Abstract
Maintenance of energy metabolism and glucose homeostasis is achieved by the regulatory effects of many hormones and their interactions. Glucocorticoids produced from adrenal cortex and adiponectin produced by adipose tissue play important roles in the production, distribution, storage, and utilization of energy substrates. Glucocorticoids are involved in the activation of a number of catabolic processes by affecting the expression of a plethora of genes, while adiponectin acts primarily as an insulin sensitizer. Both are regulated by a number of physiological and pharmacological factors. Although the effects of glucocorticoids on adiponectin expression have been extensively studied in different in vitro, animal and clinical study settings, no consensus has been reached. This report reviews the primary literature concerning the effects of glucocorticoids on adiponectin expression and identifies potential reasons for the contradictory results between different studies. In addition, methods to gain better insights pertaining to the regulation of adiponectin expression are discussed.
1. INTRODUCTION
Maintenance of energy metabolism and glucose homeostasis is crucial for proper functioning of an organism. These processes are regulated by a number of complex mechanisms occurring in different tissues and cell types. An extensive network of organs including brain, liver, muscle, adipose tissue, pancreas, and adrenal gland coordinate to maintain this homeostasis and any imbalance in this coordination can lead to metabolic disorders including diabetes, obesity, and metabolic syndrome (Besser & Jeffcoate, 1976; Tirone & Brunicardi, 2001; Yamada, Oka, & Katagiri, 2008). Hormones play an important role in communication between organs and are one of the central components controlling various metabolic processes. Many hormones including insulin, glucagon, glucocorticoids (GCs), leptin, and adiponectin control pathways and gene expression changes related to energy metabolism and its regulation (Eyster, 2011; Heppner et al., 2010; Rose, Vegiopoulos, & Herzig, 2010; Yamada et al., 2008). These different hormones are produced from different organs (insulin and glucagon from pancreatic islets, GCs from adrenal cortex, and leptin and adiponectin from adipose tissue) and affect multiple tissues that express the receptors for these hormones (Kershaw & Flier, 2004; Lukens, 1959; Rose et al., 2010). In addition to regulating overlapping processes in energy metabolism, these hormones can affect the production of each other, thereby causing feedback regulation of homeostasis. This report summarizes previous in vitro, preclinical and clinical studies pertaining to the effects of GCs on adiponectin expression and explores their implications.
2. GLUCOCORTICOIDS AND THEIR EFFECTS ON GENE EXPRESSION
GCs are catabolic steroid hormones produced from the adrenal cortex which play an important role in the production, distribution, storage, and utilization of substrates used for energy metabolism (Vegiopoulos & Herzig, 2007). In addition to their effects on energy metabolism, GCs play an important role in regulating inflammatory and immune response processes (Barnes, 1998). GCs suppress these processes, and because of their anti-inflammatory properties, synthetic GCs (such as dexamethasone, prednisolone, methylprednisolone (MP)) are extensively used for the treatment of inflammatory disorders including asthma, rheumatoid arthritis, and lupus erythematosus (Barnes, 1998; Swartz & Dluhy, 1978). However, because of their effects on systemic energy balance, chronic use results in severe side effects including steroid-induced diabetes, dyslipidemia, muscle atrophy, and metabolic syndrome (Rhen & Cidlowski, 2005; Swartz & Dluhy, 1978).
The hypothalamic–pituitary–adrenal axis plays a critical role in regulating the production of GC (Fig. 7.1; Tsigos & Chrousos, 2002). This includes the sequential and feedback regulation by a series of hormones produced by different organs. Corticotropin-releasing hormone (CRH) produced from the hypothalamus positively regulates adrenocorticotropic hormone (ACTH) production from the pituitary which in turn influences GC production in the adrenal cortex. A negative feedback loop in this axis is caused by the negative regulation in the production of CRH and ACTH by GC (Tsigos & Chrousos, 2002).
Figure 7.1.
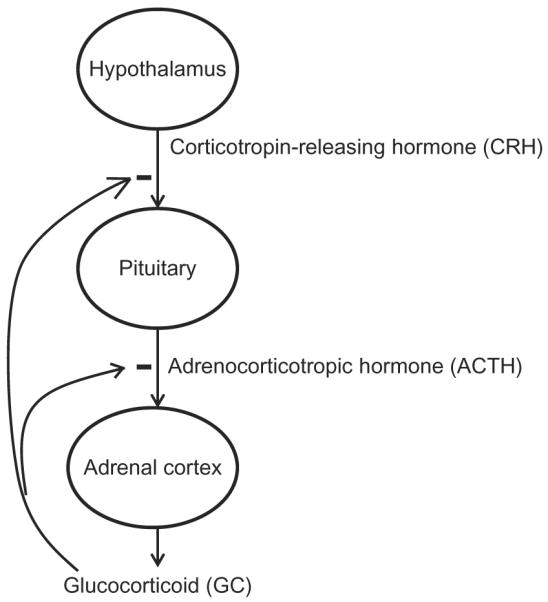
Regulation of glucocorticoids production by hypothalamic–pituitary–adrenal axis represents inhibition in the production.
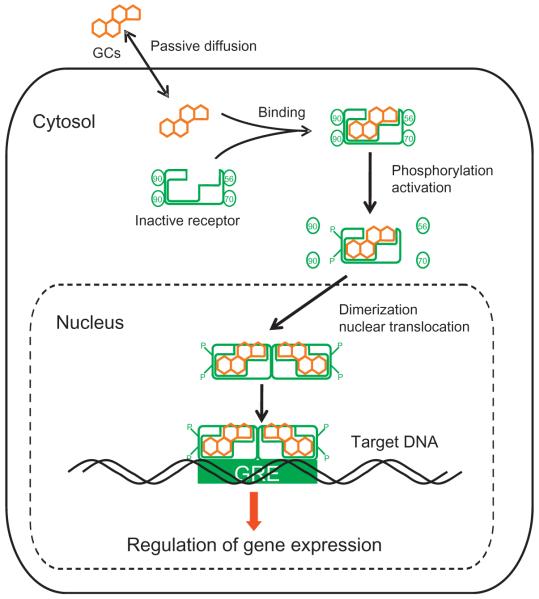
The molecular mechanism by which GCs regulate gene expression is given in Fig. 7.2 (Schaaf & Cidlowski, 2002; Sukumaran, Jusko, DuBois, & Almon, 2011). Since GCs are highly lipophilic, they can passively diffuse through cell membranes. In the absence of the hormone, the cytosolic receptor for GC is present as an inactive state complexed with heat shock proteins. Binding of GC to its cytosolic receptor results in conformational change, phosphorylation, dimerization, and activation of the receptor (Schaaf & Cidlowski, 2002). The activated GC–receptor complex rapidly translocates into the nucleus and regulates gene expression. Multiple mechanisms including direct binding, tethering, and composite binding exist by which GC can positively (transactivation) or negatively (transrepression) regulate gene expression (Oakley & Cidlowski, 2011; Schaaf & Cidlowski, 2002). On the one hand, in the case of direct binding, the activated hormone–receptor complex binds to specific DNA regions referred to as GC response elements, thereby modulating transcription rates (Schaaf & Cidlowski, 2002). On the other hand, GC–receptor complex in the nucleus can interact with other transcription factors, activators, and repressors, thereby modulating the rate of transcription of their regulated genes. For example, activated GC receptors interact with transcription factors like NfκB, AP-1, and STAT and affect the mRNA expression of many genes that are regulated by these transcription factors (Barnes, 1998; Schaaf & Cidlowski, 2002). Microarray and gene expression studies show that GC directly or indirectly regulate a vast array of genes and pathways in many tissues, suggesting its influence on many key biological processes (Almon, DuBois, & Jusko, 2007; Almon, DuBois, Pearson, Stephan, & Jusko, 2003; Almon, DuBois, Piel, & Jusko, 2004; Almon, Lai, DuBois, & Jusko, 2005; Almon et al., 2007).
