Abstract
Invasive lobular carcinoma (ILC) is a distinct type of breast carcinoma and represents 5-15% of invasive breast carcinomas in female. However, the occurrence of ILC is exceptional in male breast, and the incidence is 1.5-1.9% of male breast carcinomas. Herein, we report a case of pleomorphic lobular carcinoma in a male breast. A 76-year-old Japanese male with a history of treatment with a progestational agent for prostate cancer presented with a right breast tumor. Magnetic resonance imaging showed gynecomastia of bilateral breasts and an irregular-shaped nodule in his right breast. Histopathological study revealed infiltrative neoplastic growth of discohesive tumor cells arranged in single-filed linear cords or trabeculae. These neoplastic cells had variable-sized large nuclei containing occasional nucleoli. Immunohistochemically, these tumor cells lacked E-cadherin expression. Accordingly, an ultimate diagnosis of pleomorphic lobular carcinoma was made. This is the third documented case of pleomorphic lobular carcinoma of male breast. Our analyses of the clinicopathological features of this type of tumor revealed that patients were middle-aged or elderly men, and all cases were free from lymph node metastases or recurrence. Gynecomastia and a history of hormonal agent intake were present only in the current case. The most commonly proposed risk factor for the development of male breast cancer is elevated level of estrogen, and a possible link between the development of male breast cancer and estrogen therapy for prostate cancer has been suggested. The clinicopathological features of ILC of male breast remains unclear; therefore, additional studies are needed to clarify them.
Keywords: Lobular carcinoma, pleomorphic variant, male breast
Introduction
Male breast cancer is an uncommon neoplasm, accounting for 0.6% of all breast carcinomas, and < 1% of all malignancies in men [1,2]. Both carcinoma in situ and invasive carcinoma can occur in the male breast. Ductal carcinoma in situ has been reported in up to 10% of all breast carcinoma in males, whereas lobular carcinoma in situ is extremely rare [1]. Invasive ductal carcinoma of no special type is the most common type of male breast carcinoma. Invasive papillary carcinoma is more common in males than in females, accounting for 2-4% of male breast cancers [1,2]. Invasive lobular carcinoma (ILC), characterized histopathologically by the organization of noncohesive carcinoma cells that are either individually dispersed or arranged in a single-filed linear pattern, occurring in male breast is very rarely reported [2-6]. Herein, we report a case of pleomorphic lobular carcinoma occurring in a male breast and review the clinicopathological features of ILC in a male breast.
Case report
A 76-year-old Japanese male with a history of treatment with a progestational agent for prostatic carcinoma for 10 years presented with a right breast tumor. Physical examination revealed gynecomastia of bilateral breasts and a relatively well-circumscribed firm nodule in the subareolar region of his right breast. Mammography demonstrated a dense mass with spicula and calcification in the right breast. Magnetic resonance imaging also showed gynecomastia of bilateral breasts and an irregular-shaped nodule, measuring 30 x 25 mm in diameter, in the subareolar region of his right breast (Figure 1). Biopsy from the nodule revealed invasive carcinoma, thus, mastectomy with sentinel lymph node dissection was subsequently performed. Systemic surveillance failed to detect other tumorous lesions including in the gastrointestinal tract.
Figure 1.
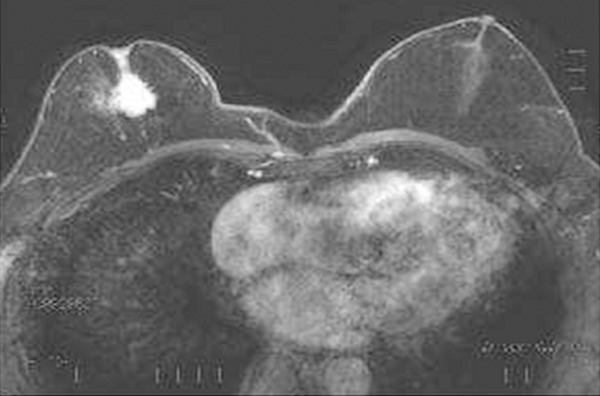
Magnetic resonance imaging shows an irregular-shaped nodule in the right breast and gynecomastia of the bilateral breasts.
