Abstract
Purpose
Systemic administration of recombinant interleukin (IL)-2 is used to support the expansion and persistence of adoptively transferred antigen-specific CTLs in patients with cancer. However, IL-2 also expands regulatory T cells (Treg) that in turn impair the antitumor activity of CTLs. As recombinant IL-15 is approaching clinical applications, we assessed the effects of this cytokine on the proliferation and antitumor activity of CTLs in the presence of Tregs. We used the model of adoptive transfer of Epstein–Barr virus (EBV)-CTLs, as these cells induce responses in patients with EBV-associated Hodgkin lymphoma, and Tregs are frequently abundant in these patients.
Experimental Design
Tregs were isolated from the peripheral blood of healthy donors and patients with Hodgkin lymphoma or from Hodgkin lymphoma tumors and assessed for their ability to inhibit the proliferation and antitumor activity of EBV-CTLs in the presence of IL-15 or IL-2. Specific molecular pathways activated by IL-15 were also explored.
Results
We found that in the presence of Tregs, IL-15, but not IL-2, promoted the proliferation, effector function, and resistance to apoptosis of effectors T cells and EBV-CTLs. IL-15 did not reverse or block Tregs but instead preferentially supported the proliferation of CTLs and effector T cells as compared with Tregs.
Conclusions
IL-15 selectively favors the survival, proliferation, and effector function of antigen-specific CTLs in the presence of Tregs, and thus IL-15, unlike IL-2, would have a significant impact in sustaining expansion and persistence of adoptively transferred CTLs in patients with cancer, including those infused with EBV-CTLs for treatment of EBV-associated malignancies.
Introduction
Adoptive transfer of antigen-specific CTLs is a promising therapy for patients with cancer (1–3). Specifically, we have previously shown that the adoptive transfer of Epstein–Barr virus–specific CTLs (EBV-CTL) in patients with EBV-associated Hodgkin lymphoma is well tolerated and induces objective clinical responses (4, 5). However, several clinical trials have shown significant correlation between the in vivo persistence of adoptively transferred CTLs and the achievement of sustained tumor regression (6, 7), indicating that strategies aimed at enhancing CTL persistence in vivo should translate in better clinical outcomes.
Growth factors play a crucial role in sustaining survival and proliferation of different T-cell subsets. In particular, CTLs expanded ex vivo are highly dependent on cytokines such as interleukin (IL)-2 or IL-15 for their growth (8). However, these cytokines are frequently unavailable in the tumor environment, rendering CTLs short living, as they are immediately starved from these crucial survival factors. To overcome this limitation, recombinant human IL-2 is frequently administered in patients to support the expansion of adoptively transferred CTLs (9, 10). Although high doses of IL-2 may be effective, their benefits are frequently hampered by severe systemic toxicities (11–13). Moreover, IL-2 also facilitates the expansion of CD4+ T lymphocytes with regulatory activity (14–17). These regulatory T cells (Treg), characterized by the constitutive expression of the high-affinity IL-2 receptor alpha (IL-2Rα, CD25) and of the forkhead box P3 (FoxP3) protein, actively inhibit the proliferation and function of effector T lymphocytes (18). Tregs are particularly relevant in patients with cancer, as these cells are frequently increased in the peripheral blood and in tumor biopsies (19–21). In addition, their presence often correlates with poor clinical outcome (20, 21). In the specific case of Hodgkin lymphoma, Tregs are found particularly abundant both in the circulation and at the tumor site (22–24), so that the use of IL-2 to sustain the in vivo expansion of adoptively transferred EBV-CTLs is highly discouraged.
IL-15 is another γ-chain cytokine that supports the proliferation and maintenance of effector T lymphocytes (25). Previous studies in mice have shown that the administration of IL-15 or the transgenic expression of IL-15 by effector T cells (26–28) enhance their antitumor effects. Recently, IL-15 has been tested in nonhuman primates, showing a favorable toxicity profile, with a preferential increase in CD8+ T lymphocyte counts and minimal effects on the absolute numbers of circulating Tregs (29, 30).
The effects of IL-15 on human Tregs remain largely unclear due to differences in the doses used and in the origin of effector T cells and Treg subpopulations tested between studies (31–41). Here, we modeled ex vivo culture conditions to assess the impact of IL-2 and IL-15 on the effector function of a specific subset of clinically relevant antigen-specific CTLs (EBV-CTLs), in the presence of Tregs isolated from both healthy donors and patients with Hodgkin lymphoma. In addition, in our experimental conditions we used doses of IL-2 and IL-15 that correspond to in vivo–measured cytokine trough levels (29, 30, 42), which makes the observed effects physiologically relevant.
Materials and Methods
Isolation of Tregs from healthy donors
We isolated peripheral blood mononuclear cells (PBMC) from the buffy coats of 10 healthy volunteers (Gulf Coast Regional Blood Center, Houston, Texas) by centrifugation on a density gradient (Ficoll, Axis-Shield PoC AS). Naturally occurring Tregs were enriched on the basis of CD4 and bright expression of CD25 using immunomagnetic selection, as previously described (43). Briefly, the CD25bright fraction was positively selected using nonsaturating concentrations (2 μL/1 × 107 PBMC) of anti-human CD25 magnetic beads (Miltenyi Biotech) and further selected using anti-human CD4 magnetic beads (Miltenyi) following the manufacturer’s instructions. The selection process resulted in a significant enrichment in cells coexpressing CD4, CD25, and FoxP3 (Supplementary Fig. S1A). As control, we used cells obtained from the CD4+ population after depletion of the CD25bright subset (CD4+CD25depleted; defined as “control cells” throughout the article), which lacked substantial FoxP3 expression (Supplementary Fig. S1B). In selected experiments, Tregs were isolated using a MoFLo sorter (Beckman Coulter Inc.) based on CD4, CD25, and CD45RA expression to obtain CD4+CD25++CD45RA+, CD4+ CD25+CD45RA+, or CD4+CD25+CD45RA− cells as previously described (44; Supplementary Fig. S2). To evaluate the effects of Tregs on specific T-cell subsets, we used immunomagnetic selection to enrich CD45RO+CD62L+, CD45RO+ CD62L−, or CD45RA+CD62L− cells from PBMC. Purity of isolated cells was more than 95% and the reanalysis included CCR7 expression (Supplementary Fig. S1C).
Isolation of Tregs from Hodgkin lymphoma samples
Peripheral blood and/or tumor biopsies from 21 patients with Hodgkin lymphoma were provided by the lymphoma bank of a National Cancer Institute–funded lymphoma SPORE (Baylor College of Medicine, Houston, TX). PBMC were isolated by centrifugation on a density gradient, whereas biopsies were gently smashed. Because of the limited number of cells usually obtained from these samples, Tregs were selected on the basis of their CD25bright expression without any further enrichment (Supplementary Table S1).
