ME, Shariat SF, Karakiewicz PI, Lotan Y, Rogers CG, Amiel GE, Bastian PJ, Vazina A, Gupta A, Lerner SP, Sagalowsky AI, Schoenberg MP, Palapattu GS. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol. 2007;51:699–706. doi: 10.1016/j.eururo.2006.11.004. discussion-8. [DOI] [PubMed] [Google Scholar]
- 28.Resorlu B, Beduk Y, Baltaci S, Ergun G, Talas H. The prognostic significance of advanced age in patients with bladder cancer treated with radical cystectomy. BJU Int. 2009;103:480–3. doi: 10.1111/j.1464-410X.2008.08033.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Raoof DA, Wang Z, Lin MY, Thomas DG, Greenson JK, Giordano TJ, Orringer MB, Chang AC, Beer DG, Lin L. Expression and effect of inhibition of the ubiquitin-conjugating enzyme E2C on esophageal adenocarcinoma. Neoplasia. 2006;8:1062–71. doi: 10.1593/neo.05832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita T, Ikeda H, Kawasaki K, Taira N, Ogasawara Y, Nakagawara A, Doihara H. Clinicopathological relevance of UbcH10 in breast cancer. Cancer Sci. 2009;100:238–48. doi: 10.1111/j.1349-7006.2008.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner KW, Sapinoso LM, El-Rifai W, Frierson HF, Butz N, Mestan J, Hofmann F, Deveraux QL, Hampton GM. Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene. 2004;23:6621–9. doi: 10.1038/sj.onc.1207861. [DOI] [PubMed] [Google Scholar]
- 32.Bose MV, Gopal G, Selvaluxmy G, Rajkumar T. Dominant negative Ubiquitin conjugating enzyme E2C sensitizes cervical cancer cells to radiation. Int J Radiat Biol. 2012;88:629–34. doi: 10.3109/09553002.2012.702299. [DOI] [PubMed] [Google Scholar]
- 33.van Ree JH, Jeganathan KB, Malureanu L, van Deursen JM. Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J Cell Biol. 2010;188:83–100. doi: 10.1083/jcb.200906147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pallante P, Berlingieri MT, Troncone G, Kruhoffer M, Orntoft TF, Viglietto G, Caleo A, Migliaccio I, Decaussin-Petrucci M, Santoro M, Palombini L, Fusco A. UbcH10 overexpression may represent a marker of anaplastic thyroid carcinomas. Br J Cancer. 2005;93:464–71. doi: 10.1038/sj.bjc.6602721. [DOI] [PMC free article] [PubMed] [Google Scholar]


