Abstract
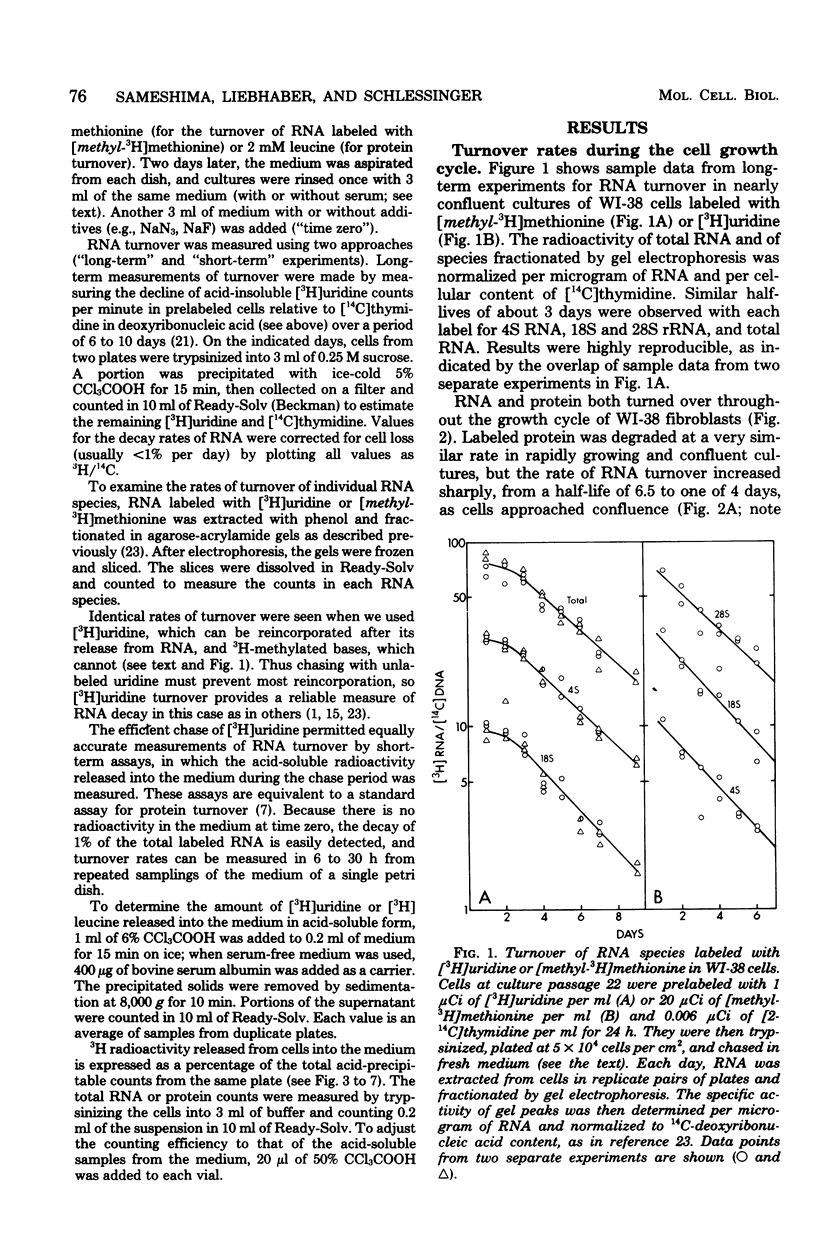
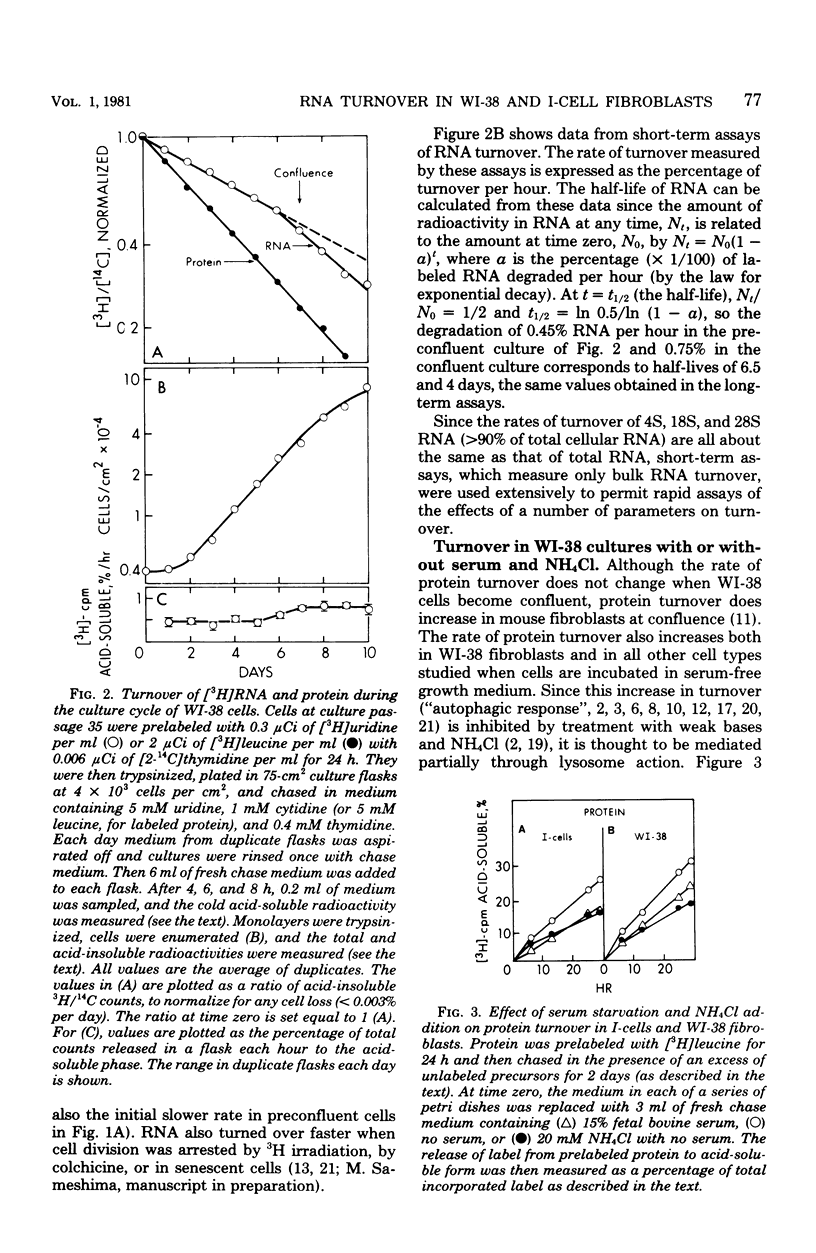
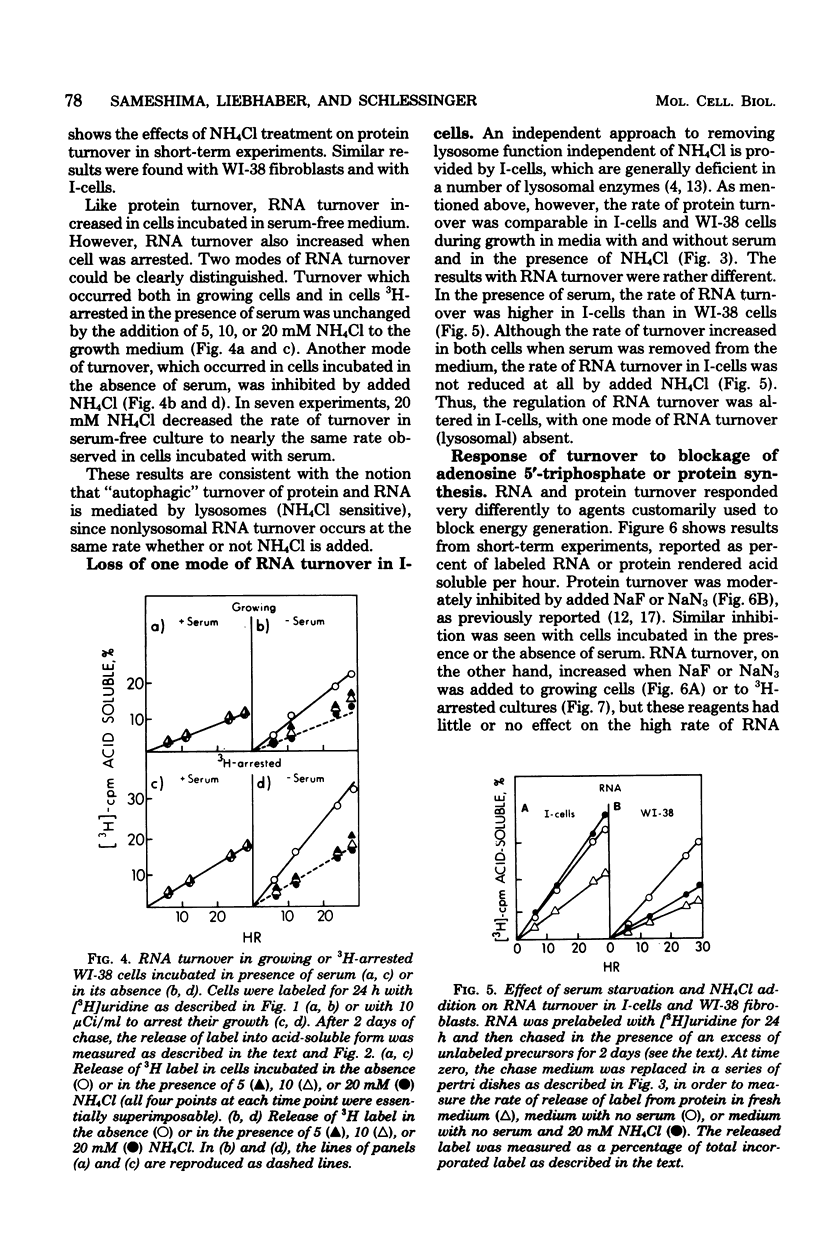
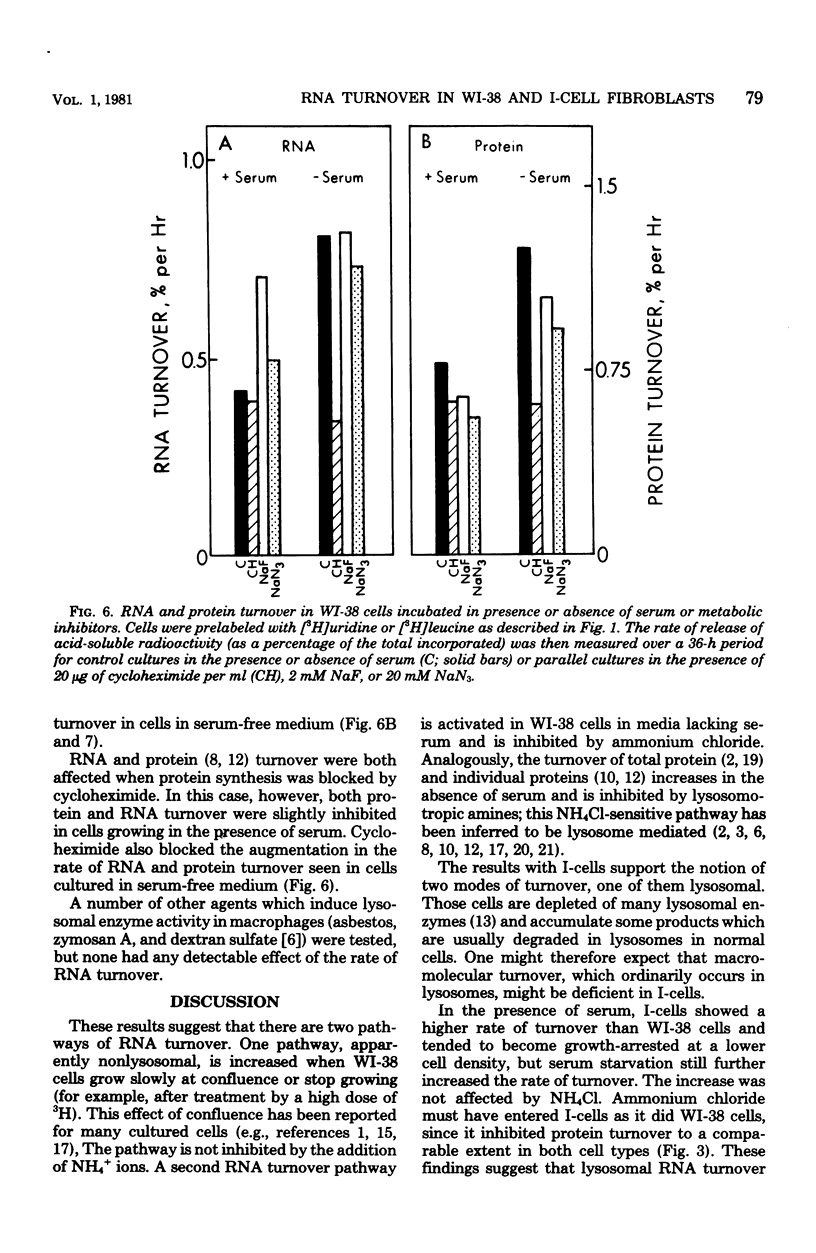
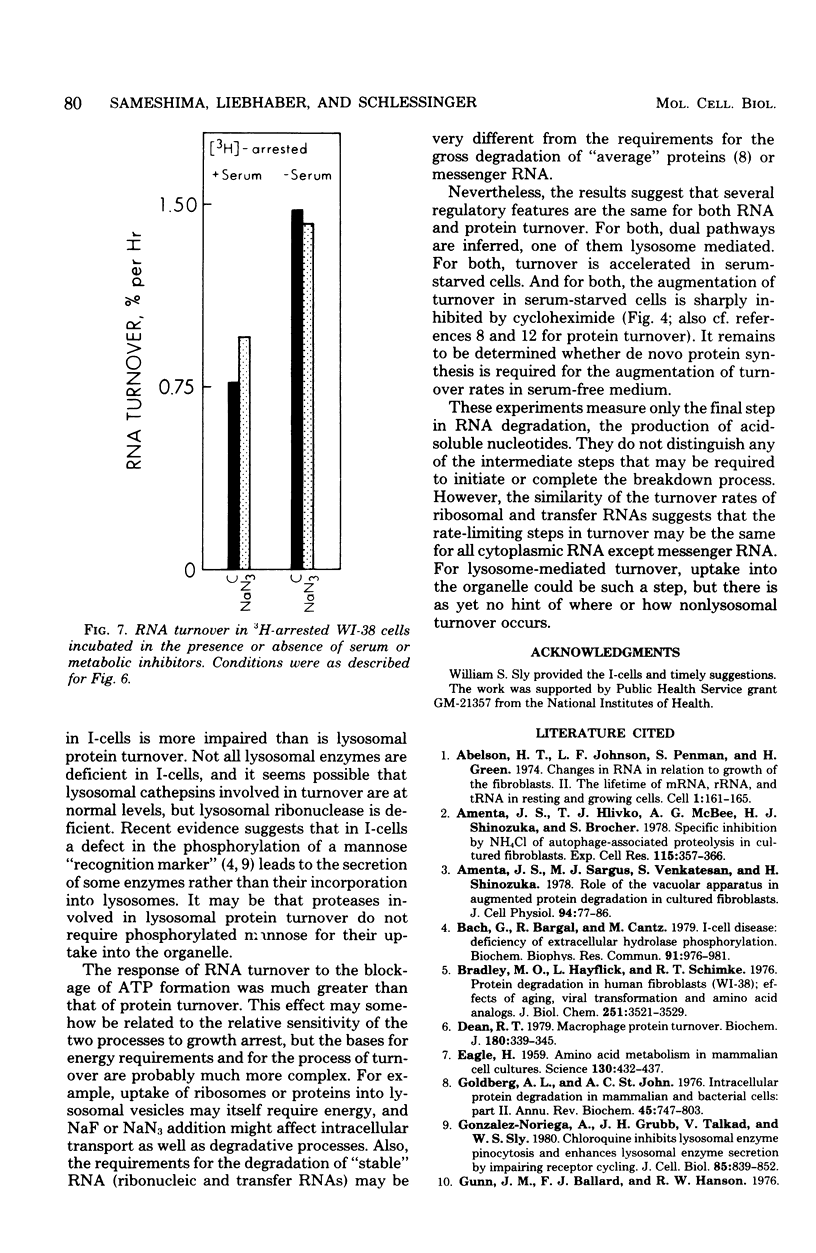
The turnover rates of 3H-labeled 18S ribosomal ribonucleic acid (RNA), 28S ribosomal RNA, transfer RNA, and total cytoplasmic RNA were very similar in growing WI-38 diploid fibroblasts. The rate of turnover was at least twofold greater when cell growth stopped due to cell confluence, 3H irradiation, or treatment with 20 mM NaN3 or 2 mM NaF. In contrast, the rate of total 3H-protein turnover was the same in growing and nongrowing cells. Both RNA and protein turnovers were accelerated at least twofold in WI-38 cells deprived of serum, and this increase in turnover was inhibited by NH4Cl. These results are consistent with two pathways for RNA turnover, one of them being nonlysosomal and the other being lysosome mediated (NH4Cl sensitive), as has been suggested for protein turnover. Also consistent with the notion of two pathways for RNA turnover were findings with I-cells, which are deficient for many lysosomal enzymes, and in which all RNA turnover was nonlysosomal (NH4Cl resistant).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Hlivko T. J., McBee A. G., Shinozuka H., Brocher S. Specific inhibition by NH4CL of autophagy-associated proteloysis in cultured fibroblasts. Exp Cell Res. 1978 Sep;115(2):357–366. doi: 10.1016/0014-4827(78)90289-6. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Venkatesan S., Shinozuka H. Role of the vacuolar apparatus in augmented protein degradation in cultured fibroblasts. J Cell Physiol. 1978 Jan;94(1):77–86. doi: 10.1002/jcp.1040940110. [DOI] [PubMed] [Google Scholar]
- Bach G., Bargal R., Cantz M. I-cell disease: deficiency of extracellular hydrolase phosphorylation. Biochem Biophys Res Commun. 1979 Dec 14;91(3):976–981. doi: 10.1016/0006-291x(79)91975-2. [DOI] [PubMed] [Google Scholar]
- Bradley M. O., Hayflick L., Schimke R. T. Protein degradation in human fibroblasts (WI-38). Effects of aging, viral transformation, and amino acid analogs. J Biol Chem. 1976 Jun 25;251(12):3521–3529. [PubMed] [Google Scholar]
- Dean R. T. Macrophage protein turnover. Evidence for lysosomal participation in basal proteolysis. Biochem J. 1979 May 15;180(2):339–345. doi: 10.1042/bj1800339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendil K. B. Intracellular protein degradation in growing, in density-inhibited, and in serum-restricted fibroblast cultures. J Cell Physiol. 1977 Sep;92(3):353–364. doi: 10.1002/jcp.1040920304. [DOI] [PubMed] [Google Scholar]
- Hershko A., Tomkins G. M. Studies on the degradation of tyrosine aminotransferase in hepatoma cells in culture. Influence of the composition of the medium and adenosine