Activation of CD4+CD25bright cells
CD4+CD25bright cells were used directly in experiments or cultured for 7 days in RPMI-1640 medium (HyClone, Thermo Scientific) supplemented with 5% human AB-serum (Valley Biomedical), 1% penicillin/streptomycin (Sigma), 1% glutamine (BioWhittaker Inc.), 0.1% 2β-mercaptoethanol (Invitrogen), anti-CD3 antibody (OKT3, Orthoclone; 0.5 μg/mL), and γ-irradiated (40 Gy) allogeneic PBMC as feeders (at a PBMC:feeder ratio of 2:1). We added recombinant human IL-2 (25 IU/mL; Teceleukin, Hoffmann-LaRoche) or recombinant human IL-15 (2.5 ng/mL; PeproTech Inc.) in experiments, as indicated.
Single-cell cloning of Tregs
In selected experiments, Tregs isolated from healthy donors and Hodgkin lymphoma samples were single-cell cloned by limiting dilution. Briefly, 1 × 103 Tregs were resuspended in RPMI-1640 medium supplemented with 5% human AB-serum, 1% penicillin/streptomycin, 2 mmol/L L-glutamine, 0.1% 2β-mercaptoethanol, OKT3 (50 ng/mL), γ-irradiated (40 Gy) allogeneic feeders, and IL-2 (100 IU/mL). Tregs were plated in 96-wells plates at the concentration of 1 Treg per well with the described activation mixture and incubated for 3 to 4 weeks. Cells were fed weekly with IL-2. Growing cells were then screened for inhibitory activity using the Cell Lab Quanta SC MPL (Beckman Coulter Inc.). Inhibitory clones were further reexpanded using allogeneic γ-irradiated feeders, irradiated EBV-immortalized lymphoblastoid cell lines (LCL), OKT3, and IL-2 (100 IU/mL) to reach sufficient numbers for further experiments, including suppression and antitumor assays in the presence of cytokines. Clonality was confirmed by Vβ-repertoire phenotype (data not shown). As control for experiments using Treg-derived clones, we expanded in parallel clones that lacked suppression activity when screened by the Cell Lab Quanta SC MPL.
Generation of EBV-CTLs
EBV-CTLs were generated from the PBMCs obtained from EBV-seropositive healthy donors and patients with Hodgkin lymphoma (peripheral blood collected according to the local Institutional Review Board–approved protocols, Baylor College of Medicine) by weekly stimulation with γ-irradiated (40 Gy) autologous LCL with the addition of IL-2 (50 IU/mL) as previously described (1, 2, 4, 5).
Immunophenotyping
Cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin–chlorophyll–protein complex, or allophycocyanin-conjugated monoclonal antibodies (mAb). We used CD3, CD4, CD8, CD19, CD45RA, CD45RO, CD62L, and CD25 from Becton Dickinson (BD Biosciences), CCR7 from R&D System, and FoxP3 from eBioscience Inc. IL-15 private receptor (IL-15Rα) expression was detected using an anti-IL-15Rα mAb (R&D Systems) in conjunction with a FITC-goat anti-mouse IgG1 (SouthernBiotech). Control samples were labeled with appropriate isotype-matched antibodies. T cell receptor (TCR) Vβ-repertoire was studied using a specific kit for flow cytometry analysis (IOTest Beta Mark TCR-Vβ Repertoire Kit, Beckman Coulter). We analyzed STAT5 phosphorylation after stimulation with IL-15 for 15 minutes using the anti-phospho-STAT5 (Y694) mAb-Alexa-Fluor-647-conjugate (BD Phosflow Reagents). We measured BCL-2 expression in the presence of IL-15 in EBV-CTLs after stimulation with autologous LCLs, as well as in T cells and Tregs after activation with OKT3 and irradiated feeders, using the PE-conjugated-hamster anti-human BCL-2 (6C8; BD Biosciences). Phosphorylation of S6K1 after T-cell activation in the presence of IL-15 was assessed using phospho-S6-ribosomal protein (Ser235/236) rabbit mAb-Alexa-Fluor-647-conjugate (Cell Signaling Technologies), according to manufacturer’s instruction. Cells were analyzed using a BD FACScalibur system equipped with the filter set for quadruple fluorescence signals and the CellQuest software (BD Biosciences). For each sample, we analyzed a minimum of 10,000 events.
Carboxyfluorescein diacetate succinimidyl ester–based assays
Proliferation of EBV-CTLs, T cells and Tregs was assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution. Cells were labeled with 1.5 μmol/L CFSE (Invitrogen) and activated with LCLs, or OKT3 (0.5 μg/mL) and irradiated feeders (ratio 2:1) in the absence or in the presence of IL-2 (25 IU/mL) or IL-15 (2.5 ng/mL). CFSE dilution was measured by flow cytometry after 7 days of culture. To evaluate suppressive activity of Tregs and Treg-clones, PBMC were labeled with 1.5 μmol/L CFSE and activated with OKT3 (0.5 μg/mL) and feeders (ratio Tregs: feeders of 2:1), in the presence of Tregs/Treg-clones or control cells at a 1:1 (T cells:Tregs) ratio, and IL-2 (25 IU/mL or at the indicated doses for the titration experiment) or IL-15 (2.5 ng/mL or at the indicated doses) or of the IL-15/IL-15Rα complex. In selected experiments, the ratio of T cells and Tregs was titrated to 2:1 and 4:1. After 7 days, cells were labeled with CD8 and CD4, analyzed by fluorescence-activated cell sorting (FACS) and cell division assessed by CSFE dilution. In selected experiments, the suppressive activity of CD4+CD25++CD45RA+, CD4+CD25+CD45RA+, or CD4+CD25+CD45RA− cells was evaluated.
Evaluation of antitumor activity
EBV-CTLs generated from 6 healthy donors were cultured in the presence of autologous LCLs with control cells or activated Tregs freshly isolated from healthy donors (at the EBV-CTLs:LCLs:Tregs ratio of 1:2:1) and in the absence or presence of IL-2 (25 IU/mL) or IL-15 (2.5 ng/mL). Similarly, EBV-CTLs generated from 3 patients with Hodgkin lymphoma were cultured in the presence of autologous LCLs with control cells or activated Hodgkin lymphoma–derived Treg-clones. After 7 days, cells were collected, stained with CD8, CD4, and CD3 to identify T cells, and CD19 to quantify residual tumor cells, and analyzed by FACS. To define tumor cells, we considered only CD19+ cells and not CD3+CD19+ cells, as the latter likely represent T cells that had acquired positivity for the CD19 through trogocytosis (45).
Evaluation of apoptosis
EBV-CTLs, T cells and Tregs were activated with LCLs or OKT3 (0.5 μg/mL) and irradiated feeders (ratio 2:1) in the absence or presence of IL-2 (25 IU/mL) or IL-15 (2.5 ng/mL) and, after 7 days, collected, washed, and labeled with Annexin-V–PE–conjugated and 7-Amino-actinomycin D (7AAD; BD Biosciences) according to the manufacture’s instruction. The percentage of Annexin-V+7AAD− cells was measured by FACS analysis. For each sample, at least 100,000 events were analyzed.
