Abstract
Persistent Müllerian duct syndrome is a rare form of internal male pseudohermaphroditism caused by defects in synthesis or action of Müllerian-inhibiting factor, due to which Müllerian duct derivatives, such as uterus, fallopian tube, and upper vagina, are normally present in 46XY males. Here, we report a 26-year-old male with right-sided obstructed inguinal hernia with left undescended testis. On exploration, hernial sac containing bowel loops, uterus with fallopian tubes, upper vagina, and testes were present.
Keywords: Persistent Müllerian duct syndrome, Transverse testicular ectopia, Hernia uteri inguinale
Introduction
Persistent Müllerian duct syndrome (PMDS) can occur due to mutation in MIS and MISR-II gene has autosomal recessive transmission. Patients often present with cryptorchidism or an inguinal hernia. Presence of uterus in the hernial sac is known as “hernia uterine inguinale.”
Transverse testicular ectopia is an uncommon anatomical abnormality in which both the gonads migrate towards the same hemiscrotum; the ectopic testis may lie either in opposite hemiscrotum, in the inguinal canal, or at the deep inguinal ring. PMDS frequently presents as cryptorchidism, inguinal hernia, and very rarely transverse testicular ectopia [1].
Case Report
A 26-year-old male presented with right-sided groin swelling and pain. He had history of swelling since 2 years, which was initially reducible on its own and suddenly became irreducible following trauma. He was married since 3 years and had no children and had no sexual dysfunction.
On examination, he was a normally virilized man. He had right-sided inguinoscrotal swelling of 10 × 6 cm in size, tender, and irreducible. Secondary sexual characteristics were well developed and penis was normal with normal pubic and axillary hairs. Scrotum was well developed and was clinically diagnosed to be right obstructed inguinal hernia with left undescended testis.
Semen analysis showed azoospermia. Ultrasound examination showed bowel loops in hernial sac, and bilateral testes were not visualized in scrotum/inguinal canal/abdomen. On exploration, hernial sac containing bowel loops, uterus with fallopian tubes, upper vagina, and testes were present (Fig. 1).
Fig. 1.
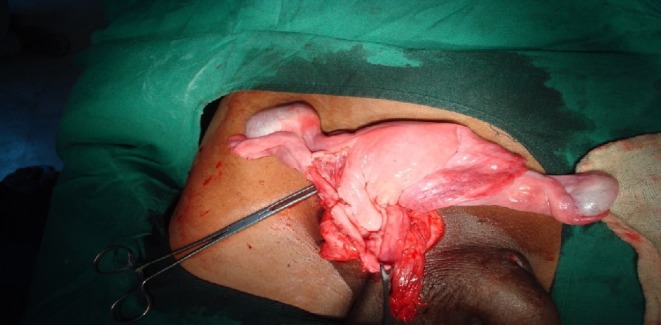
Intraoperative photograph showing hernial sac containing uterus, fallopian tubes, and testes
The patient was told about the same, on table, as he was under spinal anesthesia and not sedated at that moment. He and his wife were fully explained about the situation and the various options available, and consent was taken from both of them to remove the rudimentary uterus and fallopian tubes. Following this, cord structures along with testes were separated from uterus and fallopian tube, taking care not to damage the vasa deferentia and blood supply to cord structures and the testes. Both the testes were placed in bilateral hemiscrotum by making a rent in the median raphe to place the left testis in left hemiscrotum, as both the cord structures and their blood supplies were coming from the right side only. Uterus along with fallopian tube was removed completely and was sent for histopathology (Fig. 2). Hernia was repaired by polypropylene mesh.
Fig. 2.
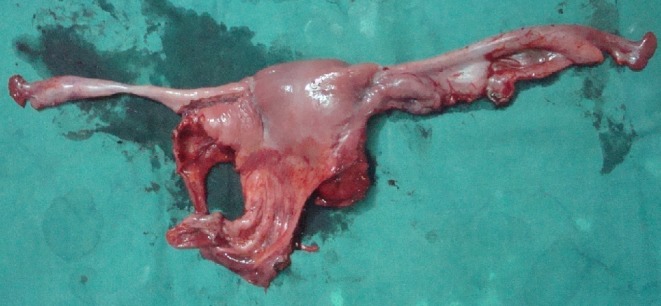
Specimen showing uterus and fallopian tube
Microscopy
Biopsy of both the testes revealed 60 % of seminiferous tubules showing spermatogenesis with maturation up to spermatozoa.
Section studied through uterus showed straight endometrial glands with compact stroma, and unremarkable myometrium, and structure of fallopian tube on both sides was seen.
Postoperatively, the patient underwent Doppler scan of both the testes, which were shown to be viable. The patient was also subjected to semen and hormonal analyses, and a bit of tissue from the buccal mucosa was sent for karyotyping. The results were as follows:
- Karyotype
46XY
- Barr body count
17 (borderline)
- Serum anti-Müllerian hormone (AMH)
Undetectable
- Serum testosterone
Total, 20.46 nmol/L; free, 0.412 nmol/L
- Serum HBG
30.6 nmol/L
- Semen analysis
Azoospermia
Virilization was maintained in a 1-year follow-up period.
