Abstract
A 70-year-old woman visited a local hospital complaining of a nodulein the right breast, present since 1 month. She was referred to our hospital for further evaluation. Following mammotome (MMT) biopsy, the nodule was diagnosed as myxoid/round cell liposarcoma. She underwent total mastectomy of the right breast. Histological analysis indicated that the tumor was almost entirely composed of proliferating small round mesenchymal cells in amyxoid matrix background with capillary-like vessels with partial necrosis (<10%). Immunohistochemically, p53 positive cells were seen focally (<1%) only, and the Ki-67 labeling index was approximately 20%. Sincethe surgical margin was histologically positive despite pathologic findings of high-grade malignancy, adjuvant treatment involving local radiation therapy (60Gy) was administered. The patient was free from any symptoms of local recurrence and metastases 1 year and 8 months after surgery.
Keywords: Myxoid liposarcoma, round cell component, breast
Introduction
Breast sarcomas are malignant tumors arising from the mesenchymal tissue of mammary glands and are regarded as extremely rare, representing approximately 0.1–0.3% of all malignant breast tumors [1-3]. Microscopically, most breast sarcomas comprisemalignant fibrous histiocytomas, followed by liposarcomas and fibrosarcomas [3]. Liposarcoma of the breast was first described by Neumann in 1862 [4] and accounts for 3–24% of all breast sarcomas [3].
Liposarcoma is one of the most common soft tissue sarcomas in adults and frequently arises in the superficial and deep soft tissues of the extremities and the retroperitoneum. Although liposarcoma shows variable histologic features, currently, it is classified into 4 major histologic subtypes: well-differentiated, myxoid/round cell, dedifferentiated, and pleomorphic [5]. More than 95% myxoid/round cell liposarcomas have either the characteristic t(12;16)(q13;p11) or t(12;22)(q13;q12) chromosomal translocationthat results in thefusion of TLS-CHOP or EWS-CHOP [6-10]. The fusion proteins derived from both of these translocations have been shown to play an important role in the oncogenesis of myxoid/round cell liposarcoma. In addition, identification of the fusion genes associated with specific tumor types aids molecular diagnosis ofvarious types of sarcomas, including myxoid/round cell liposarcoma [11].
This report describes an extremely rare case of myxoid breast liposarcoma that was successfully treated.
Case report
Clinical history
A 70-year-old woman visited a local hospital in February 2011, complaining of a nodulein the right breast, present since 1 month. She was referred to our hospital for further evaluation in July 2011.
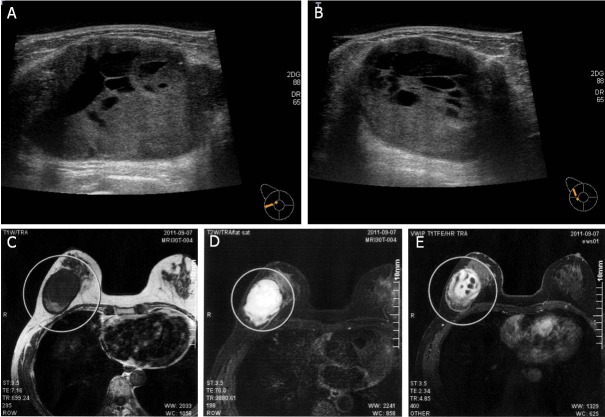
Ultrasonography revealed a relatively well-demarcated lobulated mass of 51 × 40mm withcystic change (Figure 1A, 1B). Aslightly high-intensity lesion on T1-weighted image and high-intensity lesion on T2-weigted image were observed on magnetic resonance imaging (MRI) (Figure 1C, 1D). This lesion also showed highintensity on diffusion-weighted imaging (DWI), suggesting the presence of cystic change (Figure 1E).
Figure 1.
The breast tumor as observed on ultrasonography and magnetic resonance imaging. A, B: Ultrasonography revealed a relatively well-demarcated lobulated mass of 51 × 40mm with cystic change. C, D: MRI indicating as lightly high intensity lesion on a T1-weighted image and a high intensity lesion on a T2-weighted image. E: High-intensity tumor on diffusion weighted imaging (DWI), reflecting the presence of a lesion with cystic change.
Following MMT biopsy, the tumor was diagnosed as a myxoid/round cell liposarcoma. The patient underwent total mastectomy of the right breast.
