Abstract
Gremlin 1 and noggin are inhibitors of bone morphogenetic protein (BMP) signaling. They are vital during early development but their role in adult tissues has remained largely unresolved. The BMP signaling pathway has also been implicated in tumorigenesis, however with emphasis on the role of the ligands and receptors. We performed a concurrent survey of gremlin 1 and noggin protein expression in multiple normal and cancer samples, using immunohistochemistry on tissue microarrays containing 96 samples from 34 different normal organs/tissue sites and 208 samples of 34 different tumor types. In majority of both normal and tumor samples, gremlin 1 and noggin expression was negative or weak. However, normal stomach and skin demonstrated distinct gremlin 1 and noggin expression indicating a role in adult tissues. Likewise, strong expression of both antagonists was detected in Leydig cells of testis. In the tumor panel, the expression patterns were more variable but elevated BMP antagonist expression was detected for the first time in few cases, such as glioblastoma, hepatocellular carcinoma and diffuse B-cell lymphoma for gremlin 1 and renal granular cell tumor and thyroid papillary carcinoma for noggin. Even though gremlin 1 and noggin were not widely expressed in adult tissues, in a subset of organs their expression pattern indicated a potential role in normal tissue homeostasis as well as in malignancies.
Keywords: Gremlin 1, noggin, cancer, normal tissue, immunohistochemistry
Introduction
Gremlin 1 and noggin are bone morphogenetic protein (BMP) antagonists that inhibit BMP signaling [1]. BMPs themselves are extracellular growth factors that were originally identified based on their ability to induce bone formation and are currently known to control various cellular processes during development [2,3]. Their signaling pathway is composed of type I and II transmembrane receptors and cytosolic SMAD proteins, which regulate transcription of BMP target genes [4]. Extracellular antagonists prevent the interaction between BMP ligands and their receptors by binding directly to BMPs [5]. There are several different BMP antagonists that share a common cysteine ring structure and that are further divided into subfamilies based on the ring size (8-10 membered ring) [6,7]. Different antagonists bind BMP ligands with diverse affinities, e.g. noggin inhibits the actions of BMP2, -4, -5, -6, -7, GDF5 and -6 and gremlin 1 inhibits BMP2, -4, and -7 [8,9].
As expected based on the function of BMP ligands, BMP antagonist have also essential roles in bone formation and homeostasis as well as in various developmental processes [10]. Transgenic mice with noggin or gremlin overexpression in bone osteoblasts suffer from bone defects [11,12], whereas knockout of gremlin 1 or noggin gene in mice are both embryonically lethal [13]. Due to the latter fact the function of BMP antagonists in fully developed tissues has been proven difficult to study. Yet BMP antagonists have been linked to different disease states in bone, in fibrotic disorders and in cancer implicating a notable role in adult tissue homeostasis [10]. For example, noggin mutations are found in rare genetic skeletal disorders and gremlin 1 is implicated in diabetic nephropathy and in idiopathic pulmonary fibrosis [10,14,15]. During the last decade BMP antagonist alongside BMP ligands have gained more attention also in the cancer research field.
Expression statuses of gremlin 1 and noggin have been studied in some cancer cell lines and there are few examples of expression data in clinical patient material. Low level expression of noggin protein in combination with high BMP6 and low sclerostin expression associated with poor prognosis in esophageal carcinoma and increased metastasis occurrence in prostate cancer [16,17]. Gremlin 1 is the only antagonist whose expression has been evaluated in different tumor samples concurrently and elevated mRNA levels were detected in carcinomas of the lung, ovary, kidney, breast, colon, and pancreas [18]. According to Sneddon and colleagues [19] gremlin 1 mRNA is expressed particularly in the tumor stromal cells, but not in the corresponding normal tissues. Functionally gremlin 1 and noggin have been more often used as surrogates of BMP action than been in focus themselves. However, there are few mouse xenograft studies showing on a functional level that the BMP antagonists can either promote or inhibit malignant properties. In lung cancer models, noggin was shown to suppress subcutaneous and bone metastatic tumor growth, whereas gremlin 1 increased tumor size [20,21]. In a prostate cancer model, noggin either enhanced or inhibited osteolytic bone metastasis formation [22-24]. In tumors of skin, noggin inhibited the BMP tumor-suppressive activity [25,26]. Taken together, the studies on BMP antagonists in cancer are still scarce but do suggest a possible important role. In this study, we have evaluated the protein expression of gremlin 1 and noggin in 34 normal tissues or organs and in 34 tumor types.
