Abstract
Background: Triple negative breast cancer (TNBC) is heterogeneous and considered as an aggressive tumor. This study was to evaluate the associated classification and its correlations with prognosis and the response to chemotherapy in Chinese women. Methods: Four hundred and twenty-eight cases of invasive TNBC were involved in this study. The expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), and cytokeratin 5/6 (CK5/6), Ki67 and p53 were analyzed by immunohistochemistry and compared with patient outcome, and its implications and chemotherapy response were evaluated in four subgroups: typical medullary carcinoma (TMC), atypical medullary carcinoma (AMC), non-specific invasive ductal carcinoma (IDC) and other types. Results: The factors of tumor grade, tumor stage, lymph node status, EGFR/CK5/6 status and p53 labeling index were different among the groups. TMC tumors had the lowest rate of relapse (5.8%), while AMC, IDC and other types were associated with an increased risk of relapse (19.1%, 26.7% and 38.2% respectively). Many factors were risk predictors of relapse for TNBC and IDC, while only positive lymph node was for AMC. For MC tumors, adjunctive chemotherapy decreased the risk of relapse in lymph node positive subgroup (36.8% and 66.7%), while not significant in lymph node negative one (8.1% and 10.0%). Conclusion: The classification based on histologic and IHC findings may be a significant improvement in predicting outcome in TNBC. The different chemotherapy response in subgroups may contribute to guiding the treatment of TNBC.
Keywords: Triple negative breast cancer, typical medullary carcinoma, atypical medullary carcinoma, relapse, chemotherapy
Introduction
Molecular and genetic studies demonstrated that breast cancer was a heterogeneous disease [1], and had been proposed to be classified into subgroups according to different immunohistochemical biomarkers [2,3]. Of which estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were the most important biomarkers. In the 2007 St. Gallen Consensus Meeting made a decision about adjuvant therapies (chemotherapy, endocrine therapy, and trastuzumab), operable primary breast cancers were recommended to be categorized based on the status of ER, PR, and HER2 [4]. TNBC was defined as a subtype of breast cancers that were negative for ER, PR and HER2. TNBC was generally considered as the most difficult subtype to treat among these newly proposed subtypes of breast cancer because of the aggressive clinical behavior and the lack of current availability of specific targeted therapy such as selective ER modulators, aromatase inhibitors, trastuzumab, and lapatinib [3].
TNBC accounted for approximately 10–20% of the whole breast cancer and was correlated with relatively early clinical relapse within 3 years, with frequent progression to distant metastasis, particularly, visceral metastasis. Although the metastatic potential in TNBC was similar to that of other breast cancer subtypes, these tumors were associated with a shorter median time to relapse and death [5]. DNA microarray analyses proved that TNBCs were composed of basal-like subtype and normal-like (or unclassified) subtype. Basal-like subtype was characterized by the expression of myoepithelial/basal markers (EGFR and/or CK5/6) and molecular changes including p53 gene mutation and many chromosomal alterations which were correlated with an aggressive clinical course. Histological types of TNBC were reported to be similar with those of basal-like subtype, comprising high-grade non-specific IDC, AMC, TMC, and other types of carcinomas, of which TMC was of particular interest for being high grade carcinoma but favorable prognosis [6-8]. At present, chemotherapy remains the main treatment of TNBC despite of many limitations that need to be overcome. There is still not a clear, proven effective agent that targets a definite vulnerability in TNBC.
TNBC was a clearly distinct subtype of the whole breast cancer and had usually been divided into subgroups by the expression of myoepithelial/basal markers [2,3]. However, further subclassification with different prognosis is needed. In this study, we retrospectively studied the histological types, traditional pathologic indices and the description of CK5/6, EGFR, Ki67 and p53 on 428 Chinese women with TNBC in predicting subclassifications and investigating the risk of relapse and the response of chemotherapy in TNBC.
Materials and methods
Patient characteristics
The cohort used for this study was derived from the archival paraffin-embedded breast cancer samples collected at the Cancer Hospital of Tianjin Medical University between January 2002 and December 2004, and all tumors were primary, operable early breast cancer. The age of patients ranged from 25 to 76 years old, with the median age of 50.3 years. All patients underwent preoperative mammography and ultrasound of the breast and abdomen, X-ray, or computed tomography (CT) scan of the thorax. If there were any signs of metastasis to the bone or brain, bone scan or brain CT was performed as a standard procedure. All patients involved in this study were stages I, II, or III and were diagnosed as invasive carcinoma based on either the core-biopsy before operation or frozen biopsy intra-operation and were given diagnosis on paraffin-embedded tissue sections after operation by two pathologists. The sections were reviewed in double blind by different pathologist. All patients underwent local and/or systemic treatments. Local treatment included surgery and radiotherapy. Surgical procedures consisted of mastectomy and breast-conserving surgery. Patients who underwent breast-conserving surgery had received adjuvant radiotherapy as a routine. The main systemic treatment in our study was chemotherapy guided by National Comprehensive Cancer Network (NCCN). The exclusion criteria were: the patients treated with neoadjuvant chemotherapy, the patients without complete follow-up, and non-tumor-mediated mortality. A total of 428 cases were identified and contributed to this study. We retrospectively reviewed 70.6-month follow-up data. The follow-up contacts were carried out at 3-month intervals during the first year, 6-month intervals during the second year and 12-month intervals thereafter. The medical work-up consisted of regular physical checkups, imaging tests such as chest X-ray, bone scan and/or ultrasound, and to look for recurrences, second primary breast cancers, or metastatic disease. Relapse was defined as radiographic or pathological evidence of regional tumor recurrence or distant metastasis at any time after initial therapy. The study protocol was approved by the Hospital Human Ethical Committee. Informed consent was obtained from all patients before their surgery and the examination of the specimens.
