Abstract
Kinesin-mediated cargo transport is required for many cellular functions and plays a key role in pathological processes. Structural information on how kinesins recognize their cargoes is required for a molecular understanding of this fundamental and ubiquitous process. Here we present the crystal structure of the tetratricopeptide repeat of kinesin light chain 2 in complex with a cargo peptide harboring a ‘tryptophan-acidic’ motif derived from SKIP, a critical host determinant in Salmonella pathogenesis and a regulator of lysosomal positioning. Structural data together with biophysical, biochemical and cellular assays allow us to propose a framework for intracellular transport based on the binding by kinesin-1 of W-acidic cargo motifs through a combination of electrostatic interactions and sequence-specific elements, providing direct molecular evidence of the mechanisms for kinesin-1:cargo recognition.
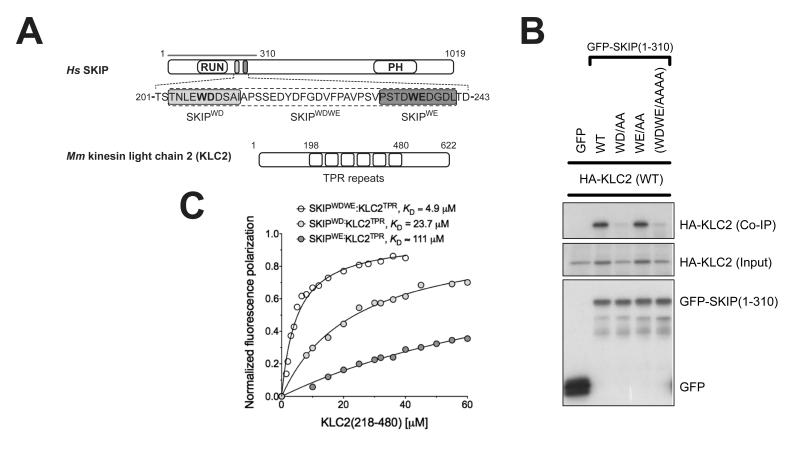
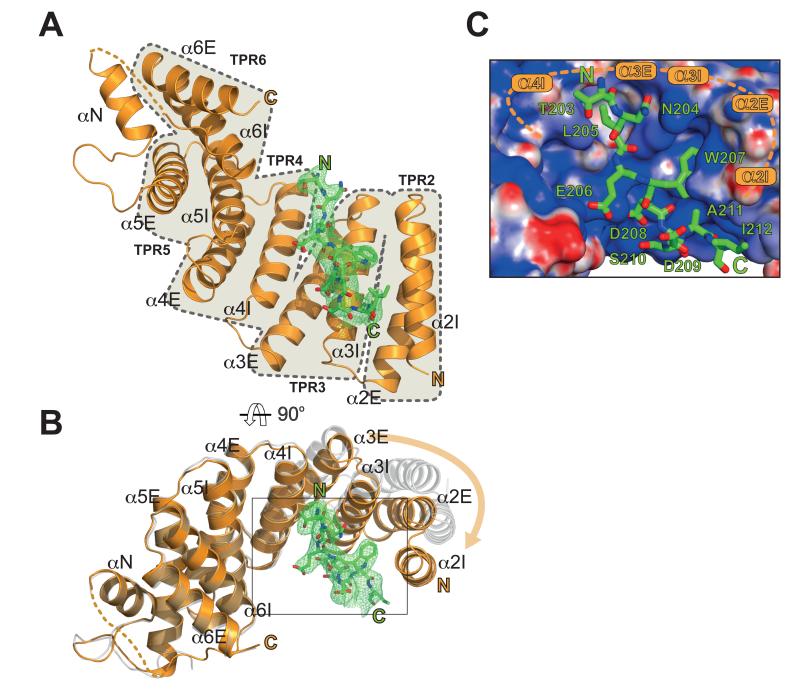
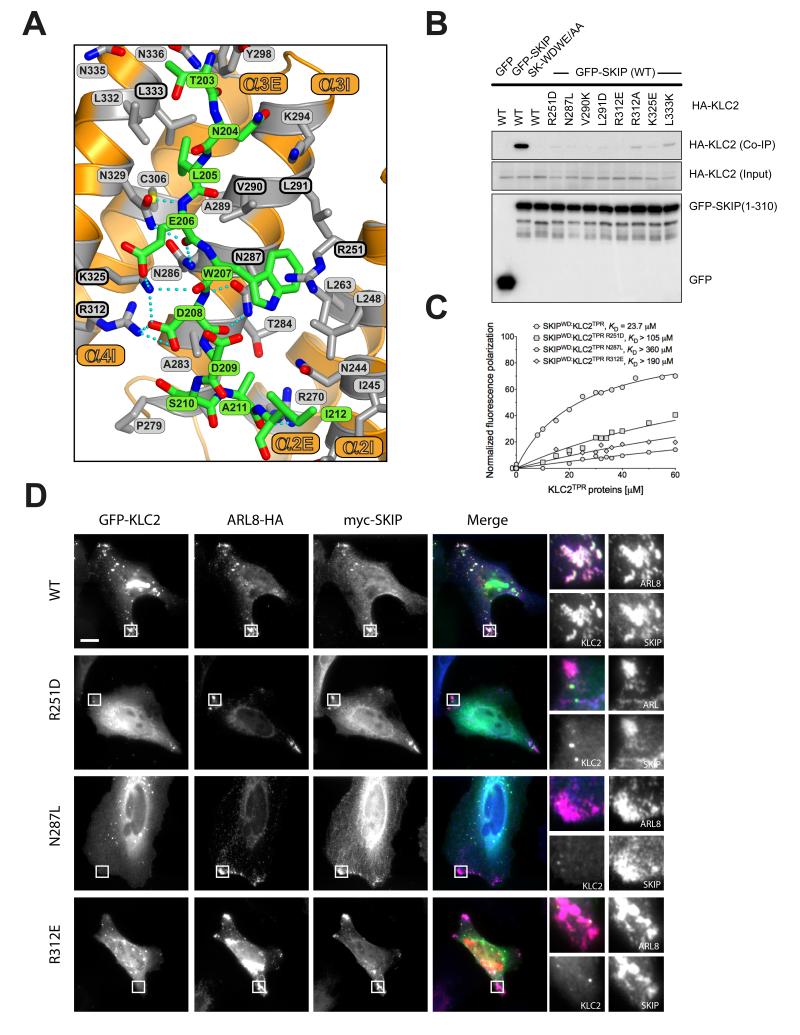
The plus-end directed motor, kinesin-1 plays a critical role in the intracellular transport of diverse protein, ribonuclear protein complexes and membrane compartments on microtubules (1). Its functions are also usurped by bacteria and viruses to aid in their replication (2, 3). Kinesin-1 can perform this diverse range of functions by virtue of its ability to interact with many different cargo proteins (4). Diversity of cargo recognition is accomplished largely through the kinesin light chains (KLC) which harbour a tetratricopeptide repeat (TPR) domain, a versatile protein interaction platform (5, 6). The KLCTPR domain can recognise short peptide stretches within relatively disordered regions of its targets. These peptides are characterized by a tryptophan residue flanked by acidic residues (e.g. EWD) and are found in a growing list of KLC binding proteins (7-16). Although W-acidic motifs often occur in pairs, single motifs are also functional and can support microtubule-based transport even when explanted from their host protein (7, 12, 17). We set out to solve the structure of a KLCTPR domain bound to a cargo W-acidic motif. We focused our attention on the SKIP cargo for its importance in Salmonella pathogenesis. SKIP contains a pair of W-acidic motifs centred at amino acid positions 207-208 (WD) and 236-237 (WE) that fall within the N-terminal kinesin-1 binding region (residues 1-310)(3, 7, 13) (Fig. 1A). To assess the relative importance of the SKIP W-acidic motifs for KLC binding we co-transfected HeLa cells with wild-type and WD/WE mutant constructs expressing GFP-SKIP(1-310) and HA-KLC2 (Fig. 1B). Disruption of the WD motif significantly reduced GFP-SKIP