Abstract
Retrorectal cystic hamartoma is a rare type of congenital cystic lesion usually diagnosed in middle-aged women. Although mostly asymptomatic, patients may present with symptoms resulting from local mass effect or with a complication. The most important complications of these cysts are infection with secondary fistulization and malignant degeneration. Because of such non-specific nature of symptoms and rare incidence, it is not unusual to have a delay in clinical diagnosis. MRI has evolved to be the investigation of choice for the evaluation of presacral tumors as it can provide excellent anatomic detail and soft tissue contrast. Role of preoperative biopsy is controversial especially with improvements in the imaging techniques. When diagnosed these lesions should be excised because of the risk of malignant transformation.
Keywords: Retrorectal mass, Tailgut cyst, Surgical resection
Retrorectal cystic hamartoma (tailgut cyst, TGC) is a rare subtype of congenital cystic lesion of the presacral region [1, 2]. Most commonly, it is incidentally discovered in middle-aged women. Embryologically, it arises from the aberrant remnant of postnatal hindgut due to incomplete involution during development [3].
Although these developmental cysts are often asymptomatic, patients may present with symptoms resulting from local mass effect (e.g., constipation, rectal fullness, lower abdominal pain, and dysuria). Initial presentation may also be delayed until complications occur, including infection with fistulization, bleeding, and malignant degeneration [4]. Complete surgical excision is indicated not only to establish the diagnosis, relieve symptoms, and avoid complications but also to rule out underlying malignancy [5]. Fewer than 100 cases describing TGC have been reported in the literature so far and the investigation modalities and treatment strategies continue to evolve. We describe here a case of TGC, discuss the management, and review the relevant literature with special emphasis on diagnostic modalities, role of biopsy, and definitive management.
Case Report
A 46-year-old woman presented to the surgery department with a 6-year history of chronic constipation and lower abdominal discomfort. She also reported increasing severity of symptoms for the past 6 months. She denied any history of rectal bleeding, mucus discharge, or genitourinary symptoms. She was otherwise healthy with no significant surgical, medical, or obstetric history. She had an average build with an unremarkable general physical examination. Digital rectal examination revealed a non-tender, extraluminal well-defined mass in the presacral region causing extrinsic compression. The overlying rectal mucosa was smooth and mobile. The exact dimensions of the mass could not be ascertained as the upper limit of the lesion could not be reached. These findings were confirmed on flexible sigmoidoscopy. Transrectal ultrasonography showed a large unilocular cystic lesion in the retrorectal space measuring 6 cm × 6 cm × 6 cm. Magnetic resonant imaging confirmed a well-circumscribed unilocular cystic lesion in the presacral region with well-defined planes, without any associated lymphadenopathy (Fig. 1).
Fig. 1.
Sagittal section of the pelvic MRI showing the tailgut cyst and its relationships to the surrounding structures (c, cyst; b, urinary bladder)
The patient underwent trans-sacral excision of this retrorectal cystic lesion. This procedure was performed in the jack-knife position after dividing the 4th and 5th sacral segments in the midline posteriorly without excision of the coccyx (Fig. 2). Intraoperatively, the lesion was cystic in nature with well-defined planes and was excised without difficulty. The patient had an uneventful post-operative course and was discharged on the 5th post-operative day. On 6-month follow-up, the patient was doing well and was symptom-free.