Materials and methods
Tissue arrays
Commercially available multiple organ normal and tumor tissue microarrays (TMA) containing formalin-fixed paraffin-embedded tissue samples with a core diameter of 1-1.5 mm were obtained from US Biomax (Rockville, MD, USA; cat. No. MNO961, MC2082). Information on TNM, clinical stage, and pathology grade were available from the tumor specimens. The normal TMA contained 96 tissue specimens from 34 different types of organs or anatomical sites: adrenal gland, bladder, bone marrow, breast, cerebellum, cerebral cortex, colon, esophagus, eye, fallopian tube, heart, kidney, liver, lung, ovary, pancreas, parathyroid, pituitary gland, placenta, prostate, rectum, skin, small intestine, spinal cord, spleen, stomach, striated muscle, testis, thymus, thyroid, tonsil, ureter, uterine cervix, and uterus endometrium. The tumor TMA contained 208 tumor samples representing the following tumor types: adenocarcinomas of colon, endometrium, esophagus, lung, ovary, pancreas, prostate and stomach, astrocytoma, ductal and lobular breast carcinomas, fibrosarcoma of the skin, glioblastoma, hepatocellular carcinoma, Hodgkin and non-Hodgkin lymphomas, kidney clear cell and granular cell carcinomas, liposarcoma, lung large cell and small cell carcinomas, lymphoma of the spleen, malignant melanoma, ovarian germ cell and stromal tumors, papillary and follicular thyroid carcinomas, seminoma, squamous cell carcinomas of esophagus, lung, skin, uterine cervix, and the head and neck, and transitional cell carcinoma of the bladder.
Immunohistochemistry
Immunohistochemistry was used to evaluate the gremlin 1 and noggin protein expression in both normal and tumor samples. The immunostainings were performed using an automated Lab Vision staining system (Fremont, CA). The TMA slides were first deparaffinized and rehydrated, and pretreated with a PT-Module (Lab Vision, Fremont, CA) at 98°C for 15 min in 50 mM Tris-HCl buffer, pH 9.0 containing 1 mM EDTA. Endogenous peroxidase activity was blocked with H202 for 5 min at RT. The following primary antibodies were used: gremlin 1 (FL-184, 1:100 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), and noggin (1:1000 dilution, Abcam, Cambridge, UK). The staining was visualized with a PowerVision+ polymer kit (Leica Biosystems Newcastle Ltd., Newcastle, UK) and diaminobenzidine was used as chromogen (DABImmPact, Vectorlabs, Burlingame, CA). The tissue sections were counterstained with hematoxylin. A control without the primary antibody showed no staining. To further verify the specificity of the antibodies, immunocytochemical stainings of breast cancer cell lines with high and low/no mRNA expression (data not shown) of the corresponding antagonists were performed and they revealed an expected staining pattern.
The entire tissue core for each sample was evaluated by pathologists, and the staining was classified as negative, weak, moderate or strong based on the staining pattern observed in the majority (at least 75%) of the cells. Data from different tissue compartments, e.g. epithelium and stroma, were recorded separately when applicable. For cancer samples, the same scoring criteria were applied but only tumor cells were evaluated.
Results
Gremlin 1 and noggin protein expression in normal tissues
Gremlin 1 and noggin protein expression was studied first in the normal TMA that contained 34 different organs or tissue sites. The protein expression was classified into four different staining categories: negative, weak, moderate, and strong staining (Figure 1).
Figure 1.