Because the tumor was pathologically diagnosed as amyxoid liposarcoma, and the surgical margin was histologically considered positive, adjuvant treatment involvinglocal radiation therapy (60Gy) was administered. During the radiation therapy, the patient complained of dizziness. MRI scan indicated the presence of a tumor at the left cerebellopontine angle that had weak low intensity on a T1-weighted image and weak high intensity on T2-weighted image. In view of the clinical history of myxoid liposarcoma, a metastatic tumor or meningioma were also suspected. The tumor was successfully resected and histologically diagnosed as a meningioma with no evidence of metastatic myxoid liposarcoma.
The patientdid not experience any symptoms of local recurrence and metastases 1 year and 8 months after surgery.
Pathological findings
The gross section showed a yellowish tumor with focal cystic change (Figure 2). This tumor was histologically almost entirely composed of proliferating small round mesenchymal cells in amyxoid matrix background with capillary-like vessels (Figure 3A, 3B). Mitosis was frequently seen (Figure 3C), and lipoblasts were scattered throughout the tumor (Figure 3D). Partial necrosis was also noted (<10%). Immunohistochemical staining was also performed using the streptavidin- biotinmethod with p53 (Clone: DO-7, Dako) and Ki-67 (Clone: MIB-1, Dako) antibodies. Immunohistochemically, p53 positive cells were seen focally (<1%) only, and the Ki-67 labeling index was approximately 20% (Figure 3E, 3F).
Figure 2.
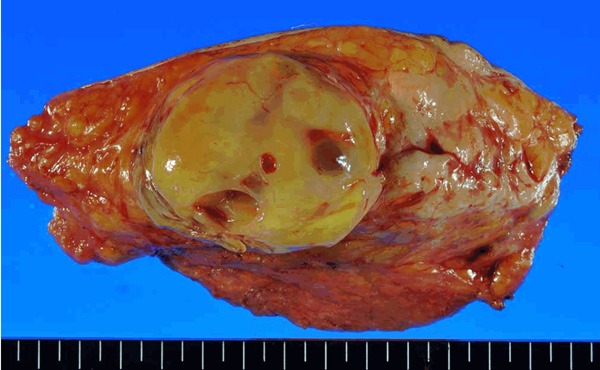
Gross section of the tumor shows a well-demarcated yellowish tumor with focal cystic change.
Figure 3.
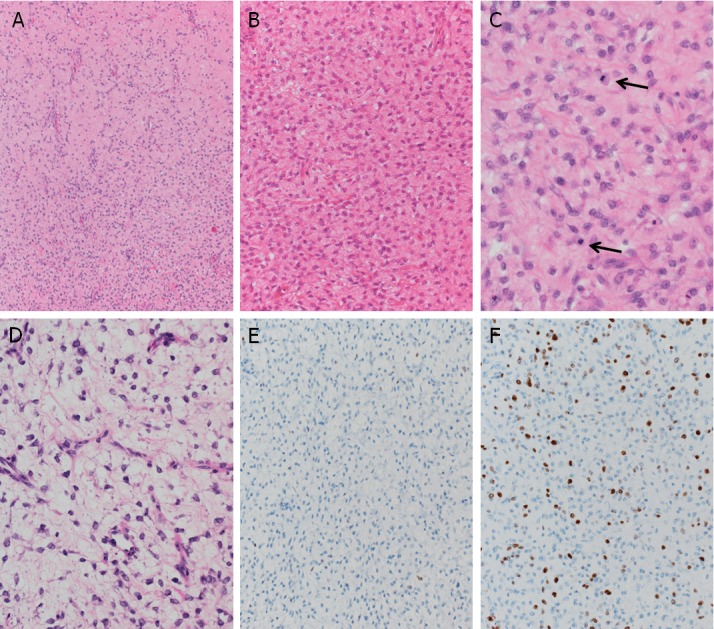
Histologic features of the breast tumor. A, B: The tumor was histologically almost entirely composed of proliferating small round mesenchymal cells in amyxoid matrix background with capillary-like vessels. C: Frequently seen mitosis (arrows). D: Lipoblasts scattered throughout the tumor. E: p53-positive cells seen only focally (<1%). F: The Ki-67 labeling index was approximately 20%.
Detection of fusion transcript
RNA extraction from paraffin-embedded tissue was performed as previously described [12]. The primer pairs used to identify the chimeric fusion genes are listed in Table 1 [11]. After polymerase chain reaction (PCR), an aliquot of the PCR product was electrophoresed on a 2% agarose gel and stained with ethidium bromide. As a positive control to show the integrity of mRNA in the sample, PCR of the ubiquitously expressed GAPDH gene transcript was performed. However, neither TLS-CHOP nor EWS-CHOP was detected in this case, although the integrity of the mRNA as assessed by the expression of GAPDH was confirmed.
Table 1.