Figure 7.2.
Molecular mechanism for the regulation of gene expression by glucocorticoids.
3. EFFECTS OF GC AND ADIPONECTIN ON ENERGY METABOLISM
One of the most important functions of GC is to maintain proper plasma glucose concentrations to feed neuronal cells under conditions of decreased glucose availability from the diet (Heppner et al., 2010; Vegiopoulos & Herzig, 2007). To accomplish this effect, GCs affect a plethora of processes ranging from production to utilization and disposal of carbohydrates, lipids, and other energy substrates involving different organs and cell types (Vegiopoulos & Herzig, 2007). GC concentrations in plasma increase during starvation and under stress. During normal conditions, GCs show robust circadian oscillations with peak concentrations occurring at the early active period (early morning for humans and early dark period for nocturnal animals), thus preparing the body for the upcoming period of activity (Sukumaran, Almon, DuBois, & Jusko, 2010). GCs increase glucose production by primarily upregulating gluconeogenesis in liver (and sometimes in kidney) (Gunn, Hanson, Meyuhas, Reshef, & Ballard, 1975; Iynedjian, Ballard, & Hanson, 1975; Vegiopoulos & Herzig, 2007). On the utilization side, GCs cause insulin resistance in many tissues including skeletal muscle and adipose tissue, thereby reducing insulin-directed disposition of glucose (Andrews & Walker, 1999). GCs stimulate protein breakdown in skeletal muscle to provide the carbon backbone from amino acids to fuel gluconeogenesis in liver (Hasselgren, 1999). In addition, GCs stimulate lipolysis in white adipose tissue producing free fatty acids for many tissues and glycerol for gluconeogenesis. In addition, GCs also inhibit lipid synthesis (Vegiopoulos & Herzig, 2007). All these processes are regulated through changes in gene expression (which eventually causes change in protein expression) and signaling cascades caused by GC (Vegiopoulos & Herzig, 2007).
Adiponectin, the adipokine produced primarily from white adipose tissue, is a very important hormone involved in metabolic processes (Kadowaki & Yamauchi, 2005). The effects of adiponectin are primarily mediated through their membrane-bound G protein-coupled receptors, AdipoR1 and AdipoR2 are expressed in multiple tissues and organs and regulate MAP kinase, AMP kinase, and PPARγ signaling cascades (Dridi & Taouis, 2009; Kadowaki & Yamauchi, 2005). The primary function of adiponectin is to act as an endogenous insulin sensitizer, and it has a major influence on free fatty acids metabolism. Studies show that adiponectin increases glucose utilization in skeletal muscle, decreases gluconeogenesis in liver, and increases fatty acid oxidation in both liver and skeletal muscle (Dridi & Taouis, 2009). The plasma concentrations of adiponectin are inversely proportional to the fat mass present and hence adiponectin concentrations are decreased in obese patients which serves as one cause for their insulin resistance (Swarbrick & Havel, 2008).
Thus, GC and adiponectin play opposite roles in regulating energy metabolism with GC primarily involved in activating catabolic processes and insulin resistance, but adiponectin acting primarily as an insulin sensitizer (Kadowaki & Yamauchi, 2005; Vegiopoulos & Herzig, 2007). However, with respect to immune and inflammatory processes, both GC and adiponectin are anti-inflammatory in their effects (Barnes, 1998; Ouchi & Walsh, 2007).
4. IN VITRO AND ANIMAL STUDIES ON THE EFFECTS OF GC ON ADIPONECTIN EXPRESSION
Initial studies on the regulation of adiponectin expression by GC and other hormones were done in the early 2000s, mainly in in vitro systems using human or murine adipocytes. Such studies produced conflicting results. For example, studies by Halleux et al. showed that in human visceral adipose tissue explants dexamethasone (dex) treatment downregulated adiponectin mRNA and protein expression, while insulin had the opposite effect of upregulating the expression in a dose-dependent manner (Halleux et al., 2001). Similarly, dex treatment decreased adiponectin mRNA expression in 3T3 murine adipocytes (Fasshauer, Klein, Neumann, Eszlinger, & Paschke, 2002). In contrast, in a study by Oliveira et al., dex treatment did not cause any change in adiponectin mRNA expression in 3T3-L1 adipocytes (de Oliveira et al., 2011b). Similar results were observed in studies on canine adipocytes, where dex treatment did not cause any changes in adiponectin expression (Ryan et al., 2010). Likewise, in a recent study using human bone marrow adipocytes, dex treatment did not have any effect on either adiponectin gene expression or secretion (Hozumi et al., 2010). The results from these in vitro studies are summarized in Table 7.1. Data from animal studies also yield inconsistent results on the effects of GC on adiponectin expression (Table 7.2). A study by Combs et al. shows that Swiss Webster mice treated with prednisolone from a slow release pellet for 28 days showed a significant increase in plasma adiponectin concentrations (Combs et al., 2003). Similarly, in neonatal Sprague–Dawley rats, dex treatment resulted in increased plasma adiponectin concentrations (Raff & Bruder, 2006). However, other studies give contrasting results on the effects of GC on adiponectin expression. 11β-Hydroxysteroid dehydrogenases (11βHSD) are enzymes involved in tissue-specific activation or deactivation of GC (Tomlinson et al., 2004). 11βHSD1 is involved in the activation of GCs and 11βHSD2 is involved in the inactivation of GC. Inactivation of 11βHSD1 results in decreased intracellular GC concentrations. Morton et al. showed that tissue-specific knockout of 11βHSD1 in adipose tissue in mice results in increased adiponectin gene expression (Morton et al., 2004). Similarly, Kershaw et al. showed that transgenic tissue-specific overexpression of 11βHSD2 in adipose tissue in high-fat fed mice results in a significant increase in adiponectin gene expression, although no significant changes in expression were observed in normal chow fed animals (Kershaw et al., 2005). In addition, a study by Shi et al. showed that chronic GC treatment results in decreased adiponectin mRNA expression and serum adiponectin concentrations (Shi et al., 2010). In this study, 7-week-old Sprague–Dawley rats were given either 5 or 15 mg/kg/day of hydrocortisone via intraperitoneal injection for 20 days with normal chow diet or high-fat diet. Low-dose treatment in normal chow diet fed animals (5 mg/kg) showed only modest decreases in mRNA expression and serum concentrations which were not statistically significant, but high-dose treatment in normal chow diet fed and both dosing regimens in high-fat fed groups showed significant decreases in both mRNA expression and serum concentrations of adiponectin.