triphosphate dependence. J Biol Chem. 1971 Feb 10;246(3):710–714. [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Kaftory A., Hershko A., Fry M. Protein turnover in senescent cultured chick embryo fibroblasts. J Cell Physiol. 1978 Feb;94(2):147–160. doi: 10.1002/jcp.1040940204. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Wolf S., Schlessinger D. Differences in rRNA metabolism of primary and SV40-transformed human fibroblasts. Cell. 1978 Jan;13(1):121–127. doi: 10.1016/0092-8674(78)90143-5. [DOI] [PubMed] [Google Scholar]
- Poole B., Wibo M. Protein degradation in cultured cells. The effect of fresh medium, fluoride, and iodoacetate on the digestion of cellular protein of rat fibroblasts. J Biol Chem. 1973 Sep 10;248(17):6221–6226. [PubMed] [Google Scholar]
- Scott J. F. Turnover of ribosomal RNA in cells in culture. Exp Cell Res. 1977 Aug;108(1):207–219. doi: 10.1016/s0014-4827(77)80027-x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Stark M. J., Frenkel R. Turnover of rat liver malic enzyme during induction by protein deprivation. Biochim Biophys Acta. 1978 Sep 27;520(2):452–459. doi: 10.1016/0005-2787(78)90242-3. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Poole B. Effect of medium composition on protein degradation and DNA synthesis in rat embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2427–2431. doi: 10.1073/pnas.74.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J. Ribosomal RNA turnover in contact inhibited cells. Nat New Biol. 1972 Jan 12;235(54):58–61. doi: 10.1038/newbio235058a0. [DOI] [PubMed] [Google Scholar]
- Wolf S., Sameshima M., Liebhaber S. A., Schlessinger D. Regulation of ribosomal ribonucleic acid levels in growing, 3H-arrested, and crisis-phase WI-38 human diploid fibroblasts. Biochemistry. 1980 Jul 22;19(15):3484–3490. doi: 10.1021/bi00556a012. [DOI] [PubMed] [Google Scholar]