Clinicopathological evaluation
We retrospectively evaluated conventional clinicopathological factors, including age at diagnosis, tumor size, tumor grade, lymph node status, tumor stage, menopausal status, family history (family history of breast cancer within first and second-degree relatives). The pathological tumor stage was assessed according to the criteria established by the 6th edition of the American Joint Committee on Cancer (AJCC) staging manual. Histological grade of the tumors were classified into grades I–III according to the Nottingham combined histological grade. Histologic type and pertinent histologic features included tumor border, glandular formation, mitotic index, degree of stromal lymphocytic infiltration, lymphovascular invasion, axillary lymph node (ALN) status, and results of IHC assess. The histologic type was assigned according to the 2003 World Health Organization criteria, and notably, diagnosis of TMC was made when the tumor showed predominantly (greater than 75%) syncytial architecture, absence of glandular structure, diffuse lymphoplasmacytic infiltrate (moderate to marked), nuclear pleomorphism (moderate to marked), and complete histological circumscription. Diagnosis of AMC was made when the tumor showed predominantly (greater than 75%) syncytial architecture with only two or three of the preceding four criteria. Tumor border was graded as circumscribed or infiltrative. Glandular formation was absent when there was no tubule formation; Mitotic index was graded as high (grade 3) when 20 or more mitotic figures were seen within 10 high-power fields, and as intermediate (grade 2) when the mitotic count was between 10 and 19, and low (grade 1) when less than 10 per 10 high power fields respectively. The degree of stromal lymphocytic infiltrate was graded as strong when more than two-thirds of the stroma of tumor mass had lymphocytic infiltration and weak when less than two-thirds of the stroma had lymphocytic infiltration. Metaplastic carcinomas were known to be a rare but characteristic subgroup of TNBC. They were aggressive, chemoresistant tumors characterized by concurrence of a high-grade carcinoma component (poorly differentiated ductal carcinoma) and extensive metaplastic component comprising squamous and/or mesenchymal (spindle-cell, cartilaginous, and/or osseous) metaplasia.
Immunohistochemistry and evaluations of the staining
IHC was performed on formalin-fixed, paraffin-embedded samples obtained from the pathology registry. Tissue sections (4 μm) were deparaffinized in xylene and rehydrated in a graded series of ethanol. The slides were treated with methanol containing 0.3% hydrogen peroxide to block any endogenous peroxidase activity. Heat-mediated antigen retrieval with the pressure cooker method was used for all staining except that no antigen epitope retrieval was necessary for EGFR. All the antibodies were used for IHC studies on serial tissue sections from each case; Primary antibodies used in this study included ER (SP1, 1:200 dilution; Zymed), PR (SP2, 1:200 dilution; Zymed), HER2 (CB11, 1:600 dilution; Zymed), EGFR (31G7, 1:100 dilution; Zymed), CK5/6 (D5/16B4, 1:200 dilution; Zymed), p53 (BP53.12 1:100 dilution; Zymed) and Ki67 (K-2, 1:100 dilution; Zymed). The immunostaining was scored in double blind by two different pathologists, who were blinded to patients’ clinicopathologic characteristics and outcomes. For each antibody, the location of immunoreactivity, percentage of stained cells, and intensity were determined. The evaluation of each protein expression was determined from the mean of the individual cases. ER and PR stains were assessed using Allred scores, with positive scores ranging from 2 to 8 [9]. CK5/6 and EGFR stains were considered positive if any cytoplasmic and/or membranous staining was observed, whereas HER2+ was defined as the whole membrane strong staining in >30% of the tumor cells, and Ki67 status was expressed in terms of percentage of positive cells, with a threshold of 14% of positive cells [2]. p53 was defined as positive when more than 10% of tumor cells were positive for nuclear staining [10].
Statisticcal analysis
All statistical analyses were carried out using SPSS software (Version 17.0 for Windows). The χ2 test and Fisher exact test were performed for group comparisons. For univariable survival analysis, Overall survival (OS) and Relapse-free survival (RFS) were conducted using the Kaplan-Meier curves. The log-rank test was used to compare survival differences among the subtypes. Cox proportional hazards models were used to calculate relative risk accounting for covariates. A 2-sided P<0.05 was considered statistically significant in all the analyses.
Results
The intrinsic histologic types were illustrated in (Figure 2). Three hundred (70.09%) patients were diagnosed as non-specific IDC, 26 (6.07%) were TMC, 68 (15.89%) were AMC and 34 (7.94%) were other types carcinoma including apocrine carcinoma (4.44%), invasive lobular carcinoma (1.40%), metaplastic carcinoma (1.64%) and squamous cell carcinoma (0.47%) (Table 1). The distribution of clinicopathological characteristics among the subtypes were listed in Table 2, MC was associated with small tumor size, lower percentage of node positivity, but higher tumor grade and higher family history of BC. The differences in tumor grade, tumor stage, lymph node status, EGFR/CK5/6 status and p53 labeling index among the subtypes were significant (P<0.05).
Figure 2.