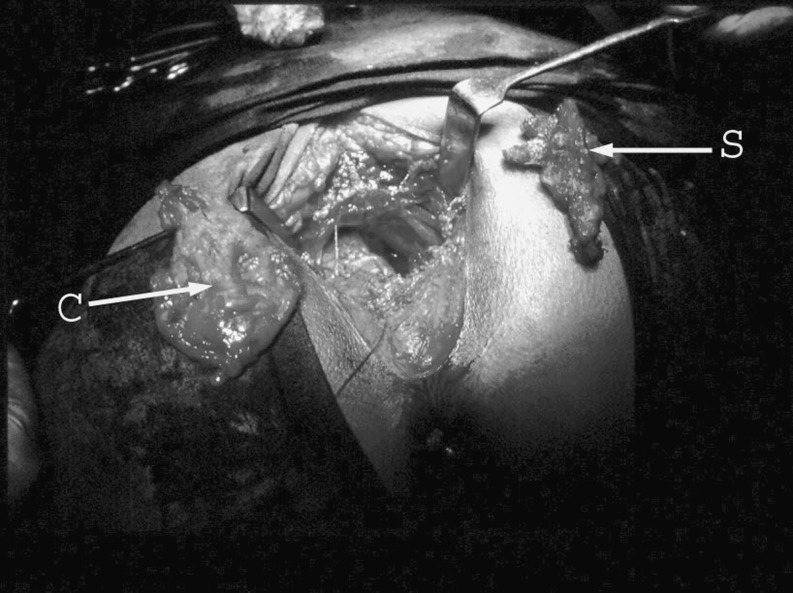
Fig. 2.
Intraoperative presentation of the resected cyst (C) and the 4th and 5th segments of sacrum (S) with the patient in jack-knife position
Histologically, the cyst showed a 5 cm × 6 cm unilocular cyst with stratified columnar epithelium. The wall contained fibrous tissue and a few scattered smooth muscle fibers. The findings were consistent with the diagnosis of TGC. No heterologous elements to suggest a teratoma were seen. There was no evidence of a malignant change or infiltration.
Discussion
Presacral tumors are a very rare entity that is mostly diagnosed in children. Among the different subtypes of presacral tumors, developmental cysts are the rarest kind [1]. In the adult population, they are seen most commonly in middle-aged women (female-to-male ratio 3:1) and are classified as epidermoid cysts, dermoid cysts, enteric cysts (tailgut cysts and cystic rectal duplication), and neurenteric cysts [6]. Although most cases are asymptomatic, patients may present with symptoms resulting from local mass effect or complication. The most important complications of these cysts are infection with secondary fistulization and malignant degeneration.
The retrorectal space is a potential space that becomes a true space when a mass grows within it. The boundaries of the retrorectal region include the posterior wall of rectum anteriorly and the sacrum posteriorly. This space extends superiorly to peritoneal reflection and inferiorly to the rectosacral fascia and the supralevator space. Laterally, ureters, iliac vessels, and sacral nerve roots bind this area [7].
Early in the development, the embryo possesses a true tail. This tail develops at about the fifth week of gestation and persists up to the eighth week of gestation. The anus forms cephalad to the tail via invagination of the ectoderm (thus giving rise to the term “tailgut” or “postanal gut”). The remaining postanal gut is a tubular structure lined by two to four layers of stratified cuboidal epithelium. This eventually fills up with epithelial debris and atrophies as the tail region regresses during the eighth week of development [1, 3, 8].
According to Peyrons’ criteria, TGCs are usually multilocular and multicystic. The cysts are lined by a wide variety of epithelia varying even within the same cyst. These include stratified squamous, transitional, stratified columnar, mucinous, or ciliated columnar, ciliated pseudostratified columnar, and gastric types. In contrast to enteric duplication cysts, TGCs have disorganized smooth muscle fibers within the cyst wall and do not contain neural plexuses [9]. The presence of some glandular or transitional epithelium is required to exclude the diagnosis of epidermoid and dermoid cysts [10]. Acute or chronic inflammation occurs in about 58 % of cases especially with history of prior infection.
As mentioned earlier, uncomplicated lesions are asymptomatic and are usually found on routine physical examinations. When symptomatic, patients typically complain of vague, longstanding pain in the perineum or low back, and change in bowel habit. These patients may also present with history of recurrent anal sinus, fistulae, or abscesses. Other symptoms described include urinary retention, dysuria, changes in the caliber of stools, and rectal bleeding.
