Case study
A 43-year-old man with a long history of obesity presented to our Weight Management Center 5 years after being disabled in a motor vehicle accident and gaining weight to a lifetime high of 269 kg and body mass index (BMI) of 85 kg/m2. His comorbidities were hypertension, obstructive sleep apnea, gastroesophageal reflux disease, gout, and osteoarthritis, and he had recently developed type 2 diabetes mellitus. Medications used included metformin, glyburide, losartan, hydrochlorothiazide, and diltiazem. He was motivated and met criteria for weight loss via a surgical intervention. Preoperatively, he was placed on a high-protein diet plus an appetite suppressant (phentermine) to achieve 10% weight loss. His weight declined, but he developed new-onset atrial fibrillation 3 weeks later, which was thought to be related to phentermine use and was cardioverted back to sinus rhythm.
Several months later, the patient underwent gastric bypass bariatric surgery with a preoperative weight of 252 kg. His type 2 diabetes mellitus resolved immediately after surgery, as did his gastroesophageal reflux and hypertension. Ten months after surgery, his weight was down to 177 kg (BMI=56 kg/m2) with hemoglobin A1c of 5.9%, fasting blood glucose of 82 mg/dL, and blood pressure of 137/ 82 mm Hg, and he was no longer taking any medication.
Obesity: Relationship to Cardiovascular Disease
Although there are multiple long-term deleterious health effects of excess weight, obesity as defined by BMI ≥30 kg/m2 is associated with premature atherosclerosis, increased risk of myocardial infarction and heart failure, and decreased survival, largely because of cardiovascular deaths, particularly in extreme weight categories.1,2 Factors that contribute to cardiovascular disease in obesity are multifactorial and include metabolic dysregulation with increased prevalence of atherogenic risk factors, including insulin resistance, hypertension, and dyslipidemia; adverse cardiac remodeling characterized by hypertrophy, chamber enlargement, and impaired ventricular systolic and diastolic function; vascular endothelial dysfunction; premature coronary artery disease; increased sympathetic tone; pulmonary hypertension with right-sided heart strain; and arrhythmias.3 Additionally, obesity is linked to a chronic state of inflammation evidenced by increased circulating levels of proinflammatory cytokines, derived largely from hepatic and adipose sources that may play roles in mechanisms of insulin resistance, plaque activation, myocardial hypertrophy, and cardiovascular disease progression. We recently observed that inflammatory changes in fat tissue are linked to systemic arterial dysfunction and abnormal clinical phenotypes, which suggests the possibility of a pathogenic adipose-cardiovascular axis.4 Although BMI is useful in predicting overall risk, other factors, including adiposity distribution, degree of visceral or ectopic fat burden, percent body fat, genetic factors, sex, and possibly qualitative features of adipose tissue, may be germane to clinical disease expression.5,6
Treatment of Obesity: Surgery and Pharmacotherapy
The case illustrated in this clinician update provides an example of the risks and benefits of various kinds of obesity treatments and highlights how potential options should be chosen with the patient’s medical history in mind.
Weight control is the cornerstone of therapy for both primary and secondary prevention of cardiovascular disease. Standard diets and exercise strategies have had limited efficacy because of low rates of long-term success in sustaining weight losses of 5% to 10% of initial body weight beyond 6 months. The use of appetite suppressants, which typically work on the hypothalamus, to decrease food intake can potentiate weight loss as long as treatment is sustained; however, weight is quickly regained on cessation of the agent. These drugs are also fraught with side effects such as blood pressure and pulse elevations, anxiety, and insomnia and thus can be extremely difficult to use, especially in patients with cardiovascular disease or risk factors. With a high BMI (class V obesity) and comorbidities such as hypertension, sleep apnea, and type 2 diabetes mellitus, the patient in this Clinician Update is certainly at increased cardiovascular risk. One of his prescribed medications, phentermine, acts primarily as a norepinephrine-releasing agent and centrally suppresses appetite in the hypothalamus. Because of its amphetamine-like actions, it also exerts peripheral effects of pulse and blood pressure elevation. In a patient with this degree of obesity, it is likely that the combination of sleep apnea, possibly right-sided heart overload, and phentermine effects triggered atrial fibrillation.
The medical and surgical treatments for obesity have a core of behavioral approaches in common: diet and exercise modification. These modalities need to be included concomitantly with medical or surgical options, likely lifelong, for successful outcomes.