Primer sequences
| PCR product size | ||
|---|---|---|
| TLS-CHOP type1, 2-F | GACAGCAGAACCAGTACAACAGCAG | TLS-CHOP type1: 379bp |
| TLS-CHOP type1, 2-R | GCTTTCAGGTGTGGTGATGTATGAAG | TLS-CHOP type1: 103bp |
| TLS-CHOP type3-F | gaagtgaccgtggtggcttcaa | 208bp |
| TLS-CHOP type3-R | ggcaagctggtctgatgcct | |
| TLS-CHOP type4-F | cctcagggctatggacagcagaa | 208bp |
| TLS-CHOP type4-R | ggctggaacaagctccatgtagc | |
| TLS-CHOP type5-F | gcagtcctcctaccctggct | 106+αbp |
| TLS-CHOP type5-R | gctgctttcaggtgtggtgat | |
| TLS-CHOP type8-F | cccctaaaccagatggccca | 190bp |
| TLS-CHOP type8-R | ggcaagctggtctgatgcct | |
| TLS-CHOP type9-F | acggacacttcaggctatgg | 159bp |
| TLS-CHOP type9-R | ctggaatacagccacatctgtt | |
| EWS-CHOP-F | tggatcctacagccaagctc | EWS-CHOP type1: 111bp |
| EWS-CHOP-R | gctgctttcaggtgtggtgat | EWS-CHOP type1: 363bp |
| GAPDH-F | GAAGGTGAAGGTCGGAGTC | 226bp |
| GAPDH-R | GAAGATGGTGATGGGATTTC | |
Discussion
Sarcomas arising from other sites have shown to have similar clinical history and biological behavior to breast sarcomas [1], suggesting that similar therapeutic protocols can be used. Postoperative radiotherapy has proved to alleviate local recurrence, especially when involved margins show an infiltrative growth pattern [13]. Furthermore, radiotherapy has shown to be effective in preventing local recurrence and should be delivered as a neoadjuvant therapy [14]. On the other hand, the impact of chemotherapy on patients with primary extremity soft tissue sarcomas remains controversial; however, the chemotherapeutic effect on myxoid/round cell liposarcoma has been gradually shown [15,16]. Patients who received ifosfamide-based chemotherapy showed significant improvement with respect to survival compared to those who did not receive chemotherapy [15]. In addition, neoadjuvant trabectedin has been demonstratedto be effective in patients with locally advanced myxoid liposarcoma [16].
In this case, surgical resection was performed without neoadjuvant preoperative chemotherapy, and the surgical margin of the resected specimen was considered positive. Postoperative radiotherapy was performed in this case to prevent local recurrence. Adverse prognostic factors in localized soft tissue myxoid liposarcoma include a high-histological grade >10% round cell component, necrosis >5%, and overexpression of p53 >10% of positive cells [17]. In addition, p53 overexpression is relatively rare (5–30%) in myxoid liposarcoma [17-19] and tends to be seen in conjunction with TLS-CHOP type 2 fusion [17]. In this case, extensive areas with round cell component were observed; although p53 overexpression was not seen and only focal (<5%) necrosis was observed.
Thus far, at least 9 different types of TLS-CHOP and 2 types of EWS-CHOP fusion genes have been reported, and either can be detected in almost all myxoid liposarcoma cases [10,11,17]. However, neither of the fusion geneswere detected in this case.
The patient is undergoing follow-up by close examination, and thus far, there is no evidence of local recurrence or metastasis 1 year and 6 months after surgery.
Acknowledgements
This work was supported in part by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Science, Sports and Culture (#23590434 to TS), Tokyo, Japan.
Disclosure of conflict of interest
The authors declare no conflicts of interest.