Because of such nonspecific nature of symptoms and rare incidence, it is not unusual to have a delay in clinical diagnosis. Interestingly, Singer et al. reported seven patients, who underwent an average of 4.7 invasive procedures or operations, before a correct diagnosis of a retrorectal lesion was made [11].
The clinical significance of tailgut cysts is primarily related to the morbidity that can result if the lesion is not suspected or nondefinitive surgery is undertaken. More importantly, the possibility of malignant transformation emphasizes the importance of early complete surgical excision of these lesions.
Over the past few years, there has been significant evolution in the diagnosis and management of these tumors mostly due to improved imaging modalities especially magnetic resonant imaging (MRI). MRI has evolved to be the investigation of choice for the evaluation of presacral tumors as it can provide excellent anatomic detail and soft tissue contrast. Additionally, it has the advantage over CT of being able to offer multiplanar reconstructions. Several series have documented these advantages of MRI in assisting with the management of these lesions [7, 12]. Overall, MRI is a valuable tool for preoperative evaluation, imaging and characterizing lesions, estimating their extent and the risk of malignancy, distinguishing organ-confined disease from tumor spread into adjacent structures, and deciding on the most appropriate intervention strategies and imaging follow-up requirements.
The differential diagnosis of retrorectal masses includes a variety of congenital, inflammatory, neurogenic, and osseous or other miscellaneous tumors. Among the presacral cystic masses, epidermoid cysts, dermoid cysts, rectal duplication cysts, and meningoceles are usually unilocular. The presence of fat content on fat-saturated images on MRI is suggestive of a dermoid cyst. Anterior meningocele is a well-defined unilocular thin-walled, fluid-filled lesion of the retrorectal space with a stalk that may be seen communicating with the thecal sac.
Fewer than 30 cases of malignant degeneration in TGC have been described in literature. Malignant degeneration of TGC has been described in isolated reports and includes adenocarcinomas, carcinoids, and sarcomas [13]. The largest series in the literature, by Hjermstad and Helwig, reported a 2 % incidence of malignant degeneration [3]. On the other hand, a recent report from Mayo Clinic reported 13 % incidence of malignant change, suggesting that the risk of malignant change may be higher than previously thought [13].
The role of preoperative biopsy is controversial especially with improvements in the imaging techniques. There is significant concern among physicians as well as patients about biopsy-related complications such as infection, hematoma, and needle track implantation. For purely cystic lesions, biopsy is not indicated. On the other hand, for lesions with mixed solid and cystic appearance, where concern about malignant degeneration is high, a percutaneous preoperative biopsy may be carried out. The advantage of preoperative biopsy in such cases not only helps with optimal planning of surgical approach and adjuvant therapy but also ensures that the patient is well informed about the prognosis.
Surgical excision is the definitive treatment for this condition. Excision may be accomplished by a transanal, transrectal, transabdominal, or combined approach [14]. The most commonly described surgical approach is via a posterior parasacral incision. Various factors need to be taken into consideration in deciding the most appropriate approach to these lesions. These include the degree of proximal extension, infection and possibility of being adherent to surrounding structures, and the suspicion of malignancy, which requires en bloc resection. The issue of whether to perform a coccygectomy in the setting of benign congenital presacral cysts is controversial. The current trend is toward preserving the coccyx unless en bloc resection is required for malignancy or the cyst is densely adherent to the coccyx [13]. Recently, the laparoscopic approach with successful extirpation of TGCs has been reported in the literature. Such an approach at this time is limited to the centers of expertise both because of the technical challenges secondary to anatomical location of these lesions and careful attention to the oncological principles as these lesions may harbor malignancy and mandate en bloc resection [15, 16].
In conclusion, retrorectal cystic hamartoma is a very rare condition usually diagnosed in middle-aged women. When diagnosed these lesions should be excised because of the risk of malignant transformation.