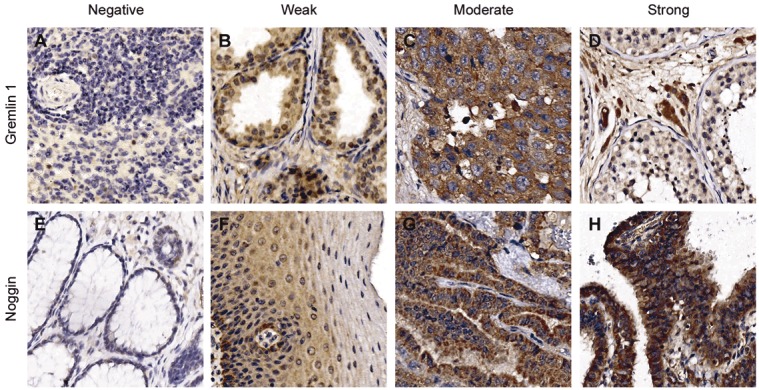
Gremlin 1 and noggin expression in normal and tumor tissues. Images representing the four staining categories are shown for gremlin 1 (A) negative staining in spleen, (B) weak staining in prostate, (C) moderate staining in ovarian dysgerminoma and (D) strong staining of the Leydig cells in testis. E. Negative staining of noggin is shown in rectum, (F) weak in esophagus, (G) moderate in papillary carcinoma of thyroid, and (H) strong staining in fallopian tube.
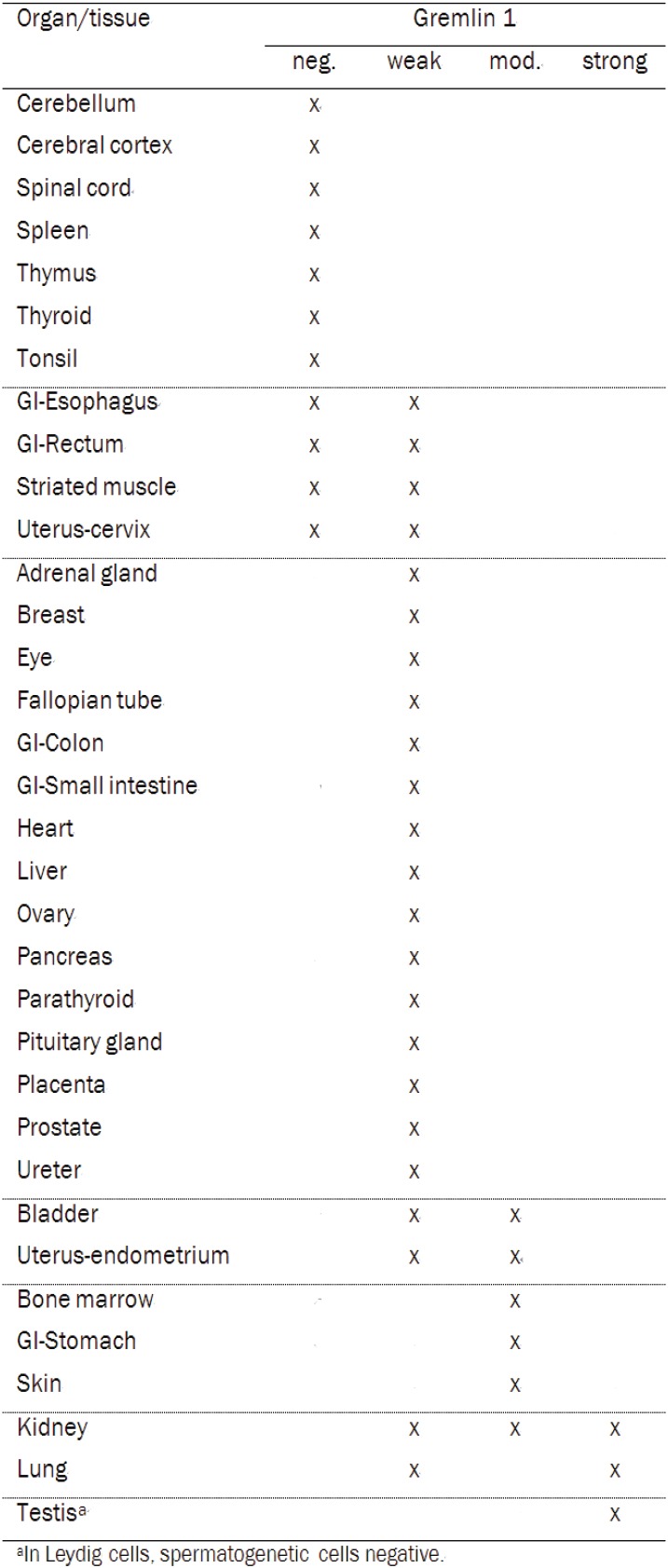
Gremlin 1 expression was negative or weak in 76% of organs or tissue sites (Table 1, Figure 1). Moderate expression was detected altogether in 15% of the tissues and for the most part in the epithelial cells when present (Table 1, Figure 1). In the stomach, gremlin 1 was moderately expressed in the parietal and chief cells of the gastric gland and in the skin in the non-keratinized epithelial cells of epidermis. Moderate gremlin 1 expression was also detected in the bone marrow. Expression pattern was variable between the samples of bladder and endometrium, where weak gremlin 1 staining was also detected. Strong staining was observed in the Leydig cells but not in the spermatogenetic cells of testis (Figure 1). The lung pneumocytes and all cells types of nephron in kidney also demonstrated strong staining, however not in all samples.