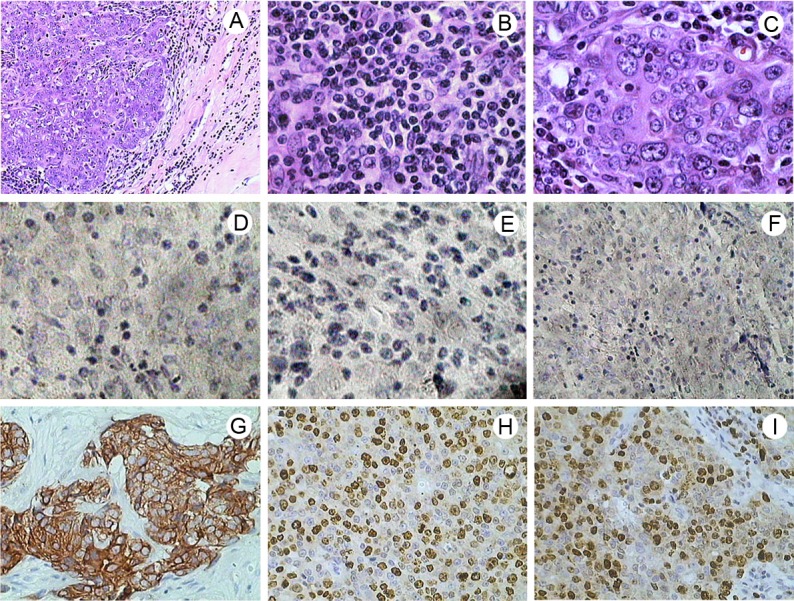
Hematoxylin - eosin staining and immunohistochemical staining in TNBC tissues. A. TMC with a clear boundary between tumor tissue and normal breast tissue, original magnification ×100. B. TMC with many lymphocytes and plasma cells at the edges of the tumor, original magnification ×400. C. TMC with large-sized cancer cells and high grade appearance, cells also tend to blend together, original magnification ×400. D. Immunohistochemical staining of ER revealed negative staining in TMC, original magnification ×400. E. Immunohistochemical staining of PR revealed negative staining in TMC, original magnification ×400. F. The tumor showed negative staining of HER2, original magnification ×200. G. Diffuse cytoplasmic and membrane staining of CK5/6, original magnification ×200. H. Immunohistochemical staining of Ki67 revealed nuclear staining, original magnification ×200. I. Immunohistochemical staining of p53 revealed nuclear staining, original magnification ×200.
Table 1.
Histologic types and the classification of TNBC
| Histologic diagnosis | Number of cases | Percentage |
|---|---|---|
| IDC | 300 | 70.09% |
| TMC | 26 | 6.07% |
| AMC | 68 | 15.89% |
| Other types | 34 | 7.94% |
| Apocrine carcinoma | 19 | 4.44% |
| Invasive lobular carcinoma | 6 | 1.40% |
| Metaplastic carcinoma | 7 | 1.64% |
| Squamous cell carcinoma | 2 | 0.47% |
| Total | 428 | 100 |
Abbreviations: TNBC, triple negative breast cancer; IDC, Invasive ductal carcinoma; TMC, Typical medullary carcinoma; AMC, Atypical medullary carcinoma.
Table 2.
Distribution of clinicopathological characteristics among the subtypes of TNBC
| Characteristics | TMC | n=26 | AMC | n=68 | IDC | n=300 | Other types | n=34 | χ2 | P-values |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| No. | % | No. | % | No. | % | No. | % | |||
| Age at diagnosis, years | 8.125 | 0.229 | ||||||||
| <40 | 5 | 19.2 | 8 | 11.8 | 40 | 13.3 | 6 | 17.6 | ||
| 40-55 | 17 | 65.4 | 52 | 76.5 | 185 | 61.7 | 21 | 61.8 | ||
| >55 | 4 | 15.4 | 8 | 11.8 | 75 | 25.0 | 7 | 20.6 | ||
| Tumor size, cm | 6.313 | 0.389 | ||||||||
| ≤2 | 13 | 50.0 | 20 | 29.4 | 89 | 29.7 | 9 | 26.5 | ||
| 2-5 | 11 | 42.3 | 44 | 64.7 | 193 | 64.3 | 24 | 70.6 | ||
| >5 | 2 | 7.7 | 4 | 5.9 | 18 | 6.0 | 1 | 2.9 | ||
| Tumor grade | NA | 32.471 | 0.000 | |||||||
| 1-2 | 6 | 8.8 | 121 | 40.3 | 19 | 55.9 | ||||
| 3 | 62 | 91.2 | 179 | 59.7 | 15 | 44.1 | ||||
| Tumor stage | 15.551 | 0.007 | ||||||||
| AJCC stage I/II | 25 | 96.2 | 56 | 82.4 | 215 | 71.7 | 19 | 55.9 | ||
| AJCC stage III | 1 | 3.8 | 12 | 17.6 | 85 | 28.3 | 15 | 44.1 | ||
| Lymph node status | 32.696 | 0.000 | ||||||||
| Negative | 24 | 92.3 | 48 | 70.6 | 142 | 47.3 | 12 | 35.3 | ||
| Positive | 2 | 7.7 | 20 | 29.4 | 158 | 52.7 | 22 | 64.7 | ||
| Menopausal status | 1.230 | 0.746 | ||||||||
| Premenopausal | 15 | 57.7 | 34 | 50.0 | 144 | 48.0 | 15 | 44.1 | ||
| Postmenopausal | 11 | 42.3 | 34 | 50.0 | 156 | 52.0 | 19 | 55.9 | ||
| Family history | 2.855 | 0.415 | ||||||||
| Yes | 6 | 23.1 | 11 | 16.2 | 39 | 13.0 | 4 | 11.8 | ||
| No | 20 | 76.9 | 57 | 83.8 | 261 | 87.0 | 30 | 88.2 | ||
| EGFR/CK5/6 | 10.633 | 0.014 | ||||||||
| Positive | 23 | 88.5 | 62 | 91.2 | 222 | 74.0 | 27 | 79.4 | ||
| Negative | 3 | 11.5 | 6 | 8.8 | 78 | 26.0 | 7 | 20.6 | ||
| Ki-67 labeling index | 5.087 | 0.166 | ||||||||
| ≥14% | 19 | 73.1 | 33 | 48.5 | 155 | 51.7 | 18 | 52.9 | ||
| <14% | 7 | 26.9 | 35 | 51.5 | 145 | 48.3 | 16 | 47.1 | ||
| p53 labeling index | 57,871 | 0.000 | ||||||||
| Positive | 23 | 88.5 | 45 | 66.2 | 102 | 34.0 | 5 | 14.7 | ||
| Negative | 3 | 11.5 | 23 | 33.8 | 198 | 66.0 | 29 | 85.3 | ||
Abbreviations: TNBC, triple negative breast cancer; TMC, typical medullary carcinoma; AMC, atypical medullary carcinoma; IDC, invasive ductal carcinoma; NA, not applicable; AJCC, American Joint Committee on Cancer. P-values were calculated to compare the four groups.
