Abstract
Here we report a case of a placental site trophoblastic tumor in a 36 year old Chinese woman, 31 months following a prior normal pregnancy. Her clinical presentation and ultrasound findings were uncharacteristic; and the final definitive diagnosis was established based on histological examination in conjunction with immunohistochemistry studies and a normal beta human chorionic gonadotropin level. The tumor exhibited high grade histological features with tumor necrosis, nuclear atypia and high mitosis. The patient was successfully treated with hysterectomy with pre- and post-operative chemotherapy.
Keywords: Placental site trophoblastic tumor (PSTT), gestational trophoblastic neoplasm (GTN), diagnosis, treatment
Introduction
Placental site trophoblastic tumor (PSTT) refers to a special type of gestational trophoblastic neoplasm (GTN) that originates from placental implantation site. Its incidence is approximately 1 per 100,000 pregnancies, about 1-2% of all the GTNs, mortality rate is 25% [1], About 200 cases of PSTT have been reported all over the world [2]. Due to its infrequent occurrence and uncharacteristic clinical presentation, to reach a correct diagnosis and management can be challenging. Here we report a case of PSTT to illustrate the diagnostic features of this type tumor, and proper management based on histological findings.
Case presentation
A 36-year-old Chinese woman, gestation 4, production 2, miscarriage 2, gave birth to a full-term healthy baby in February 2007 through vaginal delivery. Her postpartum course was unremarkable and she had regained her normal menstrual cycle. On September 26 2009 (her last menstrual period is on August 26, 2009), 31 months postpartum, she experienced irregular menstrual bleeding. The bleeding soon became so severe that she was sent to a local hospital where she underwent an emergency endometrial curettage. Post the procedure, she was given antibiotic prophylaxis infection and medications to promote uterine contraction. Pathological examination of the endometrial curettage specimen reported a proliferation of Sertoli cells, with no chorionic villi identified. The pathology diagnosis rendered by local hospital pathologist was gestational trophoblastic neoplasm (GTN). After the emergency curettage, her bleeding gradually reduced and became intermittent. With the diagnosis of GTN, she was referred to our hospital, a tertiary center, for further management.
At the hospital visit, physical examination showed no abnormality in heart, lungs or extremities. Gynecological examination reveal-ed normal vulva, little vagina bleeding, mild uterine cervical erosion and hypertrophy, but an enlarged uterus of about 50-day pregnancy size, the uterus was soft on palpation. Double attachment is normal. Pelvic color Doppler ultrasound showed that the uterus measured about 60 × 55 × 44 mm. There was a 31 × 38 × 23 mm heterogeneous irregular echo in the anterior wall of the uterus with no clear border. Also found was a honeycomb echo within the uterus. The uterine cavity contained large amount of fresh blood, and uterine endometrial line move back 3 mm. The ultrasound findings were suggestive of a “Sertoli cell tumor”. Laboratory test showed blood beta human chorionic gonadotropin (β-hCG) was within normal limits (7.79 mIU/ml).
The pathology slides were obtained from the referring hospital and reviewed at our hospital. Pathological sections showed a proliferation of medium spindled or polyhedral mononuclear cells with irregular borders, with cellular features consistent with intermediate trophoblasts (nourish cells). In addition, some multinucleated cells are also present (Figure 1A). Chorionic villi and cytotrophoblasts were not identified. These intermediate trophoblasts showed nuclear atypia with high mitotic figures (5/10 HPF). The tumor cells infiltrated between muscle fibers, and showed apparent tumor hemorrhage and necrosis.
Figure 1.
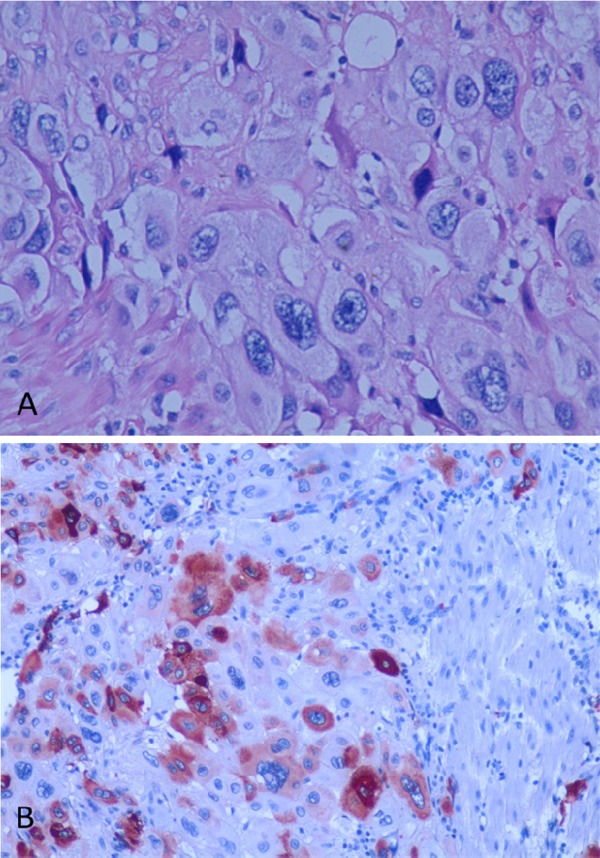
A. Endometrial curetting specimen demonstrates a proliferation of trophoblasts, with some multinucleated cells (Hematoxylin and Eosin, original magnification X200). There is marked nuclear atypia. B. The tumor cells are strongly positive for human placental lactogen (hPL) by immunohistochemistry stain (SP X100).