Table 7.1.
Summary of in vitro studies on the effects of GC on adiponectin expression
| References | Type of GC | Concentration and duration of exposure | Culture system | Effects on adiponectin mRNA/protein expression |
|---|---|---|---|---|
| Downregulation | ||||
| Fasshauer et al. (2003) | Dexamethasone | 100 nM for 16 h | 3T3-L1 preadipocytes | Decrease in protein expression/secretion |
| Fasshauer et al. (2002) | Dexamethasone | 100 nM for 16 h | 3T3-L1 preadipocytes | Decrease in mRNA expression |
| Halleux et al. (2001) | Dexamethasone | 50 nM for 20 h | Isolated adipocytes from human visceral adipose tissue | Decrease in mRNA expression |
| Degawa-Yamauchi et al. (2005) | Dexamethasone | 100 nM for 48 h | Human subcutaneous adipocytes | Decrease in protein secretion |
| No regulation | ||||
| Hozumi et al. (2010) | Dexamethasone | 1 μM for 24 h | Human bone marrow adipocytes | No change in protein secretion |
| Sakamoto et al. (2011) | Dexamethasone | 1 μM for 24 h | Human bone marrow adipocytes | No change in mRNA expression |
| Ryan et al. (2010) | Dexamethasone | 2 and 20 nM for 2 and 24 h | Differentiated canine adipocytes | No change in mRNA expression |
| de Oliveira, Iwanaga-Carvalho, et al. (2011b) | Dexamethasone | 1 μM for 24 h | 3T3-L1 adipocytes | No change in mRNA expression |
Table 7.2.
Summary of animal studies on the effects of GC on adiponectin expression
| References | Type of GC | Dosing regimen | Model system | Effects on adiponectin mRNA/protein expression |
|---|---|---|---|---|
| Negative regulation | ||||
| Shi et al. (2010) | Hydrocortisone | Peritoneal injection of 5 or 15 mg/kg/day for 20 days | SPF Sprague–Dawley rats | Decrease in mRNA expression and plasma protein concentrations |
| Morton et al. (2004) | Predicted decrease in intracellular GC in adipose tissue | NA | 11βHSD1 knockout in adipose tissue and matched wild type controls | 11βHSD1 KO increases adiponectin mRNA expression |
| Kershaw et al. (2005) | Predicted decrease in intracellular GC in adipose tissue | NA | 11βHSD2 overexpression in adipose tissue with high-fat feeding | 11βHSD2 overexpression in adipose tissue of high-fat fed animals results in increase in adiponectin mRNA expression |
| Positive regulation | ||||
| Combs et al. (2003) | Prednisolone | 2.1 mg/kg/day for 28 days | Swiss Webster mice | Increase in plasma protein concentrations |
| Raff and Bruder (2006) | Dexamethasone | Subcutaneous tapering dosing regimen (from 0.5 mg/kg to 0.05 mg/kg) | Neonatal Sprague–Dawley rats | Increase in plasma protein concentrations |
| Effects of adrenalectomy | ||||
| Makimura, Mizuno, Bergen, and Mobbs (2002) | Removal of endogenous GC | NA | ob/ob and matched wild type mice | Increase in serum protein and mRNA expression in ob/ob mice but decrease in serum protein and no change in mRNA expression in wild type |
| de Oliveira et al. (2011a) | Removal of endogenous GC and supplementation with dexamethasone | Subcutaneous dosing of 2 mg/kg, twice daily for 3 days | Male Wistar rats | Decrease in serum adiponectin and mRNA expression in epididymal fat with dex dosing |
| de Oliveira, Iwanaga-Carvalho, et al. (2011b) | Removal of endogenous GC and supplementation with dexamethasone | Subcutaneous dosing of 2 mg/kg, twice daily for 3 days | Male Wistar rats | Decrease in serum adiponectin with dex dosing and decrease in mRNA expression in epididymal fat with adrenalectomy |
4.1. Effects of adrenalectomy on adiponectin expression in murine models
Adrenalectomy is the surgical removal of adrenal gland which produces GC. In addition to GC, the adrenal gland also produces mineralocorticoids, epinephrine, and norepinephrine (Randall, 2004). Makimura et al. first explored the effects of adrenalectomy on adiponectin expression in wild type and obese ob/ob mice (Makimura et al., 2002). Adrenalectomy did not have any significant effect on adiponectin mRNA expression in epididymal fat pads from wild type animals but causes a significant increase in the mRNA expression in obese ob/ob rats. However, serum adiponectin concentrations were decreased in wild-type animals after adrenalectomy, while the concentrations are increased in ob/ob mice after adrenalectomy in accordance with its mRNA expression. Recent studies by Oliveira et al. in male Wistar rats show some interesting results on the effect of adrenalectomy and exogenous GC supplementation on the regulation of adiponectin expression (de Oliveira, de Mattos, et al., 2011a,b, de Oliveira, Iwanaga-Carvalho, et al., 2011). Adrenalectomy did not have any significant effect on the serum adiponectin concentrations, but subcutaneous injection of 2 mg/kg of dex, twice a day for 3 days to these adrenalectomized rats, caused a significant decrease in adiponectin. However, the effects of adrenalectomy and dex on adiponectin mRNA expression in adipose tissue are highly dependent on the type of fat depot studied. For example, adrenalectomy has no significant effect on adiponectin mRNA expression in retroperitoneal and epididymal adipose tissue but significantly upregulates the expression in subcutaneous adipose tissue. In addition, dex supplementation does not affect adiponectin mRNA expression in retroperitoneal and subcutaneous adipose tissue but significantly downregulates the expression in epididymal fat. These results suggest that regulation of adiponectin expression is not only tissue specific but also depot specific in adipose tissue.