Histopathological study of the resected breast tissue revealed infiltrative neoplastic growth composed of discohesive tumor cells arranged in single-filed linear cords or trabeculae. These tumor cells had variable-sized large nuclei containing occasional nucleoli, and pale to slightly eosinophilic cytoplasm (Figure 2A). Mitotic figures were observed (2/10 high-power fields). Neither tubule formation nor intracytoplasmic lumen was observed. In situ component was noted. The tumor had invaded into the surrounding fatty tissue and skin. In the juxtaposing breast tissue, gynecomastia, which is characterized by an increased number of ducts showing dilatation surrounded by a loose myxoid stroma, was observed (Figure 2B). Sentinel lymph nodes had no metastatic carcinoma.
Figure 2.
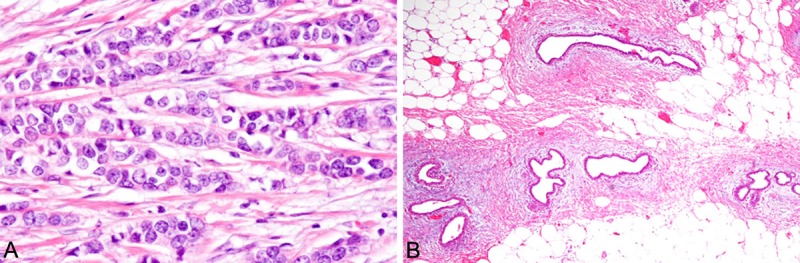
Histopathological findings of the resected breast. A. Linear cord of atypical epithelial cells. The neoplastic cells have variable-sized large round nuclei with occasional nucleoli. HE, x 400. B. Gynecomastia is observed in the surrounding breast tissue. HE, x 100.
Immunohistochemically, the neoplastic cells lacked E-cadherin expression (Figure 3). Gross cystic disease fluid protein-15 was not expressed. Estrogen receptor was expressed in all tumor cells, but progesterone receptor was not expressed. HER2 score was 1+. Ki-67 labeling index was 12.7%.
Figure 3.
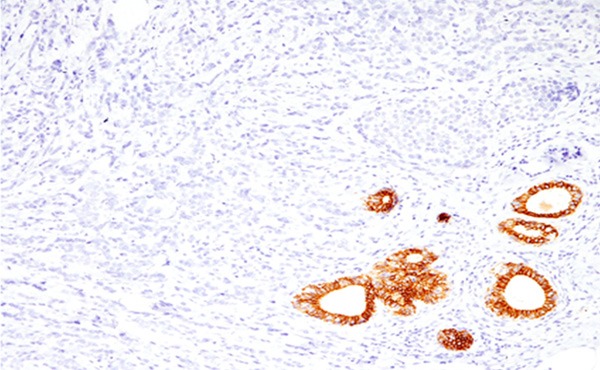
Immunohistochemical findings of the breast nodule. E-cadherin is not expressed in the neoplastic cells (Note: non-neoplastic ductal cells are positive for E-cadherin). x 200.
According to these results, an ultimate diagnosis of pleomorphic lobular carcinoma in a male breast was made.
Discussion
ILC is a distinct type of breast carcinoma based on its characteristic histopathological features and represents 5-15% of invasive breast carcinoma in females. However, the occurrence of ILC is exceptional in male breast because the adult male breast consists primarily of branching ducts and terminal ductules and usually lacks lobular development. The incidence of ILC is reported to be 1.5-1.9% of male breast carcinomas [2,3]. It has been suggested that negative immunostaining for E-cadherin should be required for a diagnosis of lobular carcinoma in males. However, E-cadherin expression was not examined in most of the previously reported cases of ILC of male breast, and only a few cases of ILC with negative E-cadherin expression have been documented [7-10]. Therefore, the incidence of ILC of male breast may be lower than previously reported.
Pleomorphic lobular carcinoma is a distinct variant of ILC, which is characterized histopathologically by a greater degree of cellular atypia and pleomorphism and a higher mitotic rate than classic ILC. This variant was first defined by Eusebi et al. in 1992 [11], and they revealed that this variant showed an aggressive clinical course as compared to classical ILC [11]. Albeit extremely rare, this variant has also been reported in the male breast. Only two cases with clinicopathological features have been documented [9,10], although Tawail et al. reported that ILC represents 6% of male breast carcinomas in Lebanon and all of them were the pleomorphic variant (the detailed clinicopathological features were not available) [6]. Table 1 summarizes the clinicopathological features of the previously reported cases of pleomorphic lobular carcinoma of the male breast as well as the present case. All cases were middle-aged or elderly men, and they were free from lymph node metastases or disease recurrence (follow-up period ranges from 2 months to 2 years).