Table 1.
Gremlin1 expression in 34 normal organs and tissue sites
|
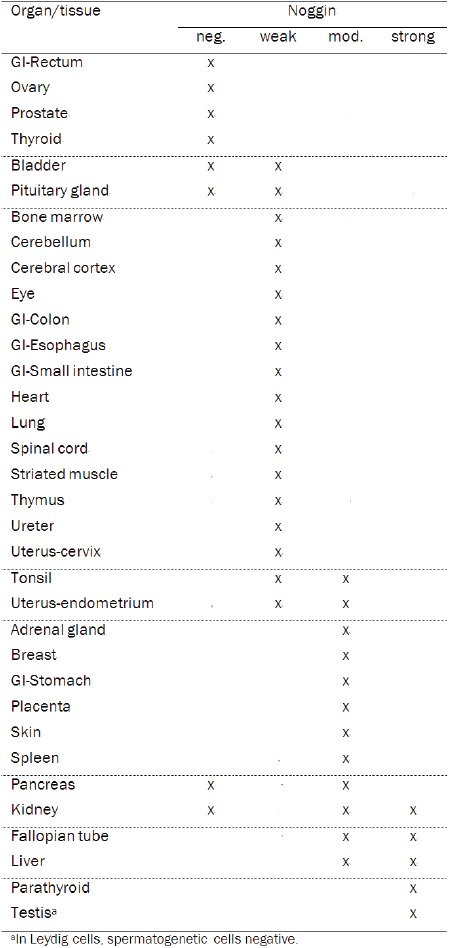
For noggin, the majority of organs or tissues demonstrated negative or weak staining (59%), whereas moderate expression of noggin was detected in 24% of samples (Table 2, Figure 1). In the adrenal gland, moderate expression was particularly detected in the cells of zona reticularis, the inner zone of cortex. In the stomach, moderate noggin staining was observed in the base of the gastric glands, whereas the cells in the neck of the gland and gastric pit stained more weakly. Moderate staining was likewise detected in the syncytiotrophoblasts of placenta, in the epithelial cells of mammary gland and in spleen. In the skin, all epithelial cells including the outermost layer harbored moderate noggin staining. Strong noggin expression was detected in parathyroid gland, and in the Leydig cells but not in the spermatogenetic cells of testis. Strong staining was also detected in some but not all samples of fallopian tube in epithelial cells, and of liver in hepatocytes (Figure 1). Inconsistent staining for noggin was observed in the samples of pancreas and kidney.
Table 2.
Noggin expression in 34 normal organs and tissue sites
|
Gremlin 1 and noggin protein expression in tumor tissues
Next the expression of gremlin 1 and noggin were analyzed in multiple cancers. Gremlin 1 was expressed at weak level in 72% and at moderate level in 22% of the tumor samples (Figure 2). No expression was detected in 6% of samples and none of the samples demonstrated strong expression. In six tumor types gremlin 1 was expressed at moderate level in at least half of the samples: invasive lobular carcinoma of breast, glioblastoma of brain, granular cell tumor of kidney, hepatocellular carcinoma of liver, ovarian dysgerminoma, and diffuse B-cell lymphoma of spleen (Figures 1 and 2). The frequency of low gremlin 1 expression was most evident in the small cell carcinoma of lung.
Figure 2.