5. CLINICAL STUDIES ON THE EFFECTS OF GC ON ADIPONECTIN EXPRESSION
Because of the clinical importance of adiponectin and GC in conditions like insulin resistance, diabetes, and metabolic syndrome, many studies on the effects of GC on adiponectin were done in healthy human volunteers and patients (Table 7.3). Similar to in vitro and animal studies, results from clinical studies are also somewhat contradictory, although many of the studies actually suggest that GCs increase adiponectin expression. Jang et al. studied the effects of type 2 diabetes and GC treatment on plasma adiponectin concentrations (Jang et al., 2008). Plasma adiponectin was lower in diabetes patients compared to healthy volunteers, and the concentrations were significantly increased after 4 mg daily oral dosing of dexamethasone for 4 days in both healthy volunteers and diabetics. Similarly, in a double-blinded, randomized crossover study by Rieth et al., prednisolone dosing caused a significant increase in the blood adiponectin concentrations compared to placebo (Rieth et al., 2009). In another study by Uchida et al., 500 mg/day MP dosing for 3 days resulted in a significant increase in serum adiponectin concentrations in patients with IgA nephropathy (Uchida et al., 2006). Correlation studies by Hjelmesaeth et al. in patients with renal transplantation suggest that GC treatment is associated with increased serum adiponectin concentrations (Hjelmesaeth et al., 2006). Kreiner et al. showed that plasma adiponectin concentrations were lower in polymyalgia rheumatic patients, and 20 mg/day of prednisolone dosing for 14 days significantly increases plasma concentrations of adiponectin in both polymyalgia rheumatic and control patients (Kreiner & Galbo, 2010). Similar observations were reported in the study done by Cimmino et al. where volunteers were dosed with 25 mg/day prednisone for a period of 1 month (Cimmino et al., 2010).
Table 7.3.
Summary of clinical studies on the effects of GC on adiponectin expression
| References | Type of GC | Dosing regimen | Model system | Effects on adiponectin mRNA/protein expression |
|---|---|---|---|---|
| Negative regulation | ||||
| Fallo et al. (2004) | Hydrocortisone for normal volunteers and increased cortisol in Cushing's syndrome patients | 25 mg I.V bolus and blood taken from 0–180 min | Healthy volunteers and Cushing's syndrome patients | Decrease in plasma adiponectin in the time series study with concentrations significantly low at 30 and 60 min after drug dosing. Plasma concentrations low in Cushing's syndrome patients |
| Krsek et al. (2004) | Increased cortisol in Cushing's syndrome patients | NA | Female patients with Cushing's syndrome | Lower adiponectin concentrations in patients but difference is not significant |
| Positive regulation | ||||
| Uchida et al. (2006) | Methylprednisolone | 500 mg/day dosing for 3 days | Patients with IgA nephropathy | Increase in serum adiponectin concentrations |
| Hjelmesaeth et al. (2006) | Prednisolone | 15±7 mg | Patients with renal transplantation | Dosing associated with increased serum adiponectin concentrations |
| Jang, Inder, Obeyesekere, and Alford (2008) | Dexamethasone | 4 mg daily oral dosing for 4 days | Healthy volunteers and diabetes patients | Increase in plasma adiponectin concentrations |
| Rieth et al. (2009) | Prednisolone | 60 mg/day for 1 week | Healthy volunteers with regular sport practice | Increase in blood adiponectin concentrations |
| Cimmino et al. (2010) | Prednisone | 25 mg/day for 1 month | Polymyalgia rheumatic patients | Increase in plasma adiponectin concentrations |
| Kreiner and Galbo (2010) | Prednisolone | 20 mg/day for 14 days | Normal and polymyalgia rheumatic patients | Increase in plasma adiponectin concentrations |
| No regulation | ||||
| Libe et al. (2005) | Increased cortisol in Cushing's syndrome patients | NA | Female patients with Cushing's syndrome and normal volunteers | No difference in plasma adiponectin concentrations |
| Lewandowski, Szosland, and Lewinski (2006) | Dexamethasone | Oral dosing of 0.5 mg every 6 h for 1 or 2 days | Normal female volunteers | No change in serum adiponectin concentrations |
| Darmon et al. (2006) | Hydrocortisone | 3 h infusion of 1.5 μg·kg−1 min−1 | Normal and obese subjects | No change in plasma adiponectin concentrations |
In contrast to the above studies, Fallo et al. showed that GC dosing decreased plasma adiponectin concentrations in healthy volunteers (Fallo et al., 2004). This was a time series study where a single dose of 25 mg hydrocortisone was given intravenously with blood samples taken from −15 to 180 min after dosing. Hydrocortisone caused a slight but significant decrease in plasma adiponectin at 30 and 60 min after dosing, and the levels eventually returned to baseline control values. This study also showed that plasma adiponectin concentrations were lower in patients with Cushing's syndrome who have elevated endogenous GC concentrations. Another study looking at adipokine concentrations in Cushing's syndrome patients shows that adiponectin concentrations are lower in these patients compared to normal subjects although the differences were not statistically significant (Krsek et al., 2004). A study by Libe et al. reported that the plasma adiponectin concentrations were not different between Cushing's syndrome and healthy volunteers (Libe et al., 2005). In another study by Darmon et al., 3-h infusion of 1.5 μg·kg−1 min−1 hydrocortisone in normal and obese subjects did not cause any significant change in plasma adiponectin concentrations (Darmon et al., 2006). Similarly, a dose of 0.5 mg of dexamethasone every 6 h for 48 h in human subjects did not cause any significant change in serum adiponectin concentrations (Lewandowski et al., 2006).