interaction with HA-KLC2 whereas abrogation of the WE motif had no obvious effects. A double mutant with both motifs disrupted displayed HA-KLC2 binding similar to the single WD mutant. Thus the WE motif has a very low affinity for KLC2. Indeed, a ten amino acid long peptide centered on the WD motif (SKIPWD, Figure 1A) bound to KLC2TPR with a KD of 24 μM whereas the affinity of the equivalent SKIPWE peptide was above 110 μM (Fig. 1C). The presence of both motifs improves the affinity for KLC2TPR because a 40 amino acid long peptide SKIPWDWE which encompasses the W-acid pair sequence bound with an apparent affinity higher than that of the single SKIPWD motif (KD = 4.9 μM). Binding affinity measurements at varying NaCl concentrations showed that electrostatic interactions play an important role in the recognition process (Fig. S1). For structural studies we focused on the SKIPWD motif and to facilitate the crystallization process, we engineered a chimeric construct in which the SKIPWD peptide was fused N-terminal to KLC2TPR via a flexible (TGS)4 linker (18). Crystals of SKIPWD-KLC2TPR grew using vapour diffusion techniques and diffracted at 2.9 Å using synchrotron radiation. The final model of the cargo complex is characterized by R and Rfree value of 20.3 and 24.5%, respectively (Table S1). KLC2TPR consists of six TPR repeats (TPR1-6) each contributed by a classical helix-turn-helix structural motif arranged in a right-handed super-helical conformation with a non-TPR helix is positioned between TPR5 and TPR6 (14). The structure of SKIPWD-KLC2TPR revealed the cargo peptide bound in an extended conformation at the N-terminal portion of KLC2TPR concave surface with a direction parallel to the external helix of the repeat (Fig. 2A,B and Fig. S2). Although our construct includes the external helix of TPR1, this region, as well as other flexible stretches, could not be unambiguously interpreted in the electron density. Thus, KLC2TPR starts from TPR2 in our model. A comparison between apo KLC2TPR (3CEQ) and cargo-bound KLC2TPR revealed structural differences considered to arise from a rigid jaw movement of the N-terminal TPR2-3 region which closes upon cargo recognition engendering the binding surface and pockets for the SKIPWD peptide. (Fig. 2B, S3). An analysis using the PISA algorithm (19) indicates that all residues of the SKIPWD peptide are involved in formation of the complex, which is stabilized by residues from TPR2-3 and the internal helix of TPR4 (Fig. 2C). This concave groove surface displays a positive electrostatic charge ideally poised to complement the negatively charged W-acidic cargo motifs (Figure 2C and Fig. S4). Overall, the interface area of the SKIPWD-KLC2TPR complex is about 770 