ELISA
The presence of the complex IL-15/IL-15Rα in our culture supernatant was quantified using a commercially available specific-ELISA kit (R&D Systems). PBMC (1 × 106/well) were activated with OKT3, feeders, IL-15 (2.5 ng/mL), and supernatants harvested every 24 hours for 7 days and analyzed for the presence of the IL-15/IL-15Rα complex. As positive control, we preincubated IL-15 (2.5 ng/mL or 0.6 ng/mL) with recombinant human IL-15Rα/Fc Chimera (R&D System) for 30 minutes at room temperature (46).
Statistical analysis
Unless otherwise noted, data were summarized by means and SEM. Student t test was used to determine the statistical significant differences between samples, with P value less than 0.05 indicating a significant difference.
Results
Putative Tregs are increased in peripheral blood and tumor samples from patients with Hodgkin lymphoma
We first analyzed peripheral blood samples and/or lymphonode biopsies from 21 patients with Hodgkin lymphoma for the presence of putative Tregs based on the expression of CD4, CD25, and FoxP3 markers, and compared it with that of peripheral blood samples obtained from healthy donors. We observed a significant increase in the frequency of circulating and tumor-infiltrating CD4+ CD25bright cells (7.4% ± 1.7% and 8.7% ± 0.9%, respectively) and CD4+FoxP3+ cells (5.3% ± 1.6% and 6.2% ± 1.2%, respectively) in samples from patients with Hodgkin lymphoma as compared with those detected in the peripheral blood of healthy donors (2.5% ± 1.1% for CD4+ CD25bright and 2.4% ± 0.96% for CD4+FoxP3+ cells, respectively; Fig. 1A). To ensure that CD4+CD25bright cells detected in peripheral blood and tumor biopsies were indeed functional Tregs, we isolated these cells by immunomagnetic selection and conducted suppression assays to assess their inhibitory properties. Although limited by the starting amount of blood/tissues from patient’s samples (Supplementary Table S1), we isolated sufficient numbers of enriched putative Tregs from the majority of samples for further functional characterization. When tested in a CFSE-based suppression assay, CD4+CD25bright cells from both healthy donors (Fig. 1B) and patients with Hodgkin lymphoma (Fig. 1C) showed inhibitory activity. Specifically for healthy donors, the addition of Tregs to the cultures inhibited the proliferation of CD8+ and CD4+ cells by 68% ± 6% and 71% ± 7%, respectively, as compared with the addition of control cells (P < 0.001 for both; Fig. 1B). Similarly, the addition of Tregs isolated from patients with Hodgkin lymphoma inhibited the proliferation of CD8+ and CD4+ cells by 43% ± 14% and 50% ± 11%, respectively, as compared with control cells (P < 0.05 for both; Fig. 1C). Although not statistically significantly, we attributed the relatively less efficient T-cell inhibition by Tregs isolated from patients with Hodgkin lymphoma to the isolation procedures used when starting from patients versus healthy donors samples, which lacked the CD4 selection step due to the limited size of the samples (Supplementary Table S1).
Figure 1.
Phenotypical and functional characterization of Tregs isolated from healthy donors and patients affected by Hodgkin lymphoma (HL). Phenotype and inhibitory function of Tregs freshly isolated from the peripheral blood of 10 healthy donors, 21 biopsies collected from patients with Hodgkin lymphoma, and peripheral blood from 7 patients with Hodgkin lymphoma were assessed by FACS. A, plots for CD4 versus CD25, CD4 versus FoxP3, and CD25 versus FoxP3 of circulating Tregs from a representative healthy donor and a patient with Hodgkin lymphoma. The graph shows the summary of percentages of Tregs quantified by FACS. *, P = 0.05. B, the proliferation of CD8+ or CD4+ cells in the presence of control or Tregs freshly isolated from 1 representative healthy donor. The graph shows the percentage of inhibition (mean ± SEM) for 10 independent experiments. C, the proliferation of CD8+ or CD4+ cells in the presence of control cells or Tregs freshly isolated from 1 representative patient with Hodgkin lymphoma. The graph shows the percentage of inhibition for 4 independent experiments.
IL-15 but not IL-2 sustains the proliferation of T cells in the presence of Tregs
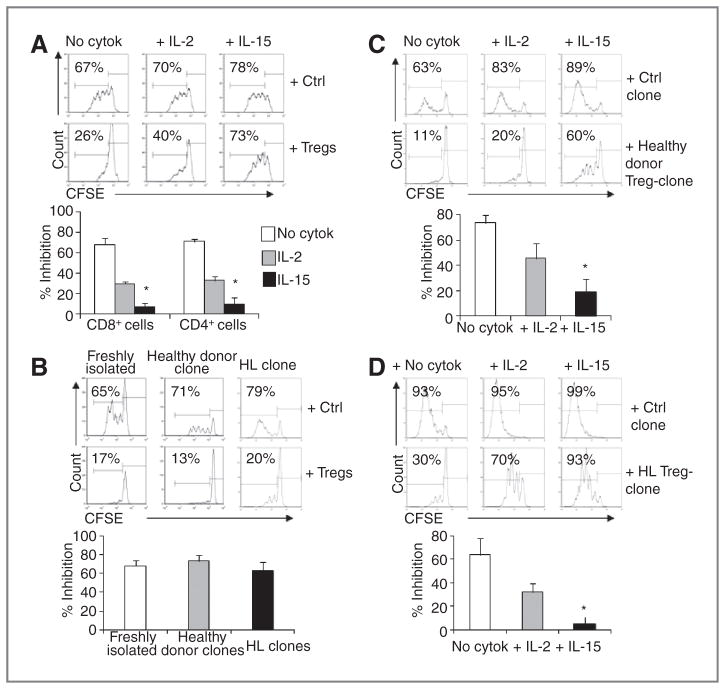
As the γ-chain cytokines IL-2 and IL-15 can be used to sustain the in vivo persistence of adoptively transferred CTLs (9, 10), we compared the effects of these cytokines on effector T cells in the presence of Tregs. We selected doses of IL-2 and IL-15 of 25 IU/mL and of 2.5 ng/mL, respectively, as they correspond to in vivo–measured cytokine trough levels (29, 30, 42). For these experiments, we first used Tregs isolated from healthy donors and then validated the results using Treg-clones generated from patients with Hodgkin lymphoma. The establishment of Treg-clones allowed us to overcome the difficulties in carrying out these experiments using the limited amount of Tregs isolated from patient’s samples. As shown in Fig. 2A, we found 30% ± 2% inhibition of CD8+ cell proliferation and 33% ± 4% inhibition of CD4+ cell proliferation in the presence of IL-2 and Tregs isolated from healthy donors as compared with the addition of IL-2 and control cells (P < 0.01 for both). In contrast, the inhibition was negligible for both CD8+ and CD4+ cells in the presence of IL-15 and Tregs as compared with the addition of IL-15 and control cells (7% ± 4% and 10% ± 6%, respectively; P = 0.1 and P = 0.2, respectively; Fig. 2A).