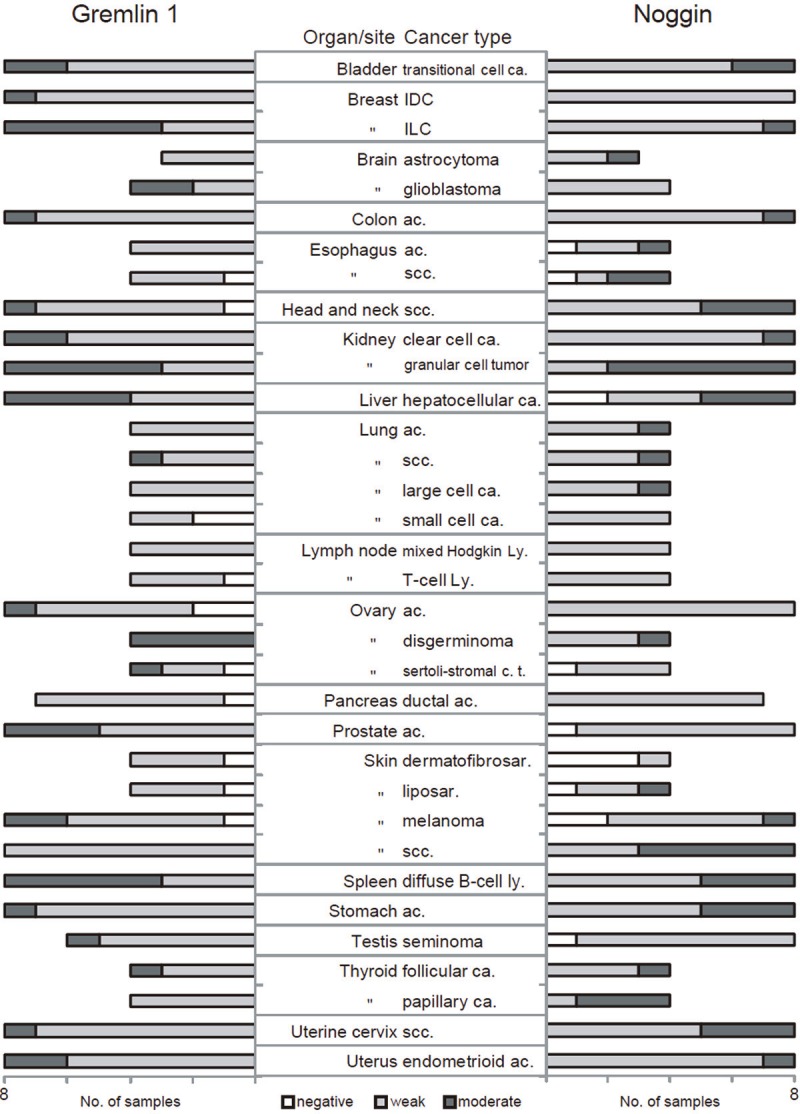
Gremlin1 and noggin expression in 208 tumor samples. White bar denotes negative, light gray bar weak, and dark grey bar moderate gremlin 1 (left) or noggin (right) expression. Abbreviations, ca. carcinoma, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, ac. Adenocarcinoma, scc. squamous cell carcinoma, ly. lymphoma, c.t. cell tumor, sar. sarcoma.
Altogether, noggin was expressed at weak level in 71% and at moderate level in 22% of tumor samples (Figure 2). No expression was detected in 7% of samples and none of the samples demonstrated strong expression. When compared with the overall staining distribution, moderate noggin expression was detected in majority of samples in the case of the squamous cell carcinoma of esophagus and skin, the granular cell tumor of kidney and the papillary carcinoma of thyroid (Figures 1 and 2). In skin dermatofibrosarcoma, no noggin expression was detected in three out of four tumor cases whereas the general frequency of noggin negative samples was notably lower (7%) (Figure 2).
Discussion
BMP antagonists are essential regulatory proteins in development and have been rarely studied in normal adult tissues. Comprehensive analysis in different cancers is likewise currently missing. We have now for the first time shown gremlin 1 and noggin protein expression patterns concurrently in variety of human adult normal tissues and cancer types.
Both gremlin 1 and noggin expression was either negative or weak in the majority of normal and tumor tissues, and none of the tumor samples demonstrated strong expression. However, it is important to note that the patterns of expression across different tissues were not identical with e.g. ovary and prostate being negative for noggin and showing moderate expression of gremlin 1. Similarly, patterns differed in the tumors e.g. frequent moderate noggin expression was detected in papillary carcinoma of thyroid and squamous cell carcinoma of skin that demonstrated weak gremlin 1 expression. Interestingly, strong expression of both antagonists was observed in the Leydig cells of testis, possibly indicating an involvement in sex hormone production.