Of the 428 cases involved in this study, 88.87% received chemotherapy, with a follow-up period of 70.6 months, the actuarial OS of TMC, AMC, IDC and other types were 92.3%, 85.3%, 82.0% and 67.6% respectively (Figure 1A). The RFS of TMC, AMC, IDC and other types were 92.3%, 80.9%, 73.3% and 61.8% respectively (Figure 1B, Table 5). Patients with TMC and AMC tumors had favorable prognosis, with relapse rates of 5.8% and 19.1%, conversely, the IDC and other types exhibited high rates of relapse (26.7% vs. 38.2%). The difference between these types was significant (P=0.030) (Figure 1B, Table 5).
Figure 1.
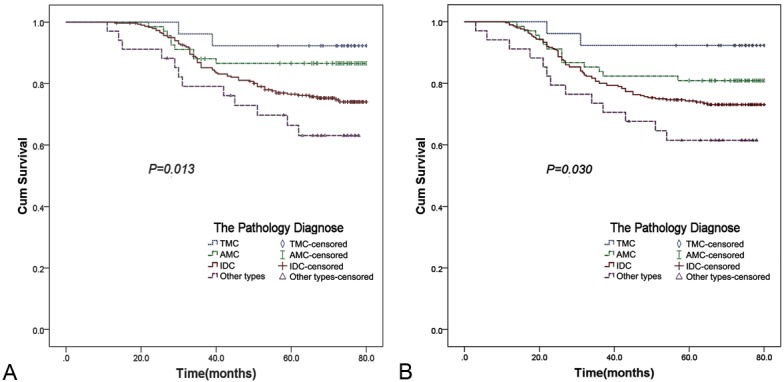
Survival curves: (A) The OS curve of the groups in TNBC (B) The RFS curve of the groups in TNBC.
Table 5.
Relapse among the various groups
| Type | No. of Patients | Relapse | RFS% | χ2 | P-values |
|---|---|---|---|---|---|
| TMC | 26 | 2 | 92.3 | 8.962 | 0.03 |
| AMC | 68 | 13 | 80.9 | ||
| IDC | 300 | 80 | 73.3 | ||
| Other types | 34 | 13 | 61.8 | ||
| AMC-G3 | 62 | 12 | 80.6 | 7.602 | 0.006 |
| IDC-G3 | 179 | 69 | 61.5 |
Abbreviations: TMC, typical medullary carcinoma; AMC, atypical medullary carcinoma; IDC, invasive ductal carcinoma; RFS, relapse-free survival.
Multivariable Cox analysis revealed that family history positive, large tumor size, lymph node positive, tumor stage III, EGFR/CK5/6 positive, high Ki-67 labeling index and high p53 labeling index were risk predictors of relapse for TNBC (P<0.05). The differences were also significant for IDC while only lymph node positive was risk predictor for AMC (P=0.016) (Tables 3, 4). The relapse hazard ratios (HR) of the subtypes were: 2.6 (AMC), 3.3 (IDC), 6.9 (other types) (Table 3). Although AMC-G3 have many overlapping histologic features with IDC-G3, the relapse of the two groups were significantly different (P=0.006) (Table 5).
Table 3.