Å2, stabilized by a mixture of H-bonds, salt bridges and hydrophobic interactions (Figure 3A). The W residue central to the motif is generally flanked by amino acids bearing a carboxylate side-chain. SKIPW207 (position 0, p0) is buried within a leucine-rich pocket positioned roughly in the middle along the TPR length and contributed by side-chains from TPR2 (L248, R251, L263) and TPR3 (N287, L290, L291). In particular, the side-chain of N287 is in a conformation such that it serves the dual purpose of stabilizing SKIPW207 indole group by lining one side of the pocket whilst engaging at the same time in hydrogen bonds with the main chain amide and carbonyl oxygen of the residue at p+1. The latter position is almost invariably occupied by a glutamic or aspartic acid residue (7). The carboxylate side-chain of SKIPD208 points in the opposite direction to that of SKIPW207 engaging in a network of salt bridges and H-bonds with the positively charged R312 and K325 side-chains of α3E and α4I, respectively. An acidic side-chain at position p−1 (SKIPE206) of the motif is also very conserved in other W-acidic motifs. Like SKIPD208, the carboxylate side-chain of SKIPE206 faces the TPR3-4 side of the KLC2TPR recognition groove where it is stabilized by an ionic interaction with K325. Position p-2 of SKIPWD features a leucine residue (SKIPL205). The hydrophobic side chain of SKIPL205 is deeply buried in a hydrophobic pocket formed by residues from TPR3 and 4. The side-chain of N329 plays a similar dual role as N287. Whilst lining the SKIPL205 pocket it also H-bonds with the main chain at position p−1. Together, N287 and N329 act like a clamp on opposite sides of SKIPWD providing four of the ten hydrogen bonds that stabilize the complex. Residues at positions p-(3,4) are less important for complex stability consistent with their general lack of sequence conservation amongst W-acidic motif cargo (7). The C-terminal stretch of SKIPWD encompassing p+(2,3,4,5) is observed in a more compact, turn-like conformation with SKIPD209,S210 at p+(2,3) making very minor contacts with the groove. As for the N-terminal peripheral region of SKIPWD the exact nature of the amino acids at p+(4,5) does not seem critical for complex stability. Alternative side-chains can be positioned at this topological position possibly involving a rearrangement of the main-chain. To investigate the effect of amino acid replacements within the KLC2TPR region on the interaction with SKIP we used immunoprecipitation as described above. All amino acid substitutions tested resulted in abrogation or near abrogation of complex formation between KLC2 and SKIP (Fig 3B,C). The importance of electrostatic interactions in cargo recognition is underscored