Figure 2.
IL-15 sustains T-cell proliferation in the presence of Tregs or Treg-clones derived from healthy donors or patients with Hodgkin lymphoma (HL). Tregs were tested for their ability to inhibit T-cell proliferation using a CFSE-based suppression assay with or without IL-2 or IL-15. A, the proliferation of T cells in the absence of cytokines or in the presence of IL-2 or IL-15 with control cells or Tregs in 1 representative healthy donor. The graph summarizes the inhibition mediated by Tregs for CD8+ and CD4+ cells (mean ± SD) for the 10 donors. *, P < 0.01. B, representative histograms for the proliferation of activated PBMC in the presence of freshly isolated Tregs or in the presence of a healthy donor-derived Treg-clone or a Hodgkin lymphoma–derived Treg-clone. Graph summarizes mean ± SEM of 4 donors/each. C, representative histograms for the proliferation of activated PBMC in the presence of control cells or a healthy donor-derived Treg-clone with or without cytokines. The graph summarizes the inhibition mediated by Treg-clones from 4 donors. *, P < 0.05. D, representative histograms for the proliferation of activated PBMC in the presence of control cells or a Hodgkin lymphoma–derived Treg-clone with or without cytokines. The graph summarizes the inhibition mediated by Treg-clones from 4 donors. *, P < 0.05.
Because the percentage of CD4+ at the beginning of our culture conditions was 47% ± 3%, in our suppression assay, the ratio CD4 cell:Tregs was approximately 1:2. Although this excess of Tregs better reflects the unfavorable setting likely present in vivo, to ensure that the limited ability of IL-2 to drive T-cell proliferation in the presence of Tregs was not related to the consumption of the cytokine by Tregs, we titrated the number of Tregs in the suppression assays and tested increased PBMC:Tregs ratios in the presence of IL-2. As shown in Supplementary Fig. S2A, Tregs maintained their ability to inhibit the proliferation of both CD4 and CD8, despite the presence of IL-2, thus confirming the limited ability of IL-2 to support T cells proliferation in the presence of Tregs. In addition, because previous studies have shown that freshly isolated CD4+ CD25+FoxP3+ cells are functionally heterogeneous, as they may contain a subpopulation lacking suppressive activity (CD25+CD45RA− cells), in selected experiments, we isolated Tregs by flow sorting based on the expression of CD25 and CD45RA, as previously described (44; Supplementary Fig. S2B and S2C). Using these cell subsets, proliferation of activated T cells remained impaired when CD25+CD45RA+ or CD25++CD45RA− cells (also referred as resting Tregs and activated Tregs, respectively; ref. 44) were added to the culture in the presence of IL-2 but not of IL-15, suggesting that the latter cytokine protects T cells from Treg inhibition rather than preferentially favors the effects mediated by CD25+CD45RA− cells, known to lack suppressive activity despite the expression of FoxP3 (44).
On the basis of the protective effects of IL-15 using polyclonal Tregs isolated from healthy donors, we repeated the experiments using single-cell–cloned Tregs generated from the peripheral blood of healthy donors and patients with Hodgkin lymphoma. Because Treg-clones from healthy donors and from patients with Hodgkin lymphoma and polyclonal Tregs were equally effective in suppressing T-cell proliferation (Fig. 2B), we validated the effects of IL-2 and IL-15 using Treg-clones. Similarly to polyclonal Tregs, both Treg-clones from healthy donors and patients with Hodgkin lymphoma significantly inhibited T-cell proliferation either in the absence of cytokine (73% ± 6% and 64% ± 8%, respectively) or in the presence of IL-2 (46% ± 12% and 32% ± 7%, respectively) as compared with control cells (Fig. 2C and D). In sharp contrast, the addition of IL-15 sustained T-cell proliferation in the presence of Treg-clones generated from healthy donors and from patients with Hodgkin lymphoma (19% ± 10% inhibition and 5% ± 6%, respectively) as compared with control cells (Fig. 2C and D), indicating that IL-15, but not IL-2, significantly protects T cells from Treg-clone mediated inhibition (P < 0.01).
Interestingly, when we titrated the amount of IL-15 in the culture system, we confirmed that low doses of IL-15 (in the range of 1.25–2.5 ng/mL, similar to that observed in vivo; refs. 29, 30) were sufficient to preserve the proliferation of T cells in the presence of Tregs (Supplementary Fig. S3A). In contrast, T cells could proliferate despite the presence of Tregs only in the presence of very high doses (>50 IU/mL) of IL-2, which corresponds to through levels associated with sever toxic events in vivo (12, 13, 42). More importantly, we found that T-cell proliferation was preserved in the presence of Tregs even when a very low dose of IL-15 (0.6 ng/mL) was added to the culture as heterodimeric complex with the IL-15Rα (Supplementary Fig. S3B and S3C), which is the bioactive form of IL-15 detected in the serum of patients (47). This suggests that in the presence of the IL-15/IL-15Rα complex very low doses of recombinant IL-15 may be sufficient in providing the expected relief from Treg-mediated inhibition in vivo (Supplementary Fig. S3), while minimizing the systemic toxicity associated with high doses of cytokines (30).
IL-15 specifically preserves the antitumor effects of EBV-CTLs in the presence of Tregs
Because ex vivo–expanded EBV-CTLs used for adoptive T-cell therapy in Hodgkin lymphoma (4, 5) and antigen-specific CTLs in general used for antitumor therapy are mainly composed of CD45RO+ effector cells, with a majority of TEM cells and a lower and variable proportion of the TCM (23% ± 8%; refs. 48–50), we first investigated whether IL-15 protects the CD45RO+ T-cell subset from Treg-mediated inhibition, in an antigen-independent manner. Therefore, we measured the suppressive activity of Tregs against CD45RO+CD62L+, CD45RO+CD62L−, or CD45RA+ CD62L− cells, as a source of central-memory, effector-memory, and terminally differentiated T cells, respectively. We found that Tregs significantly inhibited the proliferation of TCM cells, irrespective of whether we added IL-2 (42% ± 6%) or IL-15 (40% ± 7%; Fig. 3A). In contrast, IL-15 more effectively sustained the proliferation of TEM cells and TEMRA cells in the presence of Tregs, as the percentage of inhibition was 35% ± 5% and 58% ± 8% in the presence of IL-2, but only 27% ± 5% and 36% ± 10% in the presence of IL-15 (P < 0.05; Fig. 3B and C). Hence, IL-15 seems to preferentially protect effector T cells (TEM and TEMRA) from the inhibitory effects of Tregs.
Figure 3.
IL-15 protects the proliferation of effector T cells in the presence of Tregs. Tregs were tested for their ability to inhibit proliferation of different T-cell subsets using a CFSE-based suppression assay. T-cell proliferation of CD45RO+CD62L+ cells (A; TCM), of CD45RO+CD62L− cells (B; TEM), and of CD45RA+CD62L− cells (C; TEMRA). Histograms show the proliferation in the absence or in the presence of IL-2 or IL-15 and of control cells or Tregs in 1 representative of 6 experiments. Graphs show the inhibition mediated by Tregs on each T-cell subset in the presence of IL-2 or IL-15. Shown is the average ± SEM of the 6 donors. *, P < 0.01.