6. OTHER HORMONES, FACTORS, AND CONDITIONS THAT REGULATE ADIPONECTIN EXPRESSION AND THEIR RELATIONSHIP TO GC
One of the main reasons for the contradictory results for the effects of GC on adiponectin expression is due to the fact that adiponectin is regulated by many different hormones, transcription factors, and disease conditions (Fig. 7.3). GC can regulate some of these factors, and hence based on the specific condition, the effects of GC on adiponectin expression can differ. One of the main factors that regulate adiponectin expression is the amount of adipose tissue present (Swarbrick & Havel, 2008). Adiponectin expression is negatively correlated to the fat mass, and increases in fat mass result in decreased expression of adiponectin. This is one of the primary reasons for reduced concentrations of plasma adiponectin in obese patients. Similarly, adiponectin concentrations are found to be decreased in insulin-resistant conditions and diabetes (Spranger et al., 2003; Ziemke & Mantzoros, 2010). Many of the adipogenic transcription factors including PPARγ, C/EBPα, C/EBPβ, FOXO1, SIRT1, and Sp1 are found to enhance adiponectin gene expression (Liu & Liu, 2010). In contrast, CREB, AP-2bβ, and NFAT are found to negatively regulate its expression (Liu & Liu, 2010). PPARγ agonists like thiazolidinediones which are extensively used as antidiabetic drugs are found to stimulate adiponectin gene expression in both in vitro and in vivo studies (Iwaki et al., 2003; Yu et al., 2002). Similarly, ACE inhibitors, AT1R blockers, and CB1 antagonist are also found to stimulate adiponectin expression, while β blockers are found to have inhibitory effects (Swarbrick & Havel, 2008). Plasma adiponectin concentrations are also sexually dimorphic with higher concentrations observed in females compared to males (Swarbrick & Havel, 2008).
Figure 7.3.
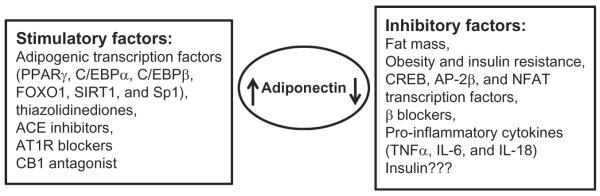
Factors regulating adiponectin expression.
Inflammatory states are another important factor found to regulate adiponectin expression (Swarbrick & Havel, 2008). Increased macrophage infiltration in adipose tissue because of increased fat mass and obesity is implicated with many clinical conditions including diabetes, insulin resistance, and metabolic syndrome (Wang, Mariman, Renes, & Keijer, 2008). Various proinflammatory cytokines including TNFα, IL-6, and IL-18 produced from these infiltrated macrophages are found to adversely affect the expression of adiponectin from adipose tissue (Fasshauer & Paschke, 2003; Liu & Liu, 2010). TNFα can inhibit adiponectin expression by suppressing the expression of adipogenic transcription factors including PPARγ, C/EBP, and SREBP, while IL-6 and IL-18 effects are mediated through MAPK signaling cascades (Liu & Liu, 2010). In spite of the fact that inflammatory factors reduce adiponectin expression, plasma adiponectin concentrations are found to be higher in inflammatory and autoimmune diseases including osteoarthritis, rheumatoid arthritis, systemic lupus erythematosus, type 1 diabetes, and inflammatory bowel disease, except for polymyalgia rheumatic where the concentrations are found to be lower in patients compared to normal subjects (Kreiner & Galbo, 2010; Otero et al., 2006; Stofkova, 2009).
Several studies have been performed on the effect of insulin on adiponectin expression, but like GC, the role of insulin on adiponectin remains controversial and different studies suggests different results ranging from positive regulation to negative regulation. Studies by Fasshauer et al. and Xu et al. in 3T3-L1 adipocytes show that insulin treatment decreases adiponectin gene expression (Fasshauer et al., 2002; Xu et al., 2004). However, the study by Halleux et al. showed that insulin treatment increased adiponectin gene expression in a concentration-dependent manner in human visceral adipose tissue (Halleux et al., 2001). Hyperinsulinemic-euglycemic clamp studies in humans suggest that the increase in insulin concentrations decreases circulating adiponectin concentrations (Brame, Considine, Yamauchi, Baron, & Mather, 2005; Mohlig et al., 2002). In addition, tissue-specific knockout of insulin receptor in adipose tissue resulted in an increase in plasma adiponectin, suggesting that insulin has inhibitory effects on adiponectin expression (Bluher et al., 2002).
7. POTENTIAL REASONS FOR CONTRADICTORY RESULTS ON THE EFFECTS OF GC ON ADIPONECTIN EXPRESSION AND METHODS THAT CAN BE USEFUL IN GAINING BETTER INSIGHT ABOUT THIS REGULATION
There are multiple potential reasons why different studies on the effects of GC on adiponectin expression show contradictory results. Some of the reasons are as follows:
A plethora of mechanisms (different transcription factors, signaling cascades, and hormones) regulate adiponectin expression (Fasshauer & Paschke, 2003; Liu & Liu, 2010; Swarbrick & Havel, 2008). GCs are known to regulate, directly or indirectly, the expression of many different genes, signaling cascades, and transcription factors, some of which are involved in regulating adiponectin expression. For example, GCs increase plasma glucose concentrations which subsequently increase plasma insulin which can regulate adiponectin expression (Jin & Jusko, 2009). Similarly, GCs influence adipogenesis and adipocyte differentiation and are also known to affect adipogenic transcription factors including C/EBP and PPARγ, all of which can influence adiponectin expression (MacDougald & Mandrup, 2002; Ringold, Chapman, Knight, & Torti, 1986). In addition, GCs are potent anti-inflammatory agents and cause reduction in proinflammatory cytokines, which again can indirectly regulate adiponectin expression (Barnes, 1998; Rhen & Cidlowski, 2005). Furthermore, the levels of these regulatory factors, their response to GC, and their effects on adiponectin can vary for different conditions, and hence the final outcome of the study of GC on adiponectin expression will be conditionally dependent. This is one of the main reasons why it is difficult to predict the outcome of the effects in vivo just based on in vitro data, to extrapolate data from animal studies to humans, and to predict the effects in disease conditions from data based on normal subjects and vice versa.
Only one-third of adipose tissue is made up of mature adipocytes, and the remaining tissue is made up of preadipocytes, mesenchymal stem cells, immune cells (predominantly macrophages), and vasculature (Wang et al., 2008). In addition, adipose tissue can be broadly classified into white and brown adipose tissue (Bjorndal, Burri, Staalesen, Skorve, & Berge, 2011; Wang et al., 2008). White adipose tissue can be divided into subcutaneous and intraabdominal adipose tissue, and intraabdominal fat is further divided into mesenteric, epididymal, and retroperitoneal fat based on their location (Bjorndal et al., 2011). All these variations can cause difference in response to external signals and hence affect the outcome of the regulation in expression. A good example was described in an earlier section, where the effects of adrenalectomy and dex treatment on adiponectin expression were highly dependent on the type of fat depot studied (de Oliveira, de Mattos, et al., 2011a).