Multivariate analysis of risk factors for relapse of TNBC
| Variable | TNBC (n=428) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Relapse (n=108) | |||||||||
|
| |||||||||
| HR | 95% CL | P-values | |||||||
| Age, years | |||||||||
| <40 | 1 | ||||||||
| 40-55 | 1.5 | 0.8 | - | 2.7 | 0.216 | ||||
| >55 | 1.3 | 0.7 | - | 2.9 | 0.307 | ||||
| Family history | |||||||||
| No | 1.0 | ||||||||
| Yes | 1.7 | 1.0 | - | 2.9 | 0.039 | ||||
| Menopausal status | |||||||||
| Postmenopausal | 1.0 | ||||||||
| Premenopausal | 0.9 | 0.6 | - | 1.5 | 0.792 | ||||
| Tumor size, cm | |||||||||
| ≤2 | 1.0 | ||||||||
| 2-5 | 2.0 | 1.0 | - | 3.7 | 0.038 | ||||
| >5 | 3.1 | 1.4 | - | 7.0 | 0.007 | ||||
| Tumor stage | |||||||||
| AJCC stage I/II | 1.0 | ||||||||
| AJCC stage III | 2.2 | 1.4 | - | 3.4 | 0.001 | ||||
| Lymph node status | |||||||||
| Negative | 1.0 | ||||||||
| Positive | 2.2 | 1.4 | - | 3.4 | 0.001 | ||||
| EGFR/CK5/6 | |||||||||
| Negative | 1.0 | ||||||||
| Positive | 5.4 | 3.0 | - | 9.7 | 0.000 | ||||
| Ki-67 labeling index | |||||||||
| ≤14% | 1.0 | ||||||||
| >14% | 2.0 | 1.3 | - | 3.1 | 0.002 | ||||
| p53 labeling index | |||||||||
| Negative | 1.0 | ||||||||
| Positive | 3.7 | 2.4 | - | 5.8 | 0.000 | ||||
| Types | |||||||||
| TMC | 1.0 | ||||||||
| AMC | 2.6 | 0.6 | - | 11.6 | 0.222 | ||||
| IDC | 3.3 | 0.8 | - | 14.4 | 0.112 | ||||
| Other types | 6.9 | 1.4 | - | 35.0 | 0.019 | ||||
Abbreviations: TNBC, triple negative breast cancer; CI, confidence interval; HR, hazard ratio; EGFR, epidermal growth factor receptor.
Table 4.
Multivariate analysis of risk factors for relapse of AMC and IDC in TNBC
| Variable | AMC (n=68) | IDC (n=300) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Relapse (n=13) | Relapse (n=80) | |||||||||
|
| ||||||||||
| HR | 95% CL | P-values | HR | 95% CL | P-values | |||||
| Age, years | ||||||||||
| <40 | 1 | 1 | ||||||||
| 40-55 | 3.5 | 0.2 | - | 60.0 | 0.392 | 1.3 | 0.5 | - | 2.9 | 0.588 |
| >55 | 2.0 | 0.2 | - | 24.3 | 0.570 | 1.2 | 0.6 | - | 2.4 | 0.611 |
| Family history | ||||||||||
| No | 1 | 1 | ||||||||
| Yes | 3.4 | 0.9 | - | 13.2 | 0.070 | 1.4 | 0.7 | - | 2.6 | 0.296 |
| Menopausal status | ||||||||||
| Postmenopausal | 1 | 1 | ||||||||
| Premenopausal | 0.8 | 0.2 | - | 3.3 | 0.781 | 1.1 | 0.6 | - | 1.8 | 0.736 |
| Tumor size, cm | ||||||||||
| ≤2 | 1 | 1 | ||||||||
| 2-5 | 1.5 | 0.3 | - | 7.3 | 0.617 | 5.5 | 1.7 | - | 17.9 | 0.005 |
| >5 | 2.4 | 0.2 | - | 35.3 | 0.533 | 9.3 | 2.5 | - | 33.7 | 0.001 |
| Tumor stage | ||||||||||
| AJCC stage I/II | 1 | 1 | ||||||||
| AJCC stage III | 1.1 | 0.2 | - | 6.0 | 0.882 | 1.7 | 1.0 | - | 2.8 | 0.044 |
| Tumor grade | ||||||||||
| G1/G2 | 1 | 1 | ||||||||
| G3 | 1.1 | 0.1 | - | 10.1 | 0.962 | 2.3 | 1.2 | - | 4.5 | 0.014 |
| Lymph node status | ||||||||||
| Negative | 1 | 1 | ||||||||
| Positive | 4.4 | 1.3 | - | 14.6 | 0.016 | 6.6 | 3.0 | - | 14.3 | 0.000 |
| EGFR/CK5/6 | ||||||||||
| Negative | 1 | 1 | ||||||||
| Positive | 1.3 | 0.1 | - | 14.7 | 0.857 | 2.1 | 1.0 | - | 4.2 | 0.040 |
| Ki-67 labeling index | ||||||||||
| ≤14% | 1 | 1 | ||||||||
| >14% | 0.9 | 0.3 | - | 3.1 | 0.871 | 2.0 | 1.2 | - | 3.4 | 0.011 |
| p53 labeling index | ||||||||||
| Negative | 1 | 1 | ||||||||
| Positive | 1.1 | 0.3 | - | 3.9 | 0.874 | 3.6 | 2.3 | - | 5.9 | 0.000 |
Abbreviations: TNBC, triple negative breast cancer; AMC, atypical medullary carcinoma; IDC, invasive ductal carcinoma; CI, confidence interval; HR, hazard ratio; AJCC, American Joint Committee on Cancer; EGFR, epidermal growth factor receptor.