References
- 1.Pyo DJ. TGC (retro rectal cyst hamartoma): case report and review. Mt Sinai Med. 1990;57:249–252. [PubMed] [Google Scholar]
- 2.Kim MJ, Kim WH, Kim NK, Yun MJ, et al. Tailgut cyst: multilocular cystic appearance on MRI. J Comput Assist Tomo. 1997;21(51):731–732. doi: 10.1097/00004728-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Hjermstad BM, Helwig WB. Tailgut cysts. Am J Clin Pathol. 1988;89:139–147. doi: 10.1093/ajcp/89.2.139. [DOI] [PubMed] [Google Scholar]
- 4.Uhlig BE, Johnson RL. Presacral tumors and cysts in adults. Dis Colon Rectum. 1975;18:581–595. doi: 10.1007/BF02587141. [DOI] [PubMed] [Google Scholar]
- 5.McCune WS. Management of sacrococcygeal tumors. Am Surg. 1964;159:911–918. doi: 10.1097/00000658-196406000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahan H, Arrive L, Wendum D, Ducou le Pointe H, Djouhri H, Tubiana JM. Clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics. 2001;21:575–584. doi: 10.1148/radiographics.21.3.g01ma13575. [DOI] [PubMed] [Google Scholar]
- 7.Yang BL, Yf Gu, Shao WJ, Chen HJ, Sun GD, Jin JY, Zhu X. Retrorectal tumors in adults: magnetic resonance imaging findings. World J Gastroenterol. 2010;16(46):5822–5829. doi: 10.3748/wjg.v16.i46.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannon J, Subramony C, Scott-Conner C. Benign retrorectal tumors in adults: the choice of operative approach. Am Surg. 1994;60:267–272. [PubMed] [Google Scholar]
- 9.Killingsworth C, Gadacz TR. Tailgut cyst (retrorectal cystic hamartoma): report of a case and review of the literature. Am Surg. 2005;71(8):666–673. [PubMed] [Google Scholar]
- 10.Prasad AR, Amin MD, Randolph TL, Lee CS. Retro rectal cystic hamartoma: report of five cases with malignancy arising in two. Arch Pathol Lab Med. 2000;124:725–729. doi: 10.5858/2000-124-0725-RCH. [DOI] [PubMed] [Google Scholar]
- 11.Singer MA, Cintron JR, Martz JE, Schoetz DJ, Abcarian H. Retrorectal cyst: a rare tumor frequently misdiagnosed. J Am Coll Surg. 2003;196:880–886. doi: 10.1016/S1072-7515(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 12.Aflalo-Hazan V, Rousset P, Mourra N, Lewin M, Azizi L, Hoeffel C. Tailgut cysts: MRI findings. Eur Radiol. 2008;18:2586–2593. doi: 10.1007/s00330-008-1028-4. [DOI] [PubMed] [Google Scholar]
- 13.Mathis KL, Dozois EJ, Grewal MS, Metzger P, Larson DW, Devine RM. Malignant risk and surgical outcomes of presacral tailgut cysts. Br J Surg. 2010;97:575–579. doi: 10.1002/bjs.6915. [DOI] [PubMed] [Google Scholar]
- 14.Localio SA, Eng K, Coppa GF. Anorectal, presacral and sacral tumors: anatomy, physiology, pathogenesis and management. Philadelphia: WB Saunders; 1987. [Google Scholar]
- 15.Lu NH, Tseng MJ. Laparoscopic management of tailgut cyst: case report and review of literature. J Minim Invasive Gynecol. 2010;17(6):802–804. doi: 10.1016/j.jmig.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Lim SW, Huh JW, Kim YJ, Kim HR. Laparoscopy-assisted resection of tailgut cysts: report of a case. Case Rep Gastroenterol. 2011;5:22–27. doi: 10.1159/000322912. [DOI] [PMC free article] [PubMed] [Google Scholar]