Moderate gremlin 1 expression was noted in stomach, bone marrow and skin. There are no previous reports of human stomach or bone marrow tissues, but reduced gremlin 1 mRNA expression have been described in non-tumor tissues adjacent to basal cell carcinomas of skin [19]. Gremlin 1 is involved in lung and kidney organogenesis [8] and strong expression in a subset of the samples of these organs suggested a role also in adult tissues. However, increased expression has been previously detected in diabetic nephropathy and in idiopathic pulmonary fibrosis, but not in their corresponding normal tissues[14,15,27].
Noggin is known to be involved in parathyroid gland during organogenesis [28], and now strong expression was demonstrated also in the adult tissue. Previously noggin has also been implicated in gastric epithelial homeostasis [29] and we have shown that BMP4 is strongly expressed in stomach [30]. Here the normal stomach samples exhibited moderate expression of noggin, as well as gremlin 1, thus indicating an important role for BMP signaling in the normal stomach. Moderate expression was detected also in the normal skin thus confirming previous observations [31]. Yet, this is the first time that moderate noggin expression has been reported in normal adult adrenal gland, breast, spleen, fallopian tube and liver.
This study demonstrated for the first time elevated gremlin 1 expression in glioblastoma, hepatocellular carcinoma and diffuse B-cell lymphoma as compared to corresponding normal tissues. Upregulated expression of gremlin 1 was also detected in ovarian dysgerminoma, confirming previous observations [18]. Moderate gremlin 1 expression was observed more often in invasive lobular breast carcinomas, as compared to invasive ductal carcinomas and normal breast. Previous reports have also detected elevated gremlin 1 expression in breast tumors, and interestingly, we have shown that BMP4 and BMP7 are also significantly more often expressed particularly in the lobular cancers [18,30,32]. In the granular cell tumors of kidney, gremlin 1 was detected at moderate level in more than half of the samples, but expression was mostly weak in the clear cell carcinomas that previously have been shown to carry methylation of the corresponding locus [33]. In the lung tumors, gremlin 1 expression was mostly weak despite its reactivation in the fibrotic diseases [15]. Similarly, the different skin tumors demonstrated mostly weak expression, indicating a downregulation of gremlin 1 in cancer as compared to normal tissue. Finally, overall we detected gremlin 1 protein expression in the tumor cells on the contrary to previous report describing gremlin 1 mRNA expression in stromal cells of different tumor types [19].
Noggin has been functionally linked to promotion of skin malignancies [25,26]. We observed frequent moderate level noggin expression in the squamous cell carcinomas of skin as compared to other tumor subtypes, such as melanoma. These data are in good concordance with previous reports on skin SCC [34]. In esophageal squamous cell carcinomas, elevated noggin expression was demonstrated resulting in the same conclusion as Yuen and colleagues [17]. Noggin protein was moderately expressed in normal breast tissues, but only weak expression levels were detected in tumor samples. Previously, microarray based patient data indicated that noggin expression in breast cancer metastatic samples was more frequent in bone metastases as compared to brain, lung or liver metastases, but expression level in the primary tumors did not influence the metastasis potential [35]. Noggin has also been in focus in prostate cancer, where it has been functionally linked to bone metastatic process [20,24]. However in concordance with our results, no major differences have been detected in noggin expression between normal and cancerous samples of prostate [16,36]. Finally, prior to this study there were no previous reports showing noggin expression in renal granular cell tumor and thyroid papillary carcinoma.
To conclude, BMP antagonists are not widely expressed in normal or cancerous tissues as compared to frequent and strong BMP4 expression in the same panel of samples [30]. Even though gremlin 1 and noggin are not considered generally to be reactivated in diseased states, there are however some very interesting examples in certain organs that defend their place also in adult tissues and in cancer. Gremlin 1 and noggin are detected in Leydig cells of testis, stomach and skin implying an involvement in the normal function of these organs. Likewise their elevated expression is detected in some tumor types indicating potential importance of BMP signaling in cancer.
Acknowledgements
The skillful technical assistance of Mrs. Sari Toivola is greatly appreciated.