For MC tumors, lymph node status played an important role. When compared given adjunctive chemotherapy or not, in lymph node positive groups the relapse percentages were 36.8% and 66.7% respectively. However, in lymph node negative groups, the relapse percentages were 8.1% and 10.0% respectively (Table 6). For the 265 patients with IDC tumors who had finished adjunctive chemotherapy in our study, the expression of CK5/6/EGFR, Ki67 and p53 markers assayed by IHC were considered having associations with increased risk of relapse: patients with positive expression of all the CK5/6/EGFR, Ki67 and p53 markers exhibited the highest relapse (59.1%). Conversely, patients with negative expression of the markers had the most favorable prognosis, with the relapse rate of only 11.1%. Patients with one or two positive expression of the markers had the middle relapses: 29.2%, 32.0%, 14.7%, 20.0%, 17.4% and 18.8% respectively (Table 7).
Table 6.
Adjunctive chemotherapy analysis in MC
| AMC & TMC | No. of Patients | No. of Events (%) | P-values | |
|---|---|---|---|---|
|
| ||||
| Lymph node status | Chemotherapy | |||
| Yes | Yes | 19 | 7 (36.8) | 0.544 |
| Yes | No | 3 | 2 (66.7) | |
| No | Yes | 62 | 5 (8.1) | 0.999 |
| No | No | 10 | 1 (10.0) | |
Abbreviations: MC, medullary carcinoma; TMC, typical medullary carcinoma; AMC, atypical medullary carcinoma.
Table 7.
Adjunctive chemotherapy (CMF or CAF) analysis in IDC (n=265)
| IDC | No. of Patients | No. of Events (%) | P-values | ||
|---|---|---|---|---|---|
|
| |||||
| CK5/6/EGFR | Ki67 | p53 | |||
| Yes | Yes | Yes | 44 | 26 (59.1) | 0.002 |
| No | 65 | 19 (29.2) | |||
| No | Yes | 25 | 8 (32.0) | 0.061 | |
| No | 68 | 10 (14.7) | |||
| No | Yes | Yes | 15 | 3 (20.0) | 0.999 |
| No | 23 | 4 (17.4) | |||
| No | Yes | 16 | 3 (18.8) | 0.999 | |
| No | 9 | 1 (11.1) | |||
Abbreviations: IDC, invasive ductal carcinoma; EGFR, epidermal growth factor receptor.
Discussion
TNBC is generally considered to be associated with aggressive clinical behavior. However, by now a limited number of studies have investigated the prevalence of the subclassifications of TNBC in the yellow race. As known, histologic type is one of the most important and clinically assessed prognostic factors in BCs. MC is particular for its features of aggressiveness but favorable prognosis. Bertucci et al. [11] obtained whole-genome oligonucleotide microarrays comparing gene expression profiles of 22 MBCs and found 95% MBCs displayed a basal profile but a distinct subgroup of basal breast cancer. Jacquemier et al. [12] used IHC on tissue-microarrays and found TMC was characterized by a high degree of basal/myoepithelial differentiation. According to molecular classification of BC, basal breast cancers were associated with poorer prognosis [2,3], and approximately 65–90% of basal tumors were currently found as TNBC [13-15]. In our study, the incidence of TNBC was about 15% in the whole cohort, approximately consistent with that in Japan [10,16]. TMC and AMC accounted for 6.1% and 15.9% respectively within the TNBC while accounted for 0.9% and 2.3% within this whole cohort. Indicating MC was closely associated and as an important part of TNBC. Weigelt et al. [17] investigated 20 metaplastic breast carcinomas using microarray-based expression profiling data and demonstrated that most of the MBCs were of basal-like molecular subtype and were reported with poor responses to chemotherapy. Nonetheless, it was a challenging issue since subtypes of TNBC were associated with different prognosis. Moreover, the subgroups of triple-negative tumors formed by TMC, AMC, IDC-NOS and other types breast carcinomas such as metaplastic carcinomas represent a pitfall for clinicians, since they share histological similarities but were heterogeneous in term of clinical outcome [18-20]. In our study, as with a high incidence, TMC, AMC and IDC were investigated respectively as independent subtypes, metaplastic carcinoma, apocrine, invasive lobular carcinoma and squamous cell carcinoma were categorized as a subtype named “other types”.
Although MC was characterized by specific histological criteria, the diagnosis of MC especially of AMC was often difficult and hardly reliable [18,19]. For the AMC group, the most common ‘‘non-medullary’’ histologic feature was the lack of a circumscribed border, followed by an infiltrative border accompanied by glandular structure; infiltrative border together with mild lymphoid infiltration. In our study, MC was associated with small tumor size, lower percentage of node positivity, but higher tumor grade, mostly expression of EGFR/CK5/6, p53 labeling index and higher family history of BC (P<0.05). It was the useful supplementary to the histological criteria and could improve the diagnostic and prognostic tools by characterising these factors.
By now, many studies demonstrated MC displayed a basal-like molecular profile, similar to those with poor prognosis in TNBC, but associated with a relatively favorable prognosis [6-8]. However, relatively few studies have performed to find an association between subtypes and prognosis in TNBC. Desmedt et al. [21] tried to differentiate MC from non-MC by gene expression profiling but failed in defining prognostic subgroups in TNBC. Miyashita et al. [16] examined histopathological subclassification of TNBC using prognostic scoring system and TNBC patients were classified into three subgroups with different prognosis. In our study, differences of relapse among the molecular subtypes were evident (P<0.05). According to the previous studies [22], TMC tumors had the most favorable prognosis and the other types had the worst. AMC-G3 was reported to prone to over-diagnose into IDC-G3 [18,19]. In our study, although the two groups had some overlapping histologic features and similar immunophenotype, the difference of prognosis between AMC-G3 and IDC-G3 was evident (P<0.05). Thus, we consider the subtype of AMC actually existed. In addition, AMC was significant in prognosis and treatment of TNBC and should be diagnosed in strict histological criteria.