by charge-reversal mutations (Fig 3B,C). When co-expressed in cells, SKIP and its small GTPase binding partner Arl8 associate strongly with lysosomes and promote their trafficking to the cell periphery. GFP-KLC2 associates with the same lysosomes (Fig. 3D, S5) (13). This is essentially abolished by KLC2 mutations (R251D, N287L and R312E) (Fig. 3D, S5). The same mutations strongly inhibited the binding of KLC2TPR to the WD motif peptide (Fig. 3C). We conclude that optimal SKIP:KLC2 stability critically depends on a very conserved cargo recognition-interaction groove. We have extended our analysis to the well characterized Calsyntenin (CSTN) cargo which also exhibits two W-acidic motifs (9, 11, 12). Differently from SKIP, both CSTN motifs bind to KLC2TPR with similar affinity (Fig S6). However, the mutations in the KLC2 binding groove which disrupt SKIPWD binding also abrogate recognition of CSTN (Fig S6). A sequence alignment shows total conservation of the KLCTPR residues interacting with the cargo across the kinesin light chain family (Fig. S7). Thus, the concave groove within the TPR domain where the SKIPWD peptide binds is likely to be the primary site of interaction for W-acidic cargo motifs in general. However, we cannot exclude that secondary sites also exist. In the context of intracellular transport where kinesin-1 functions as a tetramer containing two KLCs held together by a coiled-coil region and both SKIP motifs contribute to transport (7, 13), it is tempting to speculate that both chains can contribute to the binding of the W-acidic cargo pair. For SKIP, the higher affinity WD motif would direct the first binding event to one of the KLCTPR with avidity effect promoting the association of the WE motif to the other KLC (20, 21). It will be important to determine this relationship to the kinesin-1 tetramer and examine the importance of cargo induced TPR conformational change in motor activation.
Figure 1.
Binding of SifA-kinesin binding protein (SKIP) to kinesin light chain 2 (KLC2). (A) Scheme of SKIP and KLC2. The two W-acidic motifs and KLC2 TPR repeats are highlighted. (B) Co-immunoprecipitation shows that alanine replacement in the first W-acidic motif (WD) abrogates SKIP:KLC2 binding whereas the same substitution in the second motif (WE) has virtually no effect. (C) Fluorescence polarization measurements confirm that the first SKIP W-acidic motif (SKIPWD) has a higher affinity for the TPR domain of KLC2 than the second one (SKIPWE). A peptide encompassing both motifs (SKIPWDWE) binds with higher affinity than the best single motif. KD values reported here were calculated at 150 mM NaCl. Binding affinity values at varying ionic strength values are given in Fig. S1.
Figure 2.