One major problem in almost all of these reports is that these are single time point studies. Especially, with in vivo experiments, because of the complex matrix of factors involved in regulating adiponectin expression and since these factors have their own time course response profiles after GC dosing, adiponectin expression can show complex time course response patterns after drug dosing (with upregulation at some time points and down or no regulation other time points depending on the collective effects of the factors affected by the drug dosing). Hence the time at which the measurement was made with respect to the dosing time could make a difference.
Other factors that could possibly cause inconsistency in the results between different studies could be the dose amount, differences between the synthetic GC used for the study, routes of administration, strain, and species differences, which again could produce a difference in response based on the changes in the factors that can regulate adiponectin expression.
To address some of these concerns and to gain better insights to understand the regulation of adiponectin expression by GC, a rich time course study in rats was performed in our laboratory (Sukumaran et al., 2011). In this study, 54 male Wistar rats were given 50 mg/kg MP as an intramuscular injection and the animals were sacrificed at 18 different time points (from 0.25 to 96 h). Six animals treated with saline were used as controls. After sacrifice, different organs and plasma were harvested and stored at −80 °C. A wide range of measurements including plasma MP, glucose, free fatty acids and insulin concentrations, and gene expression of GC receptor in white adipose tissue were made along with adiponectin gene expression and plasma protein concentrations. All measurements were made at 18 time points after MP administration (three animal replicates per time point) to understand the dynamic changes in their concentrations and to characterize the direct and indirect effects of GC dosing on adiponectin expression. Because of the complexities involved in the time-dependent regulation of all these factors, a mathematical model reflecting the mechanism of GC-mediated gene expression was developed to describe these dynamics in order to better characterize the effects of GC on adiponectin expression. After intramuscular dosing, plasma concentrations of MP decline biexponentially and are not detected in plasma 8 h after dosing. Plasma glucose and free fatty acid concentrations were increased after drug dosing, which resulted in an increase in plasma insulin concentrations. The model showed that the plasma insulin concentrations peak at 16 h after MP dosing, which eventually went back to its baseline. The GCs are known to cause downregulation of their own receptor expression, which was incorporated in the model to obtain a more quantitative understanding of the drug effects. MP dosing shows an interesting effect on adiponectin gene expression and plasma adiponectin concentrations as shown in Fig. 7.4. Adiponectin gene expression showed a very quick increase with the peak occurring at around 2 h after drug dosing followed by a delayed downregulation with the concentrations falling below the controls, reaching its nadir at 15 h. Subsequently, the expression goes back to the baseline at around 36 h and stays at the baseline values after that. The plasma adiponectin concentrations showed a similar profile after GC dosing, with peak occurring almost immediately after drug dosing, the nadir occurring at 24 h with reestablishment of the baseline only after 84 h. These results suggest that GC acting through its receptor in white adipose tissue directly upregulates adiponectin gene expression, while the increase in plasma insulin after GC dosing results in the downregulation of adiponectin expression, which is supported by the data and the mathematical model. This study shows the importance of doing time series experiments because if the measurements of adiponectin mRNA expression and plasma concentrations were made only at the early time points after drug dosing, then the conclusion will be that GC upregulates the expression, while if the measurement were made only after several hours, then the conclusion will be that GC downregulates the expression, and if the measurements were made only at the later time points, then the conclusion would have been no regulation. Similar approaches can be taken to incorporate other physiological factors to get a quantitative assessment of the regulation of adiponectin gene expression by GC, which will give a better insight about the mechanisms involved in these processes.
Figure 7.4.
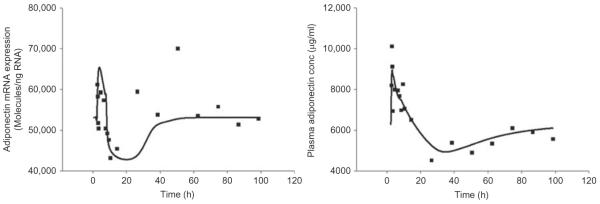
Effects of methylprednisolone dosing on adiponectin mRNA expression in white adipose tissue (left) and plasma adiponectin concentrations (right) in Wistar rats. Solid squares represent the measured data and solid curve represents the prediction from the mathematical model (Sukumaran et al., 2011).
8. CONCLUSION
Both GC and adiponectin are important hormones involved in the regulation of energy metabolism and are implicated in many clinical conditions including metabolic syndrome, diabetes, and obesity. Although extensively studied, no consensus exists on the exact effects of GC on adiponectin expression. Complex regulation of adiponectin expression by many physiological factors, conditions, tissue type, and depot-specific differences can be the cause for the discrepancy in the observation between different studies performed using different model systems, and hence caution should be exerted in extrapolating data from one system to another. One way to achieve better insights concerning the regulation of adiponectin expression is to perform rich time series experiments after GC dosing and measure all relevant physiological factors to develop a quantitative model that will differentiate the effects of different factors affected by GC on the expression of adiponectin.
ACKNOWLEGMENT
This work was supported by grant GM24211 from the National Institutes of Health.