Ki-67 was a well-known marker of proliferation which often expressed positive in breast carcinoma, while in normal breast epithelium adjacent to fibro-adenomas, it was expressed at a very low level [23]. Colleoni et al. [24] found that high Ki-67 predicted for recurrence in small size, node-negative breast cancers. Mamounas et al. [25] found that 25% of a cohort had a higher risk of relapse compared with low-risk breast cancer tumors (16% vs. 4%, respectively). Keam B et al. [26] also found Ki-67 could be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. EGFR and CK5/6 were reported as specific biomarkers to define the basal-like subtype and to reflect the cancer survival as a result of surrogating gene expression profiles analysis [2,3,27]. p53 was a marker of basal-like breast tumors and the overexpression of p53 was associated with local recurrence in TNBC [28-30]. In our study, EGFR/CK5/6 positive, high Ki-67 labeling index and high p53 labeling index were risk predictors of relapse for TNBC (P<0.05). Moreover, non-MC-TN seemed to show distinct expressions of prognostic markers compared with TMC and AMC groups. The risk predictors of relapse for TNBC were also significant for IDC while not for AMC. It suggested the prognostic biomarkers were more suitable for IDC-TN than for all of the histological types of TNBC and IDC-TN were the surrogate of the actually TNBC that one usually mentioned.
Chemotherapy is a main composition of systemic treatments and sensitive for TNBC. In clinical treatments, oncologists actually gave chemotherapy individually for the complexity of specific circumstance. In our study, adjuvant chemotherapy was given mainly decided by some clinical pathological factors (tumor size, tumor stage, and node status) based on cyclophosphamide, methotrexate and fluorouracil (CMF) and cyclophosphamide, doxorubicin, fluorouracil (CAF). For TNBC tumors, the difference between CAF and CMF was not significant [31], and the dosage was decided based on the square of the patients’ body. However, the chemotherapy responses were not always similar even in the patients with the similar clinical pathological factors. For the patients with lymph node positive in MC subtypes, the relapses were 36.8 and 66.7% respectively between the patients with and without chemotherapy (finished more than 6 cycles). While for the patients with lymph node negative in MC subtypes, compared the patients treated with chemotherapy and those without chemotherapy, the relapses were similar (8.1% vs. 10.0%), indicating those with lymph node positive had a higher relapse but can benefit more from adjuvant chemotherapy than those with lymph node negative in MC. In IDC subtype for the patients given CAF/CMF chemotherapy, those with the expression of CK5/6/EGFR, Ki67 and p53 positive had the highest relapse (59.1%), far higher than those without or with some of the expressions of the three markers. It suggested additional studies should be required to identify more effective adjuvant chemotherapy to address greater risks of relapse.
Conclusions
Of the subtypes based on histologic and IHC findings in TNBC, We demonstrated that the histologic types combined with a small panel of IHC markers can identify patients at different risk of relapse and some of different response on CMF/CAF. However, the biology underlying these observations remains poorly understood. This work suggests that we could identify biologic mechanisms associated with tumor aggressiveness, nodal metastasis, and treatment response. Additional studies will be required to identify the most effective treatment modality including more extensive surgery, radiotherapy and systemic therapy to address a greater risk of relapse; Because effective treatment modalities could exist for the local control of breast cancer, further investigation into breast cancer biomarkers, molecular subtypes, and the associated risk of relapse may profoundly affect the treatment of breast cancer. We expect the differences of relapse among the subtypes of TNBC will likely be diminished after modern systemic therapy.
Acknowledgments
This work was financially supported by National Science Foundation of China (30872519); Scientific and Technological Development Fund (09JCYBJC10100) of Tianjin Scientific and Technological Committee; Program for Chang-jiang Scholars and Innovative Research Team in University (TRT0743). The authors gratefully acknowledge Mrs Xiumin Ding and Ying Wang for technical assistance.
Disclosure of conflict of interest
The authors declare no conflicts of interest.