References
- 1.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- 2.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 3.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 4.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 5.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 6.Avsian-Kretchmer O, Hsueh AJ. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol Endocrinol. 2004;18:1–12. doi: 10.1210/me.2003-0227. [DOI] [PubMed] [Google Scholar]
- 7.Rider CC, Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J. 2010;429:1–12. doi: 10.1042/BJ20100305. [DOI] [PubMed] [Google Scholar]
- 8.Costello CM, Cahill E, Martin F, Gaine S, McLoughlin P. Role of gremlin in the lung: development and disease. Am J Respir Cell Mol Biol. 2010;42:517–523. doi: 10.1165/rcmb.2009-0101TR. [DOI] [PubMed] [Google Scholar]
- 9.Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011;43:478–481. doi: 10.1016/j.biocel.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Walsh DW, Godson C, Brazil DP, Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends Cell Biol. 2010;20:244–256. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Devlin RD, Du Z, Pereira RC, Kimble RB, Economides AN, Jorgetti V, Canalis E. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972–1978. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- 12.Gazzerro E, Pereira RC, Jorgetti V, Olson S, Economides AN, Canalis E. Skeletal overexpression of gremlin impairs bone formation and causes osteopenia. Endocrinology. 2005;146:655–665. doi: 10.1210/en.2004-0766. [DOI] [PubMed] [Google Scholar]
- 13.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 14.Dolan V, Murphy M, Sadlier D, Lappin D, Doran P, Godson C, Martin F, O’Meara Y, Schmid H, Henger A, Kretzler M, Droguett A, Mezzano S, Brady HR. Expression of gremlin, a bone morphogenetic protein antagonist, in human diabetic nephropathy. Am J Kidney Dis. 2005;45:1034–1039. doi: 10.1053/j.ajkd.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Myllarniemi M, Vuorinen K, Pulkkinen V, Kankaanranta H, Aine T, Salmenkivi K, Keski-Oja J, Koli K, Kinnula V. Gremlin localization and expression levels partially differentiate idiopathic interstitial pneumonia severity and subtype. J Pathol. 2008;214:456–463. doi: 10.1002/path.2300. [DOI] [PubMed] [Google Scholar]
- 16.Yuen HF, Chan YP, Cheung WL, Wong YC, Wang X, Chan KW. The prognostic significance of BMP-6 signaling in prostate cancer. Mod Pathol. 2008;21:1436–1443. doi: 10.1038/modpathol.2008.94. [DOI] [PubMed] [Google Scholar]
- 17.Yuen HF, McCrudden CM, Grills C, Zhang SD, Huang YH, Chan KK, Chan YP, Wong ML, Law S, Srivastava G, Fennell DA, Dickson G, El-Tanani M, Chan KW. Combinatorial use of bone morphogenetic protein 6, noggin and SOST significantly predicts cancer progression. Cancer Sci. 2012;103:1145–1154. doi: 10.1111/j.1349-7006.2012.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namkoong H, Shin SM, Kim HK, Ha SA, Cho GW, Hur SY, Kim TE, Kim JW. The bone morphogenetic protein antagonist gremlin 1 is overexpressed in human cancers and interacts with YWHAH protein. BMC Cancer. 2006;6:74. doi: 10.1186/1471-2407-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro AE, Brown PO. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci U S A. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feeley BT, Liu NQ, Conduah AH, Krenek L, Roth K, Dougall WC, Huard J, Dubinett S, Lieberman JR. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and Rank: Fc administration. J Bone Miner Res. 2006;21:1571–1580. doi: 10.1359/jbmr.060706. [DOI] [PubMed] [Google Scholar]