REFERENCES
- Almon RR, DuBois DC, Jusko WJ. A microarray analysis of the temporal response of liver to methylprednisolone: A comparative analysis of two dosing regimens. Endocrinology. 2007;148:2209–2225. doi: 10.1210/en.2006-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almon RR, DuBois DC, Pearson KE, Stephan DA, Jusko WJ. Gene arrays and temporal patterns of drug response: Corticosteroid effects on rat liver. Functional & Integrative Genomics. 2003;3:171–179. doi: 10.1007/s10142-003-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almon RR, DuBois DC, Piel WH, Jusko WJ. The genomic response of skeletal muscle to methylprednisolone using microarrays: Tailoring data mining to the structure of the pharmacogenomic time series. Pharmacogenomics. 2004;5:525–552. doi: 10.1517/14622416.5.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almon RR, DuBois DC, Yao Z, Hoffman EP, Ghimbovschi S, Jusko WJ. Microarray analysis of the temporal response of skeletal muscle to methylprednisolone: Comparative analysis of two dosing regimens. Physiological Genomics. 2007;30:282–299. doi: 10.1152/physiolgenomics.00242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almon RR, Lai W, DuBois DC, Jusko WJ. Corticosteroid-regulated genes in rat kidney: Mining time series array data. American Journal of Physiology. Endocrinology and Metabolism. 2005;289:E870–E882. doi: 10.1152/ajpendo.00196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RC, Walker BR. Glucocorticoids and insulin resistance: Old hormones, new targets. Clinical Science (London, England) 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clinical Science (London, England) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Besser GM, Jeffcoate WJ. Endocrine and metabolic diseases. Adrenal diseases. British Medical Journal. 1976;1:448–451. doi: 10.1136/bmj.1.6007.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. Journal of Obesity. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Developmental Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Brame LA, Considine RV, Yamauchi M, Baron AD, Mather KJ. Insulin and endothelin in the acute regulation of adiponectin in vivo in humans. Obesity Research. 2005;13:582–588. doi: 10.1038/oby.2005.62. [DOI] [PubMed] [Google Scholar]
- Cimmino MA, Andraghetti G, Briatore L, Salani B, Parodi M, Cutolo M, et al. Changes in adiponectin and leptin concentrations during glucocorticoid treatment: A pilot study in patients with polymyalgia rheumatica. Annals of the New York Academy of Sciences. 2010;1193:160–163. doi: 10.1111/j.1749-6632.2009.05364.x. [DOI] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- Darmon P, Dadoun F, Boullu-Ciocca S, Grino M, Alessi MC, Dutour A. Insulin resistance induced by hydrocortisone is increased in patients with abdominal obesity. American Journal of Physiology. Endocrinology and Metabolism. 2006;291:E995–E1002. doi: 10.1152/ajpendo.00654.2005. [DOI] [PubMed] [Google Scholar]
- de Oliveira C, de Mattos AB, Biz C, Oyama LM, Ribeiro EB, do Nascimento CM. High-fat diet and glucocorticoid treatment cause hyperglycemia associated with adiponectin receptor alterations. Lipids in Health and Disease. 2011;10:11. doi: 10.1186/1476-511X-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira C, Iwanaga-Carvalho C, Mota JF, Oyama LM, Ribeiro EB, Oller do Nascimento CM. Effects of adrenal hormones on the expression of adiponectin and adiponectin receptors in adipose tissue, muscle and liver. Steroids. 2011;76:1260–1267. doi: 10.1016/j.steroids.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Moss KA, Bovenkerk JE, Shankar SS, Morrison CL, Lelliott CJ, et al. Regulation of adiponectin expression in human adipocytes: Effects of adiposity, glucocorticoids, and tumor necrosis factor alpha. Obesity Research. 2005;13:662–669. doi: 10.1038/oby.2005.74. [DOI] [PubMed] [Google Scholar]
- Dridi S, Taouis M. Adiponectin and energy homeostasis: Consensus and controversy. The Journal of Nutritional Biochemistry. 2009;20:831–839. doi: 10.1016/j.jnutbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Eyster KM. Dysfunctional hormonal regulation of metabolism in obesity. South Dakota Medicine. 2011;(Spec No):18–21. [PubMed] [Google Scholar]
- Fallo F, Scarda A, Sonino N, Paoletta A, Boscaro M, Pagano C, et al. Effect of glucocorticoids on adiponectin: A study in healthy subjects and in Cushing's syndrome. European Journal of Endocrinology. 2004;150:339–344. doi: 10.1530/eje.0.1500339. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46:1594–1603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- Gunn JM, Hanson RW, Meyuhas O, Reshef L, Ballard FJ. Glucocorticoids and the regulation of phosphoenolpyruvate carboxykinase (guanosine triphosphate) in the rat. The Biochemical Journal. 1975;150:195–203. doi: 10.1042/bj1500195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleux CM, Takahashi M, Delporte ML, Detry R, Funahashi T, Matsuzawa Y, et al. Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochemical and Biophysical Research Communications. 2001;288:1102–1107. doi: 10.1006/bbrc.2001.5904. [DOI] [PubMed] [Google Scholar]
- Hasselgren PO. Glucocorticoids and muscle catabolism. Current Opinion in Clinical Nutrition and Metabolic Care. 1999;2:201–205. doi: 10.1097/00075197-199905000-00002. [DOI] [PubMed] [Google Scholar]
- Heppner KM, Habegger KM, Day J, Pfluger PT, Perez-Tilve D, Ward B, et al. Glucagon regulation of energy metabolism. Physiology & Behavior. 2010;100:545–548. doi: 10.1016/j.physbeh.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Hjelmesaeth J, Flyvbjerg A, Jenssen T, Frystyk J, Ueland T, Hagen M, et al. Hypoadiponectinemia is associated with insulin resistance and glucose intolerance after renal transplantation: Impact of immunosuppressive and antihypertensive drug therapy. Clinical Journal of the American Society of Nephrology. 2006;1:575–582. doi: 10.2215/CJN.01471005. [DOI] [PubMed] [Google Scholar]
- Hozumi A, Osaki M, Sakamoto K, Goto H, Fukushima T, Baba H, et al. Dexamethasone-induced plasminogen activator inhibitor-1 expression in human primary bone marrow adipocytes. Biomedical Research. 2010;31:281–286. doi: 10.2220/biomedres.31.281. [DOI] [PubMed] [Google Scholar]
- Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- Iynedjian PB, Ballard FJ, Hanson RW. The regulation of phosphoenolpyruvate carboxykinase (GTP) synthesis in rat kidney cortex. The role of acid-base balance and glucocorticoids. The Journal of Biological Chemistry. 1975;250:5596–5603. [PubMed] [Google Scholar]
- Jang C, Inder WJ, Obeyesekere VR, Alford FP. Adiponectin, skeletal muscle adiponectin receptor expression and insulin resistance following dexamethasone. Clinical Endocrinology. 2008;69:745–750. doi: 10.1111/j.1365-2265.2008.03242.x. [DOI] [PubMed] [Google Scholar]
- Jin JY, Jusko WJ. Pharmacodynamics of glucose regulation by methylprednisolone. II. Normal rats. Biopharmaceutics & Drug Disposition. 2009;30:35–48. doi: 10.1002/bdd.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine Reviews. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. The Journal of Clinical Endocrinology and Metabolism. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS. Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes. 2005;54:1023–1031. doi: 10.2337/diabetes.54.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner F, Galbo H. Insulin sensitivity and related cytokines, chemokines, and adipokines in polymyalgia rheumatica. Scandinavian Journal of Rheumatology. 2010;39:402–408. doi: 10.3109/03009741003631479. [DOI] [PubMed] [Google Scholar]