Based on the pathology findings and a normal β-hCG serum level, placental site trophoblastic tumor (PSTT) was highly suspected. In order to confirm the diagnosis, we requested the pathologist at our hospital to perform immunohistochemistry (IHC) studies. The IHC studies showed that the tumor cells were positive for human placental lactogen (hPL) (Figure 1B), placental alkaline phosphatase (PLAP) and smooth muscle actin (SMA). hCG positive cells were scattered and mostly of multinucleated giant cells. These IHC results confirmed a diagnosis of PSTT.
The presence of tumor necrosis, nuclear atypia and frequent mitotic figures indicated that this PSTT had high grade histological features. In addition, the tumor occurred more than two years following her prior malignancy. Based on the diagnosis and associated high risk factors, the patient was treated with EMA-CO chemotherapy regimen (etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine) for three days, followed by a total hysterectomy with preservation of ovaries. The hysterectomy specimen revealed two masses located in the anterior wall of the uterus, measuring 2 x 1 x 1 cm and 2 x 2 x 2 cm respectively (Figure 2A). The mass lesions were purple blue with hemorrhage and tumor necrosis. The tumors had ill-defined borders, and invaded into the myometrium. Pathological examination of the tumor and the IHC results were similar to that described in the curettage specimen. The tumor cells demonstrated nuclear atypia, frequent mitosis (5/10 HPF) and myometrial invasion (Figure 2B). In addition, the uterus showed chronic endometritis and chronic cervicitis. Post operation, she finished the eighth days chemotherapy and received additional four cycles of EMA-CO chemotherapy regimen with no major side effects. At the last follow-up (40 months after diagnosis), the patient was well and showed no sign of tumor recurrence.
Figure 2.
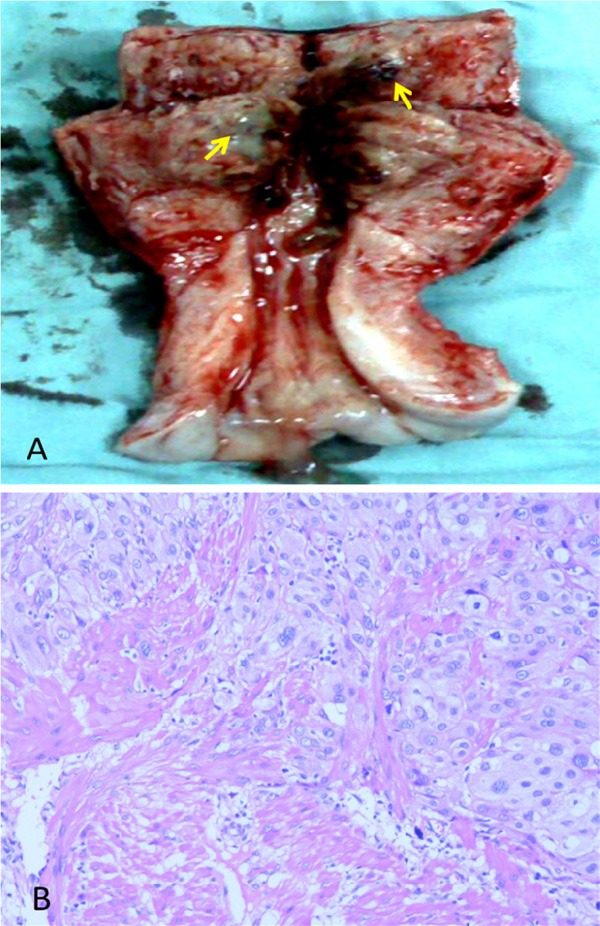
Hysterectomy specimen. A. Opening the endometrial cavity reveals two blue/purple lesions (arrows). B. Histological examination reveals a proliferation of intermediate trophoblasts with nuclear atypia and conspicuous mitotic figures (Hematoxylin and Eosin, original magnification X100). The tumor shows myometrial invasion.
Summary
Due to the heterogeneity of the clinical manifestations, the diagnosis for PSTT is usually difficult and requires a combination of blood β-hCG test, radiology examination, histology with immunohistochemical staining. Surgery has been the primary treatment option. However, chemotherapy has also played an important role for cases with high risk features.