Structure of the SKIPWD-KLC2TPR cargo complex. (A,B) Cartoon representations of the SKIPWD motif (displayed as stick model in green) bound to KLC2TPR domain (orange cartoon) in two orthogonal orientations. Simulated annealing (Fo-Fc) omit map for the W-acidic cargo motif is contoured at the 3σ level. Individual TPR repeats composed by helix-turn-helix elements (I and E for internal and external, respectively) are highlighted in A. A non-TPR helix (αN) is between TPR5 and TPR6. Panel B also shows the apo KLC2TPR structure (grey transparent cartoon) with an orange arrow indicating the movement of the TPR2-3 region with respect to the common TPR4-6 reference frame. (C) Electrostatic potential surface representation of KLC2TPR with its SKIPWD-bound cargo. Positive and negative potential is shown in blue and red, respectively. Cargo recognition is achieved by a combination of charge complementarity and sequence specificity.
Figure 3.
The SKIPWD-KLC2TPR interface and the effect of KLC2TPR mutations in cargo binding and cellular recruitment. (A) Details of the SKIPWD:KLC2TPR interface. KLCTRP side-chains stabilizing the SKIPWD cargo peptide (green) are shown as grey sticks emanating from the orange cartoon. Non-carbon elements are: nitrogen, blue; oxygen, red; sulfur, yellow. Hydrogen bonds are represented by dotted cyan lines. (B) Co-immunoprecipitation assay showing the effect of KLC2TPR mutations at the SKIPWD:KLC2TPR interface on the interaction. (C) Fluorescence polarization measurements showing that R251D, N287L and R312E mutations in KLC2 dramatically reduce the affinity of the TPR domain for the SKIPWD peptide. KD values reported here were calculated at 150 mM NaCl. (D) Replacement of key KLC2TPR residues results in loss of GFP-KLC2 association with Arl8/SKIP positive lysosomal membranes. Scale bar shows 10μm. In merge panels, GFP-KLC2, Arl8 and SKIP are shown in green, red and blue respectively.
Supplementary Material
Acknowledgements
Scientists of I24 beamline (Diamond Light Source, Didcot, UK) are gratefully acknowledged for their support during data collection. ARL8b-HA and myc-SKIP were a kind gift from Dr. S. Munro, (LMB-MRC, Cambridge). We thank Prof. T. Blundell (University of Cambridge) for useful discussions. Coordinates and structure factor files for have been deposited in the Protein Data Bank under accession number 3ZFW. M.P.D and A.L. are supported by a Wellcome Trust Research Career Development Fellowship to M.P.D. and a London Law Trust Medal Fellowship to M.P.D. S.P. is supported by a British Heart Foundation grant awarded to R.A.S.
Footnotes
The authors declare no competing financial interests.
References
- 1.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009 Oct;10:682. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 2.Dodding MP, Way M. Coupling viruses to dynein and kinesin-1. Embo J. 2011 Aug 31;30:3527. doi: 10.1038/emboj.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucrot E, Henry T, Borg JP, Gorvel JP, Meresse S. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science. 2005 May 20;308:1174. doi: 10.1126/science.1110225. [DOI] [PubMed] [Google Scholar]
- 4.Gindhart JG. Towards an understanding of kinesin-1 dependent transport pathways through the study of protein-protein interactions. Brief Funct Genomic Proteomic. 2006 Mar;5:74. doi: 10.1093/bfgp/ell002. [DOI] [PubMed] [Google Scholar]
- 5.D’Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003 Dec;28:655. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Hammond JW, Griffin K, Jih GT, Stuckey J, Verhey KJ. Co-operative versus independent transport of different cargoes by Kinesin-1. Traffic. 2008 May;9:725. doi: 10.1111/j.1600-0854.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- 7.Dodding MP, Mitter R, Humphries AC, Way M. A kinesin-1 binding motif in vaccinia virus that is widespread throughout the human genome. Embo J. 2011 Nov 16;30:4523. doi: 10.1038/emboj.2011.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyama T, et al. Cayman ataxia protein caytaxin is transported by kinesin along neurites through binding to kinesin light chains. J Cell Sci. 2009 Nov 15;122:4177. doi: 10.1242/jcs.048579. [DOI] [PubMed] [Google Scholar]