Discussion
PMDS patients are phenotypically and genotypically males with normal secondary sexual characteristics, since the testosterone secretion and function are normal.
At 7 weeks of gestation, both the Müllerian (paramesonephric) and Wolffian (mesonephric) ducts are present in a fetus. In male fetus (XY), Leydig cells secrete testosterone, which has a local effect on Wolffian duct and helps in differentiation into epididymis, vas deferens, and seminal vesicle, whereas dihydrotestosterone helps in the formation of urogenital sinus and external genitalia. Sertoli cells secrete Müllerian-inhibiting factor (MIF) or AMH, which is a glycoprotein that helps in regression of Müllerian ducts.
PMDS can occur sporadically, but most commonly, it occurs as autosomal recessive trait due to mutation in MIS and MISR-II gene [2]. Patients with type I PMDS have defect in MIS gene located at 19p13, which was seen in 45 % cases. Patients with type II PMDS have defective MIS receptor (MISR-II), located at 12q13 [3], seen in 40 % cases, and in 16 % the cause is unknown [4].
There are three anatomic variants:
In the most common male type, one testis is usually found within the scrotum; the uterus and ipsilateral fallopian tube are either in the inguinal canal or can be brought into it by gentle traction on the presenting testis [5].
In some cases, the contralateral testis and tube are also in the hernial sac; transverse testicular ectopia can also occur [1].
The least common form, or female type, is characterized by bilateral cryptorchidism with testes embedded in the broad ligaments in an ovarian position with respect to the uterus, which is fixed in the pelvis [6].
Correct management of PMDS requires recognition of the condition by the surgeon and confirmation with testicular biopsies and chromosomal studies. Distinguishing PMDS from other intersex disorders is critical. A karyotyping and assessment of testicular response to chorionic gonadotropin stimulation are essential to verify both genetic sex and the existence of functional testicular tissue [7]. As in this case, the diagnosis is often made incidentally during surgery for an inguinal hernia or during exploration for cryptorchidism. Transverse testicular ectopia should be suspected preoperatively in patients who have unilateral inguinal hernia associated with a contralateral nonpalpable testis, as in the case. In suspected cases, ultrasonography, computerized tomography, magnetic resonance imaging, and laparoscopy may be helpful in diagnosis [6]. Before puberty in patients with bilateral cryptorchidism, serum AMH levels also help in diagnosis.
After confirmation of the diagnosis, definitive surgery consists of removal of the Müllerian remnants with orchiopexy or orchiectomy [5]. Preservation of the Müllerian derivatives is incompatible with successful orchiopexies because with sexual maturation, the uterus may become hypertrophic and cause discomfort or may present as a mass whose origin is unknown [5]. In this case, the characteristic macroscopic appearance gives sufficient evidence of the PMDS. The most common presenting symptoms are inguinal hernia, undescended testes, testes tumor, and abdominal mass. Surgeons dealing with hernia should consider the possibility of PMDS, especially when it is associated with cryptorchidism.
References
- 1.Boleken ME, Kaya M, Güran S, Memetoğlu ME, Kanmaz T, Yücesan S. Persistent Müllerian duct syndrome with transverse testicular ectopia. Int Urol Nephrol. 2007;39(4):1173–1175. doi: 10.1007/s11255-006-9163-9. [DOI] [PubMed] [Google Scholar]
- 2.Hoshiya M, Christian BP, Cromie WJ, Kim H, Zhan Y, MacLaughlin DT, et al. Persistent Müllerian duct syndrome caused by both a 27-bp deletion and a novel splice mutation in the MIS type II receptor gene. Birth Defects Res A Clin Mol Teratol. 2003;67(10):868–874. doi: 10.1002/bdra.10091. [DOI] [PubMed] [Google Scholar]
- 3.Manjunath BG, Shenoy VG, Raj P. Persistent Müllerian duct syndrome: how to deal with the Müllerian duct remnants—a review. Indian J Surg. 2010;72:20–23. doi: 10.1007/s12262-010-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belville C, Josso N, Picard JY. Persistence of Müllerian derivatives in males. Am J Med Genet. 1999;89:218–223. doi: 10.1002/(SICI)1096-8628(19991229)89:4<218::AID-AJMG6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 5.di Clemente N, Belville C. Anti-Müllerian hormone receptor defect. Best Pract Res Clin Endocrinol Metab. 2006;20:599–610. doi: 10.1016/j.beem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.David AD. Sexual differentiation: normal and abnormal. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh urology. 9. Philadelphia: Saunders; 2007. pp. 3826–3827. [Google Scholar]
- 7.Dekker HM, de Jong IJ, Sanders J, Wolf RF. Persistent Müllerian duct syndrome. RadioGraphics. 2003;23:309–313. doi: 10.1148/rg.232025141. [DOI] [PubMed] [Google Scholar]