- Krsek M, Silha JV, Jezkova J, Hana V, Marek J, Weiss V, et al. Adipokine levels in Cushing's syndrome; elevated resistin levels in female patients with Cushing's syndrome. Clinical Endocrinology. 2004;60:350–357. doi: 10.1111/j.1365-2265.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- Lewandowski KC, Szosland K, Lewinski A. Short-term dexamethasone administration does not alter serum adiponectin or resistin concentrations in overweight and obese subjects despite an increase in insulin resistance. Clinical Endocrinology. 2006;65:551–552. doi: 10.1111/j.1365-2265.2006.02638.x. [DOI] [PubMed] [Google Scholar]
- Libe R, Morpurgo PS, Cappiello V, Maffini A, Bondioni S, Locatelli M, et al. Ghrelin and adiponectin in patients with Cushing's disease before and after successful transsphenoidal surgery. Clinical Endocrinology. 2005;62:30–36. doi: 10.1111/j.1365-2265.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. The Biochemical Journal. 2010;425:41–52. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- Lukens FD. The pancreas: Insulin and glucagon. Annual Review of Physiology. 1959;21:445–474. doi: 10.1146/annurev.ph.21.030159.002305. [DOI] [PubMed] [Google Scholar]
- MacDougald OA, Mandrup S. Adipogenesis: Forces that tip the scales. Trends in Endocrinology and Metabolism. 2002;13:5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. American Journal of Physiology. Endocrinology and Metabolism. 2002;283:E1266–E1271. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- Mohlig M, Wegewitz U, Osterhoff M, Isken F, Ristow M, Pfeiffer AF, et al. Insulin decreases human adiponectin plasma levels. Hormone and Metabolic Research. 2002;34:655–658. doi: 10.1055/s-2002-38248. [DOI] [PubMed] [Google Scholar]
- Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, et al. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2004;53:931–938. doi: 10.2337/diabetes.53.4.931. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. The Journal of Biological Chemistry. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clinica Chimica Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff H, Bruder ED. Adiponectin and resistin in the neonatal rat: Effects of dexamethasone and hypoxia. Endocrine. 2006;29:341–344. doi: 10.1385/ENDO:29:2:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall DC. Discovering the role of the adrenal gland in the control of body function. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2004;287:R1007–R1008. doi: 10.1152/classicessays.00017.2004. [DOI] [PubMed] [Google Scholar]
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. The New England Journal of Medicine. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rieth N, Jollin L, Le Panse B, Lecoq AM, Arlettaz A, De Ceaurriz J, et al. Effects of short-term corticoid ingestion on food intake and adipokines in healthy recreationally trained men. European Journal of Applied Physiology. 2009;105:309–313. doi: 10.1007/s00421-008-0904-6. [DOI] [PubMed] [Google Scholar]
- Ringold GM, Chapman AB, Knight DM, Torti FM. Hormonal control of adipogenesis. Annals of the New York Academy of Sciences. 1986;478:109–119. doi: 10.1111/j.1749-6632.1986.tb15525.x. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Vegiopoulos A, Herzig S. Role of glucocorticoids and the glucocorticoid receptor in metabolism: Insights from genetic manipulations. The Journal of Steroid Biochemistry and Molecular Biology. 2010;122:10–20. doi: 10.1016/j.jsbmb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Ryan VH, German AJ, Wood IS, Hunter L, Morris P, Trayhurn P. Adipokine expression and secretion by canine adipocytes: Stimulation of inflammatory adipokine production by LPS and TNFalpha. Pflügers Archiv. 2010;460:603–616. doi: 10.1007/s00424-010-0845-x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Osaki M, Hozumi A, Goto H, Fukushima T, Baba H, et al. Simvastatin suppresses dexamethasone-induced secretion of plasminogen activator inhibitor-1 in human bone marrow adipocytes. BMC Musculoskeletal Disorders. 2011;12:82. doi: 10.1186/1471-2474-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. The Journal of Steroid Biochemistry and Molecular Biology. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- Shi JH, Du WH, Liu XY, Fan YP, Hu XL, Zhou HY, et al. Glucocorticoids decrease serum adiponectin level and WAT adiponectin mRNA expression in rats. Steroids. 2010;75:853–858. doi: 10.1016/j.steroids.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- Stofkova A. Leptin and adiponectin: From energy and metabolic dysbalance to inflammation and autoimmunity. Endocrine Regulations. 2009;43:157–168. [PubMed] [Google Scholar]
- Sukumaran S, Almon RR, DuBois DC, Jusko WJ. Circadian rhythms in gene expression: Relationship to physiology, disease, drug disposition and drug action. Advanced Drug Delivery Reviews. 2010;62:904–917. doi: 10.1016/j.addr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Jusko WJ, DuBois DC, Almon RR. Mechanistic modeling of the effects of glucocorticoids and circadian rhythms on adipokine expression. The Journal of Pharmacology and Experimental Therapeutics. 2011;337:734–746. doi: 10.1124/jpet.111.179960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metabolic Syndrome and Related Disorders. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz SL, Dluhy RG. Corticosteroids: Clinical pharmacology and therapeutic use. Drugs. 1978;16:238–255. doi: 10.2165/00003495-197816030-00006. [DOI] [PubMed] [Google Scholar]
- Tirone TA, Brunicardi FC. Overview of glucose regulation. World Journal of Surgery. 2001;25:461–467. doi: 10.1007/s002680020338. [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, et al. 11beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocrine Reviews. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Uchida HA, Nakamura Y, Kaihara M, Norii H, Hanayama Y, Sugiyama H, et al. Steroid pulse therapy impaired endothelial function while increasing plasma high molecule adiponectin concentration in patients with IgA nephropathy. Nephrology, Dialysis, Transplantation. 2006;21:3475–3480. doi: 10.1093/ndt/gfl423. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Molecular and Cellular Endocrinology. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. Journal of Cellular Physiology. 2008;216:3–13. doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- Xu A, Wong LC, Wang Y, Xu JY, Cooper GJ, Lam KS. Chronic treatment with growth hormone stimulates adiponectin gene expression in 3T3-L1 adipocytes. FEBS Letters. 2004;572:129–134. doi: 10.1016/j.febslet.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Yamada T, Oka Y, Katagiri H. Inter-organ metabolic communication involved in energy homeostasis: Potential therapeutic targets for obesity and metabolic syndrome. Pharmacology & Therapeutics. 2008;117:188–198. doi: 10.1016/j.pharmthera.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: Lessons from translational research. The American Journal of Clinical Nutrition. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]