Abbreviations
- NCCN
National Comprehensive Cancer Network
- AJCC
American Joint Committee on Cancer
- ER
Estrogen Receptor
- PR
Progesterone Receptor
- HER2
Human Epidermal growth factor receptor 2
- EGFR
Epidermal growth factor receptor
- TNBC
Triple negative breast cancer
- IDC
Invasive ductal carcinoma
- MC
Medullary Carcinoma
- TMC
Typical medullary carcinoma
- AMC
Atypical medullary carcinoma
- HR
Hazard ratio
- OS
Overall survival
- RFS
Relapse-free survival
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Voduc KD, Cheang MC, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Yin Q, Yu Q, Zhang J, Liu Z, Wang S, Lv S, Niu Y. A retrospective study of breast cancer subtypes: the risk of relapse and the relations with treatments. Breast Cancer Res Treat. 2011;130:489–498. doi: 10.1007/s10549-011-1709-6. [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 5.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16:1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 6.Fulford LG, Reis-Filho JS, Ryder K, Jones C, Gillett CE, Hanby A, Easton D, Lakhani SR. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 2007;9:R4. doi: 10.1186/bcr1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J. Clin. Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 8.Reis-Filho JS, Tutt AN. Triple negative tumors: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 9.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-uvant endbinding assay for predicting response to adjocrine therapy in breast cancer. J. Clin. Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa Y, Horiguchi J, Toya H, Nakajima H, Hayashi M, Tagaya N, Takeyoshi I, Oyama T. Triple-negative breast cancer: Histological subtypes and immunohistochemical and clinicopathological features. Cancer Sci. 2011;102:656–662. doi: 10.1111/j.1349-7006.2011.01858.x. [DOI] [PubMed] [Google Scholar]
- 11.Bertucci F, Finetti P, Cervera N, Maraninchi D, Viens P, Birnbaum D. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–4644. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- 12.Jacquemiert J, Padovani L, Rabayrol L, Lakhani SR, Penault-Llorca F, Denoux Y, Fiche M, Figueiro P, Maisongrosse V, Ledoussal V, Marinez Penuela J, Udvarhely N, El Makdissi G, Ginestier C, Geneix J, Charafe-Jauffret E, Xerri L, Eisinger F, Birnbaum D, Sobol H. Typical medullary breast carcinomas have a basal/myoepithelial phenotype. J Pathol. 2005;207:260–268. doi: 10.1002/path.1845. [DOI] [PubMed] [Google Scholar]
- 13.Bidard FC, Conforti R, Boulet T, Michiels S, Delaloge S, Andre F. Does triple-negative phenotype accurately identify basal-like tumour? An immunohistochemical analysis based on 143 ‘triple-negative’ breast cancers. Ann Oncol. 2007;18:1285–1286. doi: 10.1093/annonc/mdm360. [DOI] [PubMed] [Google Scholar]
- 14.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:R65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, Birnbaum D. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- 16.Miyashita M, Ishida T, Ishida K, Tamaki K, Amari M, Watanabe M, Ohuchi N, Sasano H. Histopathological subclassification of triple negative breast cancer using prognostic scoring system: five variables as candidates. Virchows Arch. 2011;458:65–72. doi: 10.1007/s00428-010-1009-2. [DOI] [PubMed] [Google Scholar]
- 17.Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat. 2009;117:273–280. doi: 10.1007/s10549-008-0197-9. [DOI] [PubMed] [Google Scholar]
- 18.Ridolfi RL, Rosen PP, Port A. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365–1385. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Böcker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol. 2002;86:116–119. [PubMed] [Google Scholar]
- 20.Sloane JP, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W, Bussolati G, Coleman D, Connolly CE, Eusebi V, De Miguel C, Dervan P, Drijkoningen R, Elston CW, Faverly D, Gad A, Jacquemier J, Lacerda M, Martinez-Penuela J, Munt C, Peterse JL, Rank F, Sylvan M, Tsakraklides V, Zafrani B. Consistency achieved by 23 European pathologists from 12 countries in diagnosing breast disease and reporting prognostic features of carcinomas. European Commission Working Group on Breast Screening Pathology. Virchows Arch. 1999;434:3–10. doi: 10.1007/s004280050297. [DOI] [PubMed] [Google Scholar]
- 21.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, Delorenzi M, Piccart M, Sotiriou C. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 22.Martinez SR, Beal SH, Canter RJ, Chen SL, Khatri VP, Bold RJ. Medullary carcinoma of the breast: a population-based perspective. Med Oncol. 2011;28:738–744. doi: 10.1007/s12032-010-9526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J. Clin. Oncol. 2005;23:7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 24.Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Viale G, Renne G, Casadio C, Veronesi P, Intra M, Torrisi R, Goldhirsch A. Minimal and small size invasive breast cancer with no axillary lymph node involvement: The need for tailored adjuvant therapies. Ann Oncol. 2004;15:1633–1639. doi: 10.1093/annonc/mdh434. [DOI] [PubMed] [Google Scholar]
- 25.Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, Watson D, Geyer CE Jr, Wickerham DL, Wolmark N. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J. Clin. Oncol. 2010;28:1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Im SA, Lee KH, Han SW, Oh DY, Kim JH, Lee SH, Han W, Kim DW, Kim TY, Park IA, Noh DY, Heo DS, Bang YJ, Keam B. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011;13:R22. doi: 10.1186/bcr2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin BK, Lee Y, Lee JB, Kim HK, Lee JB, Cho SJ, Kim A. Breast carcinomas expressing basal markers have poor clinical outcome regardless of estrogen receptor status. Oncol Rep. 2008;19:617–625. [PubMed] [Google Scholar]
- 28.de Roos MA, de Bock GH, de Vries J, van der Vegt B, Wesseling J. P53 overexpression is a predictor of local recurrence after treatment for both in situ and invasive ductal carcinoma of the breast. J Surg Res. 2007;140:109–114. doi: 10.1016/j.jss.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Koukourakis MI, Giatromanolaki A, Galazios G, Sivridis E. Molecular analysis of local relapse in high risk breast cancer patients: Can radiotherapy fractionation and time factors make a difference? Br J Cancer. 2003;88:711–717. doi: 10.1038/sj.bjc.6600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zellars RC, Hilsenbeck SG, Clark GM, Allred DC, Herman TS, Chamness GC, Elledge RM. Prognostic value of p53 for local failure in mastectomy-treated breast cancer patients. J. Clin. Oncol. 2000;18:1906–1913. doi: 10.1200/JCO.2000.18.9.1906. [DOI] [PubMed] [Google Scholar]
- 31.Kao KJ, Chang KM, Hsu HC, Huang AT. Correlation of microarray-based breast cancer molecular subtypes and clinical outcomes: implications for treatment optimization. BMC Cancer. 2011;11:143. doi: 10.1186/1471-2407-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]