- 9.Konecna A, et al. Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell. 2006 Aug;17:3651. doi: 10.1091/mbc.E06-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt MR, et al. Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc Natl Acad Sci U S A. 2009 Sep 8;106:15344. doi: 10.1073/pnas.0904268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki Y, et al. The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport. Embo J. 2007 Mar 21;26:1475. doi: 10.1038/sj.emboj.7601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano T, et al. A small peptide sequence is sufficient for initiating kinesin-1 activation through part of TPR region of KLC1. Traffic. 2012 Jun;13:834. doi: 10.1111/j.1600-0854.2012.01350.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosa-Ferreira C, Munro S. Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev Cell. 2011 Dec 13;21:1171. doi: 10.1016/j.devcel.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, et al. Crystal structures of the tetratricopeptide repeat domains of kinesin light chains: insight into cargo recognition mechanisms. PLoS One. 2012;7:e33943. doi: 10.1371/journal.pone.0033943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006 Feb 10;281:3552. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 16.Ligon LA, Tokito M, Finklestein JM, Grossman FE, Holzbaur EL. A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J Biol Chem. 2004 Apr 30;279:19201. doi: 10.1074/jbc.M313472200. [DOI] [PubMed] [Google Scholar]
- 17.Morgan GW, et al. Vaccinia protein F12 has structural similarity to kinesin light chain and contains a motor binding motif required for virion export. PLoS Pathog. 2010 Feb;6:e1000785. doi: 10.1371/journal.ppat.1000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellegrini L, et al. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature. 2002 Nov 21;420:287. doi: 10.1038/nature01230. [DOI] [PubMed] [Google Scholar]
- 19.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007 Sep 21;372:774. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Radnai L, et al. Affinity, avidity, and kinetics of target sequence binding to LC8 dynein light chain isoforms. J Biol Chem. 2010 Dec 3;285:38649. doi: 10.1074/jbc.M110.165894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack ET, et al. Dependence of avidity on linker length for a bivalent ligand-bivalent receptor model system. Journal of the American Chemical Society. 2012 Jan 11;134:333. doi: 10.1021/ja2073033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu TC, Korczynska J, Smith DK, Brzozowski AM. High-molecular-weight polymers for protein crystallization: poly-gamma-glutamic acid-based precipitants. Acta Crystallogr D Biol Crystallogr. 2008 Sep;64:957. doi: 10.1107/S0907444908021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter G. xia2: an expert system for macromolecular crystallography data reduction. Journal of Applied Crystallography. 2010;43:186. [Google Scholar]
- 24.Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallographica Section D. 2010;66:133. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans P. Scaling and assessment of data quality. Acta Crystallographica Section D. 2006;62:72. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- 26.Vagin A, Teplyakov A. MOLREP: an Automated Program for Molecular Replacement. Journal of Applied Crystallography. 1997;30:1022. [Google Scholar]
- 27.Steiner RA, Lebedev AA, Murshudov GN. Fisher’s information in maximum-likelihood macromolecular crystallographic refinement. Acta Crystallographica Section D. 2003;59:2114. doi: 10.1107/s0907444903018675. [DOI] [PubMed] [Google Scholar]
- 28.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallographica Section D. 2011;67:355. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bricogne G, et al. BUSTER. version 2.10.0 Global Phasing Ltd; Cambridge, United Kingdom: 2011. [Google Scholar]
- 30.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallographica Section D. 2004;60:2126. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 31.Brünger AT, et al. Crystallography & NMR System: A New Software Suite for Macromolecular Structure Determination. Acta Crystallographica Section D. 1998;54:905. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 32.Davis IW, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007 Jul;35:W375. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodding MP, Newsome TP, Collinson LM, Edwards C, Way M. An E2-F12 complex is required for intracellular enveloped virus morphogenesis during vaccinia infection. Cell Microbiol. 2009 May;11:808. doi: 10.1111/j.1462-5822.2009.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.