References
- 1.Adem C, Reynolds C, Ingle JN, Nascimento AG. Primary breast sarcoma: Clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer. 2004;91:237–241. doi: 10.1038/sj.bjc.6601920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGregor JK. Liposarcoma of the breast. Case report and review of the literature. Can Med Assoc J. 1960;82:781–783. [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard SG, Marks PV, Temple LN, Thompson HH. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer. 1990;66:941–944. doi: 10.1002/1097-0142(19900901)66:5<941::aid-cncr2820660522>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Neumann E. BeitragezurCasuistik der Brustdrusengeschwutste. Virchows Arch Path Anat. 1862;24:316–328. [Google Scholar]
- 5.Enzinger FM, Weiss SW. Liposarcoma. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors. 3rd ed. St Louis: Mosby; 1955. pp. 431–466. [Google Scholar]
- 6.Aman P, Ron D, Mandahl N, Fioretos T, Heim S, Arheden K, Willén H, Rydholm A, Mitelman F. Rearrangement of the transcription factor gene CHOP in myxoid liposarcoma with t(12;16)(q13;p11) Genes Chromosomes Cancer. 1992;5:278–285. doi: 10.1002/gcc.2870050403. [DOI] [PubMed] [Google Scholar]
- 7.Knight JC, Renwick PJ, Dal Cin P, Van den Berghe H, Fletcher CDM. Translocation t(12;16)(q13;p11) in myxoid liposarcoma and round cell liposarcoma: molecular and cytogenetic analysis. Cancer Res. 1995;55:24–27. [PubMed] [Google Scholar]
- 8.Kuroda M, Ishida T, Horiuchi H, Kida N, Uozaki H, Takeuchi H, Tsuji K, Imamura T, Mori S, Machinami R, et al. Chimeric TLS/FUS-CHOP gene expression and the heterogeneity of its junction in human myxoid and round cell liposarcoma. Am J Pathol. 1995;147:1221–1227. [PMC free article] [PubMed] [Google Scholar]
- 9.Panagopoulos I, Hoglund M, Mertens F, Mandahl N, Mitelman F, Aman P. Fusion of the EWS and CHOP genes in myxoid liposarcoma. Oncogene. 1996;12:489–494. [PubMed] [Google Scholar]
- 10.Panagopoulos I, Mertens F, Isaksson M, Mandahl N. A novel FUS/CHOP chimera in myxoid liposarcoma. Biochem Biophys Res Commun. 2000;279:838–845. doi: 10.1006/bbrc.2000.4026. [DOI] [PubMed] [Google Scholar]
- 11.Hisaoka M, Tsuji S, Morimitsu Y, Hashimoto H, Shimajiri S, Komiya S, Ushijima M. Detection of TLS/FUS-CHOP fusion transcripts in myxoid and round cell liposarcoma by nested reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Diagn Mol Pathol. 1998;7:96–101. doi: 10.1097/00019606-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji S, Hisaoka M, Morimitsu Y, Hashimoto H, Shimajiri S, Komiya S, Ushijima M, Nakamura T. Detection of SYT-SSX fusion transcripts in synovial sarcoma by reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Am J Pathol. 1998;153:1807–1812. doi: 10.1016/S0002-9440(10)65695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari A, Besana-Ciani I, Rovera F, Siesto G, Dionigi G, Boni L, Dionigi R. An unusual case of breast liposarcoma with liver metastases: The role of radical surgery. Breast J. 2007;13:324–5. doi: 10.1111/j.1524-4741.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 14.Moreau LC, Turcotte R, Ferquson P, Wunder J, Clarkson P, Masri B, Isler M, Dion N, Werier J, Ghert M, Deheshi B, Canadian Orthopaedic Oncology Society (CANOOS) Myxoid/round cell liposarcoma (MRCLS) revisited: an analysis of 418 primarily managed cases. Ann Surg Oncol. 2012;19:1081–1088. doi: 10.1245/s10434-011-2127-z. [DOI] [PubMed] [Google Scholar]
- 15.Eilber FC, Eilber FR, Eckardt J, Rosen G, Riedel E, Maki RG, Brennan MF, Singer S. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg. 2004;240:686–697. doi: 10.1097/01.sla.0000141710.74073.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gronchi A, Bui BN, Bonvalot S, Pilotti S, Ferrari S, Hohenberger P, Hohl RJ, Demetri GD, Le Cesne A, Lardelli P, Perez I, Nieto A, Tercero JC, Alfaro V, Tamborini E, Blay JY. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol. 2012;23:771–776. doi: 10.1093/annonc/mdr265. [DOI] [PubMed] [Google Scholar]
- 17.Antonescu CR, Tschernyavsky SJ, Decuseara R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum JR, Ladanyi M. Prognostic impact of TP53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977–3987. [PubMed] [Google Scholar]
- 18.Pilotti S, Lavarino C, Mezzelani A, Della Torre C, Minoletti F, Sozzi G, Azzarelli A, Rilke F, Pierotti MA. Limitedrole of TP53 and TP53-related genes in myxoid liposarcoma. Tumori. 1998;84:571–577. doi: 10.1177/030089169808400512. [DOI] [PubMed] [Google Scholar]
- 19.Dei Tos AP, Piccinin S, Doglioni C, Vukosavljevic T, Mentzel T, Boiocchi M, Fletcher CDM. Molecular aberrations of the G1-S checkpoint in myxoid and round cell liposarcoma. Am J Pathol. 1997;151:1531–1539. [PMC free article] [PubMed] [Google Scholar]