Table 1.
Clinicopathological features of pleomorphic lobular carcinoma of the male breast
| Age | Gynecomastia | Hormonal agents | E-cadherin | Lymph node metastasis | Outcome | Reference | |
|---|---|---|---|---|---|---|---|
| Case 1 | 44 | - | - | - | - | Free from disease for 2 years | 9 |
| Case 2 | 55 | - | - | - | - | Free from disease for 1 year | 10 |
| Present case | 76 | + | + | - | - | Free from disease for 2 months |
The most commonly proposed risk factor for the development of male breast cancer is elevated level of estrogen, regardless of whether it is from an exogenous or endogenous source. It has been recognized that the incidence of breast cancer is higher in patients with Klinefelter syndrome because the testes are atrophic in this condition, resulting in hyperestrogenism [11]. Moreover, a possible link between the development of male breast cancer and estrogen therapy for prostate cancer has been suggested [12]. The present patient had received progestational agent for prostate cancer, resulting in gynecomastia, although the previously reported two cases had no history of hormonal therapy and gynecomastia. The incidence of male breast cancer is increasing, however, the clinicopathological features of ILC of male breast remains unclear. Therefore, additional studies are needed to clarify them.
References
- 1.Reiner A, Badve S. Carcinoma of the male breast. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. World Health Organization Classification of Tumours of the Breast. Lyon: IARC Press; 2012. pp. 168–169. [Google Scholar]
- 2.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 3.Goss PE, Reid C, Pintilie M, Lim R, Miller B. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955-1996. Cancer. 1999;85:629–639. doi: 10.1002/(sici)1097-0142(19990201)85:3<629::aid-cncr13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Erhan Y, Zekioglu O, Erhan Y. Invasive lobular carcinoma of the male breast. Can J Surg. 2006;49:365–366. [PMC free article] [PubMed] [Google Scholar]
- 5.Briest S, Vang R, Terrell K, Emens L, Lange JR. Invasive lobular carcinoma of the male breast: a rare histology in an uncommon disease. Breast Care. 2009;4:36–38. doi: 10.1159/000190078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tawil AN, Boulos FI, Chakhachiro ZI, Otrock ZK, Kandaharian L, El Saghir NS, Abi Saad GS. Clinicopathologic and immunohistochemical characteristics of male breast cancer: a single center experience. Breast J. 2012;18:65–68. doi: 10.1111/j.1524-4741.2011.01184.x. [DOI] [PubMed] [Google Scholar]
- 7.Kao L, Bulkin Y, Fineberg S, Montgomery L, Koenigsberg T. A case report: lobular carcinoma in situ in a male patient with subsequent invasive ductal carcinoma identified on screening breast MRI. J Cancer. 2012;3:226–230. doi: 10.7150/jca.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer JT, Shutter J. Synchronous bilateral invasive lobular breast cancer presenting as carcinomatosis in a male. Am J Surg Pathol. 2009;33:470–474. doi: 10.1097/PAS.0b013e318190d10d. [DOI] [PubMed] [Google Scholar]
- 9.Maly B, Maly A, Pappo I, Meir K, Pappo O. Pleomorphic variant of invasive lobular carcinoma of the male breast. Virchows Arch. 2005;446:344–345. doi: 10.1007/s00428-004-1179-x. [DOI] [PubMed] [Google Scholar]
- 10.Rohini B, Singh PA, Vastsala M, Vishal D, Mitali S, Nishant S. Pleomorphic lobular carcinoma in a male breast: a rare occurrence. Patholog Res Int. 2010 Dec 1;2010:871369. doi: 10.4061/2010/871369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinton LA. Breast cancer risk among patients with Klinefelter syndrome. Acta Paediatr. 2011;100:814–818. doi: 10.1111/j.1651-2227.2010.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson CT, Malmer B, Wiklund F, Grönberg H. Breast cancer as a second primary in patients with prostate cancer-estrogen treatment or association with family history of cancer? J Urol. 2006;176:538–543. doi: 10.1016/j.juro.2006.03.036. [DOI] [PubMed] [Google Scholar]