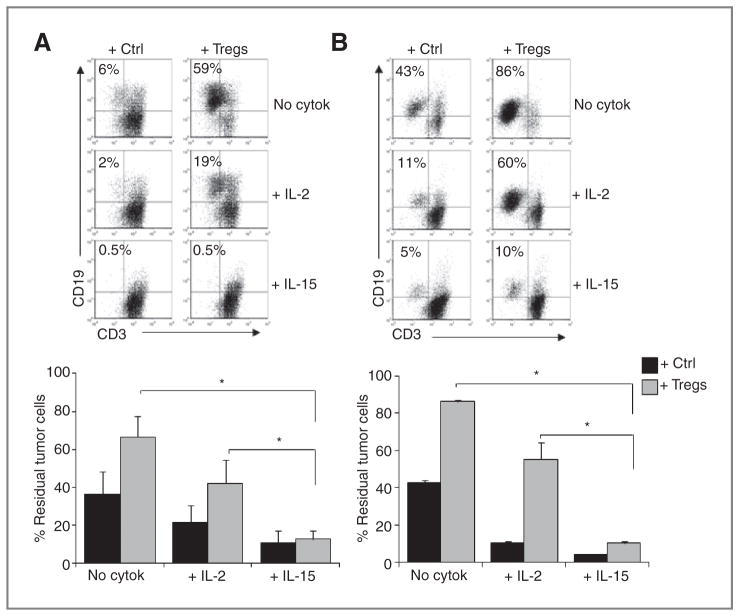
Then, IL-15–mediated protection was confirmed in our model of EBV-CTLs. As illustrated in Fig. 4A, when EBV-CTLs generated from healthy donors were cocultured with autologous LCL (1:2 CTL:LCL ratio to ensure an excess of target cells), the percentage of residual CD19+ tumor cells, as assessed by flow cytometry by day 5 to 7, was 67% ± 11% in the cocultures containing Tregs versus 36% ± 12% in control cultures (P < 0.01), indicating that Tregs significantly impair the elimination of target cells by effector CTLs. We next assessed the effects of adding IL-2 or IL-15 to these cultures. As shown in Fig. 4A, the effector function of EBV-CTLs generated from healthy donors remained impaired by Tregs in the presence of IL-2, as the percentage of residual tumor cells increased from 22% ± 9% in the cocultures containing IL-2 and control cells to 42% ± 13% in cocultures containing IL-2 and Tregs isolated from healthy donors (P < 0.01). In contrast, in the presence of IL-15, the effector function of EBV-CTLs was sustained with or without Tregs (residual tumor cells 11% ± 6% in IL-15 cultures with control cells versus 13% ± 5% in IL-15 with Tregs, P = NS; Fig. 4A). Similar results were observed when EBV-CTLs generated from patients with Hodgkin lymphoma were cocultured with autologous LCL in the presence of Hodgkin lymphoma–derived Treg-clones. As shown in Fig. 4B, residual tumor cells increased from 11% ± 1% in cocultures containing IL-2 and control cells to 56% ± 9% in cocultures containing IL-2 and Treg-clones (P < 0.01). In contrast, in the presence of IL-15, the effector function of EBV-CTLs generated from patients with Hodgkin lymphoma was sustained with or without Tregs (residual tumor cells 4% ± 1% in IL-15 cultures with control cells versus 10% ± 1% in IL-15 with Tregs). Hence, IL-15 can preserve the effector function of antigen-specific CTLs in the presence of Tregs.
Figure 4.
IL-15 sustains the antitumor effects of EBV-CTLs in the presence of Tregs. Tregs were tested for their ability to inhibit EBV-CTL antitumor activity in the presence or absence of cytokines in coculture experiments. A, a representative coculture experiment in which EBV-CTLs generated from a healthy donor were incubated with autologous LCL with or without Tregs freshly isolated from healthy donors and cytokines. Residual tumor cells were enumerated by flow cytometry based on the expression of CD19 by day 7 of culture. The graph summarizes the percentage of residual tumor cells (mean ± SEM) for the 6 independent experiments. *, P < 0.01. B, a representative coculture experiment in which EBV-CTLs generated from a patient with Hodgkin lymphoma were incubated with autologous LCL with or without Treg-clones and cytokines. The graph summarizes the percentage of residual tumor cells (mean ± SD) for 3 independent experiments. *, P < 0.01.
IL-15–mediated protection is due to a preferential increased proliferation and reduced cell death of effector T cells as compared with Tregs
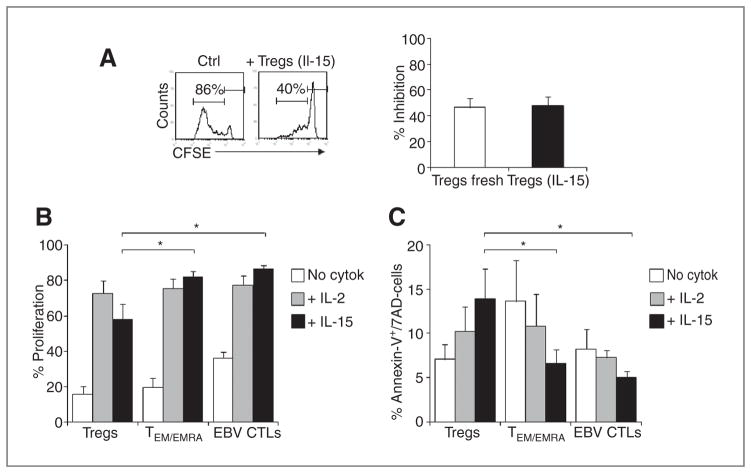
To discover the mechanisms responsible for the protective effects of IL-15, we dissected the effects of this cytokine on Tregs and on effector T cells. First, we found that the protective effects of IL-15 were not determined by a reversal of Treg-inhibitory properties. Indeed, freshly isolated Tregs that had been activated and cultured for 7 days in the presence of IL-15, when tested in the suppression assay in the absence of cytokines, retained their inhibitory functions and blocked the proliferation of activated T cells (from 61% ± 7% to 34% ± 5%, in the presence of Tregs previously expanded in IL-15; P < 0.01; Fig. 5A).
Figure 5.
IL-15 does not directly affect Treg-inhibitory function but preferentially supports the proliferation and survival of effector T cells. A, the ability of Tregs to maintain inhibitory properties after expansion for 7 days in the presence of IL-15, before being tested in a classical CFSE-suppression assay. Histograms from this representative experiment show the CFSE dilution of T cells in the presence of control cells or Tregs previously expanded in IL-15. The graph summarizes the percentage of inhibition (mean ± SEM) of 5 donors. B, the percentage of proliferating Tregs, TEM/EMRA cells, and EBV-CTLs activated in the absence or in the presence of IL-2 or IL-15 as assessed by CFSE dilution. Data are mean ± SEM of 5 independent experiments. *, P < 0.05. C, the percentage of apoptotic cells for Tregs, TEM/EMRA cells, and EBV-CTLs assessed by Annexin-V and 7AAD staining in the absence or in the presence of IL-2 or IL-15. The graph summarizes mean ± SEM of 5 independent experiments. *, P < 0.05.