In 1976, Kurman first described PSTT as syncytial endometritis, and named it sertoli cell pseudotumor. In 1981, Scully described the morphological details and recognized it as a neoplastic process, and named it PSTT. In 1983, the World Health Organization (WHO) formally acknowledged the neoplastic nature of this lesion and adopted the terminology of PSTT. PSTT can occur following term labor, abortion and rarely in molar pregnancy. The interval from prior pregnancy to tumor development is usually less than 2-years, but a longer interval is also observed in some patients. PSTT most frequently occurs within the uterus, with rare cases occurring in fallopian tube likely secondary to tubal pregnancies and resulting in large internal bleeding [3]. PSTT has also been reported concurrent with a live twin pregnancy and successfully resected at the time of C-section [4]. Interestingly, PSTT also can occur in patients without a pregnancy history. It was reported in the ovary of a young child with isosexual precocious puberty [5] and in men [6], as a subtype of non-seminomatous germ cell tumor.
The clinical presentation of PSTT is nonspecific or uncharacteristic; therefore, the diagnosis can be challenging. Patients often present with irregular vaginal bleeding or menorrhagia after a period of amenorrhea, and an enlarged uterus. Blood β-hCG was usually normal or only slightly increased, and the levels are not proportional to the tumor burden. This differs from many of the GTDs that the later often have a high β-hCG level. However, other types of GTDs with a low serum β-hCG level have also been reported [7]. Ultrasound findings are often lack of specificity. A definitive diagnosis requires histology examination in conjunction with IHC studies. PSTT are composed of intermediate trophoblasts, lack of cytotrophoblasts and chorionic villi; the tumor cells are weakly and partially positive for hCG, but strongly positive for hPL.
The standard treatment is surgery. The principle is to remove tumor and/or tumor involving organs, including total hysterectomy with or without bilateral salpingo-oophorectomy. In young women, preservation of ovaries is recommended. It has been reported in recent years that reproductive function (uterus) could be persevered after successful removal of PSTT [4]. Patients with high risk factors require adjuvant chemotherapy post surgical removal. The risk factors associated with prognosis are: (1) metastases out of uterus (2) PSTT occurs more than two years following the prior pregnancy (3) Tumor cells exhibit high grade histological features such as extensive tumor necrosis, nuclear atypia, higher mitotic figures (>5/10 HPF) and a high Ki67 proliferation index (4) patients aged over forty years [1]. Among all the risk factors mentioned above, metastases out of uterus are the most critical. The prognosis of PSTT is good if it is limited to the uterus; in contrast, the mortality can reach up to 25% if metastases occur. In our patient, although she was younger than 40 years and she showed no sign of distant metastases, her tumor occurred more than 2 years following prior pregnancy, and the tumor demonstrated a number of high grade histological features. She was treated with EMA-CO chemotherapy regimen and underwent hysterectomy. Her ovaries were persevered. She showed no sign of recurrence with more than 3 years follow-up.
Disclosure of conflict of interest
There is no conflict of interest for all authors.
References
- 1.Piura B, Shaco-Levy R. Placental site trophoblastic tumor. Harefuah. 2007;146:62–67. 77. [PubMed] [Google Scholar]
- 2.Colecchi C, Partemi S, Minelli N, Cascini F, Rossi R, Fulcheri E, Pascali VL, Oliva A. Placental site trophoblastic tumor with lung metastases as cause of death in a young patient: a case report. Placenta. 2011;32:1060–1063. doi: 10.1016/j.placenta.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Su YN, Cheng WF, Chen CA, Lin TY, Hsieh FJ, Cheng SP, Hsieh CY. Pregnancy with primary tubal placental site trophoblastic tumor--A case report and literature review. Gynecol Oncol. 1999;73:322–325. doi: 10.1006/gyno.1998.5318. [DOI] [PubMed] [Google Scholar]
- 4.Liszka L, Wilk M, Wodolazski A, Palen P, Sikora J. Successful treatment of placental site trophoblastic tumor in twin pregnancy without hysterectomy. Tumori. 2009;95:108–111. doi: 10.1177/030089160909500120. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo MR, Podda A, Cao D, Rodriguez MM. Placental site trophoblastic tumor in the ovary of a young child with isosexual precocious puberty. Pediatr Dev Pathol. 2009;12:73–76. doi: 10.2350/08-05-0462.1. [DOI] [PubMed] [Google Scholar]
- 6.Suurmeijer AJ, Gietema JA, Hoekstra HJ. Placental site trophoblastic tumor in a late recurrence of a nonseminomatous germ cell tumor of the testis. Am J Surg Pathol. 2004;28:830–833. doi: 10.1097/01.pas.0000126053.88174.b5. [DOI] [PubMed] [Google Scholar]
- 7.Okumura M, Fushida K, Rezende WW, Schultz R, Zugaib M. Sonographic appearance of gestational trophoblastic disease evolving into epithelioid trophoblastic tumor. Ultrasound Obstet Gynecol. 2010;36:249–51. doi: 10.1002/uog.7560. [DOI] [PubMed] [Google Scholar]