- 21.Kim M, Yoon S, Lee S, Ha SA, Kim HK, Kim JW, Chung J. Gremlin-1 induces BMP-independent tumor cell proliferation, migration, and invasion. PLoS One. 2012;7:e35100. doi: 10.1371/journal.pone.0035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feeley BT, Krenek L, Liu N, Hsu WK, Gamradt SC, Schwarz EM, Huard J, Lieberman JR. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006;38:154–166. doi: 10.1016/j.bone.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Virk MS, Petrigliano FA, Liu NQ, Chatziioannou AF, Stout D, Kang CO, Dougall WC, Lieberman JR. Influence of simultaneous targeting of the bone morphogenetic protein pathway and RANK/RANKL axis in osteolytic prostate cancer lesion in bone. Bone. 2009;44:160–167. doi: 10.1016/j.bone.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Secondini C, Wetterwald A, Schwaninger R, Thalmann GN, Cecchini MG. The role of the BMP signaling antagonist noggin in the development of prostate cancer osteolytic bone metastasis. PLoS One. 2011;6:e16078. doi: 10.1371/journal.pone.0016078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu MY, Rovinsky SA, Lai CY, Qasem S, Liu X, How J, Engelhardt JF, Murphy GF. Aggressive melanoma cells escape from BMP7-mediated autocrine growth inhibition through coordinated Noggin upregulation. Lab Invest. 2008;88:842–855. doi: 10.1038/labinvest.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharov AA, Mardaryev AN, Sharova TY, Grachtchouk M, Atoyan R, Byers HR, Seykora JT, Overbeek P, Dlugosz A, Botchkarev VA. Bone morphogenetic protein antagonist noggin promotes skin tumorigenesis via stimulation of the Wnt and Shh signaling pathways. Am J Pathol. 2009;175:1303–1314. doi: 10.2353/ajpath.2009.090163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang Q. Bone morphogenetic protein-7 and Gremlin: New emerging therapeutic targets for diabetic nephropathy. Biochem Biophys Res Commun. 2009;383:1–3. doi: 10.1016/j.bbrc.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 28.Patel SR, Gordon J, Mahbub F, Blackburn CC, Manley NR. Bmp4 and Noggin expression during early thymus and parathyroid organogenesis. Gene Expr Patterns. 2006;6:794–799. doi: 10.1016/j.modgep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Shinohara M, Mao M, Keeley TM, El-Zaatari M, Lee HJ, Eaton KA, Samuelson LC, Merchant JL, Goldenring JR, Todisco A. Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology. 2010;139:2050–2060. e2. doi: 10.1053/j.gastro.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alarmo EL, Huhtala H, Korhonen T, Pylkkanen L, Holli K, Kuukasjarvi T, Parkkila S, Kallioniemi A. Bone morphogenetic protein 4 expression in multiple normal and tumor tissues reveals its importance beyond development. Mod Pathol. 2013;26:10–21. doi: 10.1038/modpathol.2012.128. [DOI] [PubMed] [Google Scholar]
- 31.Singh SK, Abbas WA, Tobin DJ. Bone morphogenetic proteins differentially regulate pigmentation in human skin cells. J Cell Sci. 2012;125:4306–4319. doi: 10.1242/jcs.102038. [DOI] [PubMed] [Google Scholar]
- 32.Alarmo EL, Korhonen T, Kuukasjarvi T, Huhtala H, Holli K, Kallioniemi A. Bone morphogenetic protein 7 expression associates with bone metastasis in breast carcinomas. Ann Oncol. 2008;19:308–314. doi: 10.1093/annonc/mdm453. [DOI] [PubMed] [Google Scholar]
- 33.van Vlodrop IJ, Baldewijns MM, Smits KM, Schouten LJ, van Neste L, van Criekinge W, van Poppel H, Lerut E, Schuebel KE, Ahuja N, Herman JG, de Bruine AP, van Engeland M. Prognostic significance of Gremlin1 (GREM1) promoter CpG island hypermethylation in clear cell renal cell carcinoma. Am J Pathol. 2010;176:575–584. doi: 10.2353/ajpath.2010.090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerhart J, Hayes C, Scheinfeld V, Chernick M, Gilmour S, George-Weinstein M. Myo/Nog cells in normal, wounded and tumor-bearing skin. Exp Dermatol. 2012;21:466–468. doi: 10.1111/j.1600-0625.2012.01503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarragona M, Pavlovic M, Arnal-Estape A, Urosevic J, Morales M, Guiu M, Planet E, Gonzalez-Suarez E, Gomis RR. Identification of NOG as a specific breast cancer bone metastasis-supporting gene. J Biol Chem. 2012;287:21346–21355. doi: 10.1074/jbc.M112.355834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haudenschild DR, Palmer SM, Moseley TA, You Z, Reddi AH. Bone morphogenetic protein (BMP)-6 signaling and BMP antagonist noggin in prostate cancer. Cancer Res. 2004;64:8276–8284. doi: 10.1158/0008-5472.CAN-04-2251. [DOI] [PubMed] [Google Scholar]