When we looked at T-cell proliferation and expression of antiapoptotic molecules accompanying the exposure to IL-15 (8, 25), we found a differential effect of this cytokine on effector T cells and Tregs, which were also different from the effects that IL-2 had on these populations. First, IL-15 significantly increased the division rate of TEM/EMRA cells (82% ± 3%), and EBV-CTLs (87% ± 2%) as compared with Tregs (58% ± 9%; P = 0.02 and P = 0.01, respectively; Fig. 5B). IL-2, instead, induced proliferation of Tregs (73% ± 7%) comparable with that of TEM/EMRA cells (76% ± 5%) and EBV-CTLs (77% ± 5%; P = NS).
Second, in the presence of IL-15, apoptotic cells (Annexin-V+7AAD−) were significantly lower in TEM/EMRA cells (7% ± 2%) and EBV-CTLs (5% ± 1%) as compared with Tregs (14% ± 3%; P = 0.04 and P = 0.03, respectively; Fig. 5C). In contrast, in the presence of IL-2 the percentage of apoptotic cells was similar in CD45RO+ CD62L− effector cells (11% ± 4%), EBV-CTLs (7% ± 1%), and Tregs (10% ± 3%; P = NS). Hence, our data suggest that IL-15, unlike IL-2, has a differential effect on proliferation and apoptosis in effector and Tregs.
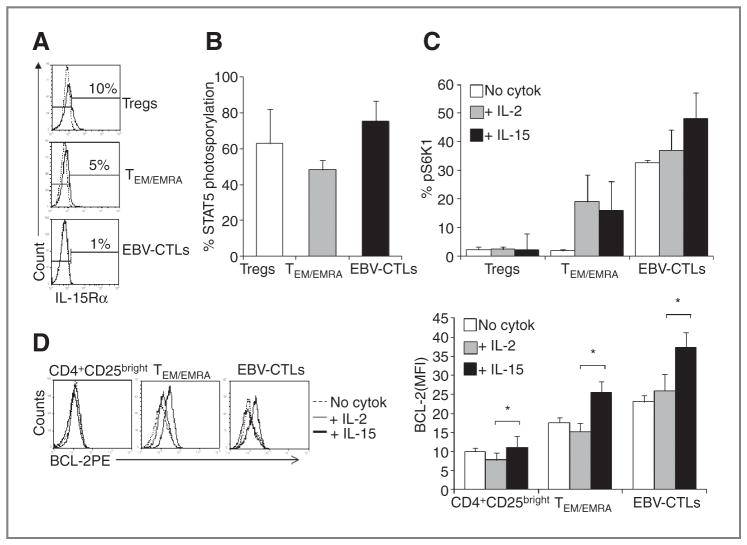
Upon further investigation, we observed that IL-15 activates different pathways in Tregs and effector T cells, despite the expression of the IL-15Rα was similar in all subsets (Fig. 6A), and STAT5 phosphorylation was comparable in Tregs (63% ± 19%) and effector T cells (TEM/EMRA, 48% ± 6%, EBV-CTLs, 76% ± 11%) following exposure to IL-15 (Fig. 6B). We found that in response to IL-15, phosphorylation of S6K1, a downstream target associated with a proliferative signature, was significantly higher in effector cells (TEM/EMRA = 16% ± 7%; EBV-CTLs = 48% ± 9%) as compared with Tregs (2% ± 0.4%; Fig. 6C). A similar pattern was, however, also observed in response to IL-2, with higher phosphorylation of S6K1 in effector T cells (TEM/EMRA = 31% ± 13%; EBV-CTLs = 37% ± 10%) as compared with Tregs (2% ± 1%; Fig. 6C). This prompted us to focus on potential differential effects of IL-2 and IL-15 in the apoptotic pathway.
Figure 6.
IL-15 upregulates pS6K1 and BCL-2 in effector T cells as compared with Tregs. A, the expression of IL-15Rα by Tregs, TEM/EMRA, and EBV-CTLs in 1 representative donor of 5 studied. B, STAT5 phosphorylation of Tregs, TEM/EMRA cells, and EBV-CTLs in the presence of IL-15 as assessed by FACS. The graph summarizes means ± SD for 4 independent experiments. C, the phosphorylation of S6K1 (pS6K1), assessed by FACS, in Tregs, TEM/EMRA cells, and EBV-CTLs in the absence or in the presence of IL-2 or IL-15. The graph summarizes data (means ± SD) from 4 experiments. D, BCL-2 expression by Tregs, TEM/EMRA cells, and EBV-CTLs after stimulation with IL-15. The histograms show BCL-2 expression in 1 representative donor after activation in the absence (dotted line) or in the presence of IL-2 (thin line) or IL-15 (bold line). The graph summarizes the mean ± SEM of 6 independent experiments. *, P < 0.05.
Indeed, effector T cells significantly upregulated BCL-2 in response to IL-15 [mean fluorescence intensity (MFI) from 17 ± 3 to 24 ± 3 for TEM/EMRA, and from 23 ± 2 to 37 ± 4 for EBV-CTLs], which was significantly better as compared with IL-2 (15 ± 2 for TEM/EMRA, and 26 ± 4 for EBV-CTLs), whereas both cytokines induced no equivalent BCL-2 upregulation in Tregs (MFI from 10 ± 1 to 9 ± 1 for IL-15 and to 8 ± 2 for IL-2; Fig. 6D). Overall, these data suggest that the simultaneous preferential proliferation and resistance to apoptosis in effector T cells than in Tregs mediated by IL-15, but not by IL-2, may provide better resilience of effector T cells to Treg-inhibitory activity.
Discussion
The toxicity associated with the systemic administration of γ-chain cytokines, used to support the in vivo persistence of adoptively transferred tumor-specific CTLs (12, 13), and the effects that these cytokines exert on the host inhibitory Tregs (14–17) remain obstacles to improve the clinical benefits of adoptive immunotherapy-based approaches. To address the specific issue of γ-chain cytokines and Tregs, we have analyzed the effects of IL-2 and IL-15 in our model of EBV-CTLs. We show that IL-15, but not IL-2, supports EBV-CTL effector function and proliferation in the presence of Tregs isolated from healthy donors or patients with Hodgkin lymphoma. This protection from Treg-suppressive effects can be related to the enhanced proliferation and reduced activation induced cell death that IL-15 provides to effectors T cells but not to Tregs, likely through the activation of different pathways in effector T cells as compared with Tregs.
The infusion of tumor-infiltrating lymphocytes and EBV-CTLs that have been expanded in vitro has shown clinical benefits in patients with melanoma and EBV-associated malignancies, including Hodgkin lymphoma, respectively (2–5). Yet, IL-2 is often required to sustain CTL persistence and expansion in vivo (9, 10). On the other hand, the administration of IL-2 induces systemic toxicities and enhances proliferation and function of Tregs, a subset of lymphocytes that suppress CTL proliferation and function (14–17). As a result of these dual properties, the administration of IL-2 can be particularly detrimental when EBV-CTLs are adoptively transferred in patients with Hodgkin lymphoma. The expansion of the transferred T cells would indeed be offset by an equivalent or superior expansion of Tregs that are particularly abundant in the peripheral blood and at the tumor site of patients with Hodgkin lymphoma (19, 22–24).
Efforts have been made to identify alternative growth factors that can promote robust engraftment of ex vivo–expanded antigen-specific CTLs without concomitant enhancement of Treg activity. IL-15, that is now entering clinical trials (29, 30), has the potential to be less toxic than IL-2 (8). However, previous preclinical models have produced somewhat contradictory results about the specific effects of IL-15 on Tregs. For example, short preincubation of human T lymphocytes with IL-15 protects their proliferation and IFN-γ production in the presence of Tregs (39). Moreover, studies in nonhuman primates, have shown that IL-15 produces excellent expansion of T cells but limited effects on Tregs (29, 30). These beneficial effects of IL-15 on T lymphocytes concur with the observation that human autoimmune disorders, including rheumatoid arthritis, psoriasis, and celiac diseases, are associated with the local overexpression of IL-15, which may favor a proinflammatory environment allowing autoreactive T cells to overcome the inhibition of functional Tregs (33–35). In contrast, other studies suggest that IL-15 can amplify both Treg number and suppressive function (37, 38), and contributes to their de novo generation (18, 32, 36–39, 51, 52). Finally, IL-2−/− X IL-15−/− mice show significantly decreased numbers of Tregs (31), and treatment with IL-15 promotes Treg activity sufficiently to protect natural killer–depleted non-obese diabetic mice against developing diabetes (38). These apparently disparate results may be in part attributable to differences in the distribution of the expression of the IL-15Rα. Unlike the IL-2α chain (CD25), which is constitutively expressed on Tregs, expression of IL-15Rα is more heterogeneous (53), so that the net effects produced on effector T cells and Tregs may be dependent on the precise target population, cytokine concentration, and read-out used.
Our study has focused on the influence of IL-15 and Tregs specifically on EBV-CTLs and CD4 and CD8 effector subsets in general, when present in a complex cellular mixture, rather than on the selected CD4+CD25depleted population, as the latter may not take into account the effects that each subset provides to the whole population (39, 54). In addition, testing the effect of the cytokines on the CD4/CD8 effector population should better predict the consequences of administering IL-15 as part of clinical studies as the majority of adoptively transferred CTLs are CD4/CD8 effector/cytotoxic T cells (2, 4, 5). In addition, to ensure that our ex vivo studies would better predict clinical relevance, we used a dose of IL-15 that is safely obtained in vivo in nonhuman primates (29, 30). Although lower than concentrations used by many other studies, this is more likely to reflect concentrations that will be obtained in humans. Moreover, we used a CFSE-based assay to measure Treg-mediated inhibition rather than thymidine-incorporation assays that may fail to discriminate between proliferation of Tregs and effector T cells.
We found that IL-15, but not IL-2, supports T-cell effector function in ex vivo coculture experiments that, similarly to tumor environment, put them simultaneously and for a prolonged period of time in contact with tumor cells and Tregs. IL-15, but not IL-2, preserves the proliferation and effector function of T cells and CTLs in the presence of Tregs either isolated from healthy donors or patients with Hodgkin lymphoma. IL-15 also protects CTLs from apoptosis. Hence, by providing IL-15 it may be possible to sustain protection of CTLs against Tregs in vivo.
Why does IL-15 produce these differential effects in antigen-specific CTLs and effector T cells in general as compared with Tregs? Although our experiments illustrate that IL-15 is functional in Tregs, this cytokine does not modify their inhibitory function. However, the proliferation and resistance to apoptosis mediated by IL-15 is greater in effector T cells than in Tregs, so that the net activity of effector T cells is increased, both in term of proliferative potential and cytotoxic activity. We and others (37) have shown that the IL-15Rα is expressed at low level both on Tregs and effector T cells, so that the differential effects of IL-15 on each subset likely lies in differences in downstream signaling and not on different expression levels of the receptor. IL-15 is also active when antigen-presenting cells trans-present the IL-15Rα, thus bypassing the need for IL-15Rα expression by target cells (53). However, this mechanism should be equally available to both effector T cells and Tregs, and again cannot explain the differential effects of IL-15 in these two cell subsets. When tested specifically, we found that IL-15 supports T-cell proliferation in the presence of Tregs, regardless of its presence in a soluble form or in a complex with IL-15Rα. This may have additional important implications in vivo, as the complex IL-15/IL-15Rα not only is more stable, has enhanced activity, and is the preferential form of IL-15 present in the serum of patients (47), but can further reduce the dose of IL-15 required to overcome the inhibitory effect of Tregs, thus further reducing the potential toxicity of this cytokine.
Exploring the downstream signaling of IL-15, we found that STAT5 was equally phosphorylated in effector T cells and Tregs after IL-15 exposure. In contrast, there was divergence in phosphoinositide 3-kinase (PI3K)–mediated signaling between effector T cells and Tregs in response to IL-15, with the former showing greater and simultaneous p70S6Kinase phosphorylation and BCL-2 expression, effects that are respectively consistent with their superior proliferation and resistance to apoptosis. It will be of future interest to analyze interactions with other modulatory pathways to provide a more detailed underpinning for the differential effects on PI3K–mediated signaling we report.
We conclude that as IL-15 benefits both the proliferative and antitumor activity of EBV-CTLs and effector T cells in general, without an equivalent increase in the inhibitory activity of Tregs, this cytokine could be of clinical value for patients with Hodgkin lymphoma and other patients receiving T-cell therapies for their disease, and should be preferred when circulating or tumor-infiltrating Tregs are increased.
Supplementary Material
Translational Relevance.
Adoptive transfer of antigen-specific CTLs represents a promising therapy for patients with cancer, and interleukin (IL)-2 is frequently administered to sustain their in vivo expansion and persistence. However, IL-2 also induces systemic toxicities and boosts the proliferation and function of regulatory T cells (Treg), known to suppress the antitumor activity of CTLs. Therefore, IL-2 administration can be detrimental in patients with cancer with abundant Tregs. In view of these challenges, we studied the benefits of IL-15, another cytokine now available for clinical use to promote the engraftment of ex vivo–expanded CTLs in supporting their antitumor effects despite the presence of Tregs. We studied the effects of IL-15 in our model of Epstein–Barr virus (EBV)–associated Hodgkin lymphomas that can be successfully treated with infusions of EBV-specific CTLs. Our study provides preclinical data, suggesting that IL-15 should be preferred for clinical use when circulating or that tumor-infiltrating Tregs are increased in patients with cancer.
Acknowledgments
Grant Support
This work was supported by grants: P50CA126752 (H.E. Heslop) and R01 CA131027 (B. Savoldo). S.K. Perna is supported by a fellowship from the EHA-ASH Translational Research Training in Hematology; H.E. Heslop is supported by a Dan L Duncan Chair; M.K. Brenner is supported by a Fayez Sarofim Chair; G. Dotti is supported by NIH R01 CA142636, a Leukemia and Lymphoma Society Translational Research grant and W81XWH-10-10425 Department of Defense, Technology/Therapeutic Development Award; B. Savoldo is supported by NIH R01CA131027 and a Leukemia and Lymphoma Society Translational Research grant.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: S.K. Perna, G. Dotti, B. Savoldo
Development of methodology: S.K. Perna, B. De Angelis, G. Dotti, B. Savoldo
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): S.K. Perna, B. De Angelis, H.E. Heslop, G. Dotti, B. Savoldo
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): S.K. Perna, D. Pagliara, G. Dotti, B. Savoldo
Writing, review, and/or revision of the manuscript: S.K. Perna, B. De Angelis, H.E. Heslop, M.K. Brenner, C.M. Rooney, G. Dotti, B. Savoldo
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S.K. Perna, L. Zhang, A. Mahendravada, G. Dotti
Study supervision: G. Dotti, B. Savoldo
Provision of technical assistance for some of the experiments: S.T. Hasan
References
- 1.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 2.Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs) Blood. 2006;108:2942–9. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein–Barr virus+ Hodgkin’s disease. J Exp Med. 2004;200:1623–33. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roskrow MA, Suzuki N, Gan Y, Sixbey JW, Ng CY, Kimbrough S, et al. Epstein–Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin’s disease. Blood. 1998;91:2925–34. [PubMed] [Google Scholar]
- 6.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 7.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–10. [PubMed] [Google Scholar]
- 9.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO, Seipp CA, et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985;135:2865–75. [PubMed] [Google Scholar]
- 12.Atkins MB. Interleukin-2: clinical applications. Semin Oncol. 2002;29:12–7. doi: 10.1053/sonc.2002.33077. [DOI] [PubMed] [Google Scholar]
- 13.Siegel JP, Puri RK. Interleukin-2 toxicity. J Clin Oncol. 1991;9:694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- 14.Zorn E, Mohseni M, Kim H, Porcheray F, Lynch A, Bellucci R, et al. Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:382–8. doi: 10.1016/j.bbmt.2008.12.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A. 2010;107:10632–7. doi: 10.1073/pnas.1000027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sereti I, Imamichi H, Natarajan V, Imamichi T, Ramchandani MS, Badralmaa Y, et al. In vivo expansion of CD4CD45RO-CD25 T cells expressing foxP3 in IL-2-treated HIV-infected patients. J Clin Invest. 2005;115:1839–47. doi: 10.1172/JCI24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–5. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 18.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Qian CN, Zeng YX. Regulatory T cells and EBV associated malignancies. Int Immunopharmacol. 2009;9:590–2. doi: 10.1016/j.intimp.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–12. [PubMed] [Google Scholar]
- 22.Schreck S, Friebel D, Buettner M, Distel L, Grabenbauer G, Young LS, et al. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27:31–9. doi: 10.1002/hon.878. [DOI] [PubMed] [Google Scholar]
- 23.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 24.Baumforth KR, Birgersdotter A, Reynolds GM, Wei W, Kapatai G, Flavell JR, et al. Expression of the Epstein–Barr virus-encoded Epstein–Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldmann TA. IL-15 in the life and death of lymphocytes: immunotherapeutic implications. Trends Mol Med. 2003;9:517–21. doi: 10.1016/j.molmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–74. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in non-human primates. Blood. 2009;114:2417–26. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–95. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. Eur J Immunol. 2007;37:1817–26. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 32.Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA. The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol. 2008;84:973–80. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanzi D, Stefanile R, Santagata S, Iaffaldano L, Iaquinto G, Giardullo N, et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in celiac disease. Am J Gastroenterol. 2011;106:1308–17. doi: 10.1038/ajg.2011.80. [DOI] [PubMed] [Google Scholar]
- 35.Benito-Miguel M, Garcia-Carmona Y, Balsa A, Perez de AC, Cobo-Ibanez T, Martin-Mola E, et al. A dual action of rheumatoid arthritis synovial fibroblast IL-15 expression on the equilibrium between CD4+CD25+ regulatory T cells and CD4+CD25− responder T cells. J Immunol. 2009;183:8268–79. doi: 10.4049/jimmunol.0900007. [DOI] [PubMed] [Google Scholar]
- 36.Imamichi H, Sereti I, Lane HC. IL-15 acts as a potent inducer of CD4(+) CD25(hi) cells expressing FOXP3. Eur J Immunol. 2008;38:1621–30. doi: 10.1002/eji.200737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu S, Sun Z, Sun Y, Zhu J, Li X, Zhang X, et al. IL-15 and dendritic cells induce proliferation of CD4(+)CD25(+) regulatory T cells from peripheral blood. Immunol Lett. 2011;140:59–67. doi: 10.1016/j.imlet.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Xia J, Liu W, Hu B, Tian Z, Yang Y. IL-15 promotes regulatory T cell function and protects against diabetes development in NK-depleted NOD mice. Clin Immunol. 2010;134:130–9. doi: 10.1016/j.clim.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Ben AM, Belhadj HN, Moes N, Buyse S, Abdeladhim M, Louzir H, et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182:6763–70. doi: 10.4049/jimmunol.0801792. [DOI] [PubMed] [Google Scholar]
- 40.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 41.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–96. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piscitelli SC, Forrest A, Vogel S, Chaitt D, Metcalf J, Stevens R, et al. Pharmacokinetic modeling of recombinant interleukin-2 in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 1998;64:492–8. doi: 10.1016/S0009-9236(98)90132-1. [DOI] [PubMed] [Google Scholar]
- 43.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–61. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 44.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 46.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–83. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 47.Bergamaschi C, Bear J, Rosati M, Beach RK, Alicea C, Sowder R, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood. 2012;120:e1–e8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dotti G, Savoldo B, Pule M, Straathof KC, Biagi E, Yvon E, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–84. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunne PJ, Faint JM, Gudgeon NH, Fletcher JM, Plunkett FJ, Soares MV, et al. Epstein–Barr virus-specific CD8(+) T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–40. doi: 10.1182/blood-2002-01-0160. [DOI] [PubMed] [Google Scholar]
- 51.Burchill MA, Yang J, Vang KB, Farrar MA. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol Lett. 2007;114:1–8. doi: 10.1016/j.imlet.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yates J, Rovis F, Mitchell P, Afzali B, Tsang JY, Garin M, et al. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–99. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- 53.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 54.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.