This patient was unable to tolerate one of the pharmacotherapeutic options for obesity treatment because of its common sympathomimetic cardiovascular side effects. Unfortunately, many of the medications for obesity have undergone intense scrutiny because of potential cardiovascular and other side effects and have come up short when evaluated for health risk versus benefit. Phentermine was approved for weight loss by the Food and Drug Administration (FDA) in 1959 for short-term (12 weeks) treatment of obesity; long-term studies are unavailable.7 It was combined with fenfluramine in a popular combination termed “phen-fen” until concerns about valvulopathy prompted the FDA to withdraw fenfluramine from the market in 1997. Another agent for obesity, sibutramine, approved in 1997 for long-term use, was withdrawn from the market recently after a study of subjects with preexisting cardiovascular disease, type 2 diabetes mellitus, or both showed increased risk of nonfatal myocardial infarction and stroke with treatment for a mean duration of 3.4 years.8
Currently, the only centrally acting appetite suppressants on the market for obesity treatment are phentermine and diethylpropion, both of which are sympathomimetics with potential cardiovascular side effects. Orlistat, a peripherally acting agent, is a pancreatic and gastric lipase inhibitor that blocks 25% to 30% of fat calories from absorption in the gastrointestinal tract. Its actions are considered mild compared with appetite suppressants, with those who take it achieving less than a 5% weight loss compared with placebo.9 The FDA guidance for industry for weight loss drugs recommends that drugs brought through the approval process show for efficacy a difference in mean weight loss between product and placebo groups of at least 5% or that the proportion of subjects undergoing active treatment who lose ≥5% of baseline body weight be at least 35%.
Other agents that did not achieve FDA approval for obesity treatment recently for safety reasons include rimonabant, a cannabinoid receptor antagonist; lorcaserin, a 5-hydroxytryptamine (serotonin) 2C receptor agonist; and the combination drugs topiramate/ phentermine and naltrexone/bupropion. Concerns about side effects were again the predominant reason for non-approval status, including anxiety and depression for rimonabant, breast tumors in rat models for lorcaserin, cardiovascular effects and teratogenicity for topiramate/phentermine, and elevations in blood pressure for naltrexone/ bupropion. These drugs did meet the FDA efficacy recommendations listed above. The approval bar for obesity agents in the United States is high because of the number of Americans who would be eligible for these agents (33.8% of the population was considered obese in the United States as of 2008).
Table 1 summarizes recent pharmacological agents used for obesity treatment.10–12 The National Institutes of Health/National Heart, Lung, and Blood Institute guidelines on the assessment and treatment of obesity recommend that pharmacotherapy for obesity be considered for those patients with a BMI ≥30 kg/m2 or ≥27 kg/m2 with at least 1 serious comorbidity. All pharmacotherapeutic options for obesity should be adjuncts to dietary and physical activity recommendations.13 Newer medical approaches to obesity management may include the addition of incretin hormones, the blood levels of which are altered after gastric bypass surgery. GLP-1 (glucagon-like peptide 1) agonists such as exenatide and liraglutide have been shown to promote weight loss in both diabetic and nondiabetic patients. Other experimental approaches include combinations of injectable agents plus leptin, a hormone secreted by adipose tissue that signals satiety in the hypothalamus. These approaches are considered an off-label use of approved agents in the case of GLP-1 agonists and experimental in the case of leptin in combination with other agents. Table 2 summarizes the recent activity of newer pharmaceutical obesity agents in the FDA approval process.14
Table 1.
| Drug | Mechanism of Action | Cardiovascular Effects | Weight Loss* | Status |
|---|---|---|---|---|
| Fenfluramine/ phentermine resin | 5HT-releasing agent and reuptake inhibitor/norepinephrine-releasing agent | Cardiac valvulopathy and pulmonary hypertension | 11.0%; 34 wk | Fenfluramine withdrawn in 1997; phentermine still available |
| Fenfluramine, dexfenfluramine | 5HT-releasing agent and reuptake inhibitors | Cardiac valvulopathy and pulmonary hypertension | 3.0%; 1 y | Both withdrawn from market in 1997 |
| Sibutramine | Norepinephrine/serotonin reuptake inhibitor; induces satiety/increases energy expenditure | BP and pulse elevations, MI and stroke risk | 3.7% to 5.0%; 1 y | Withdrawn from market in 2010 |
| Phentermine resin diethylpropion | Norepinephrine releasing agents | BP and pulse elevations | 8.1%; 36 wk | Approved in 1960s for short-term use |
| Mazindol | Norepinephrine reuptake inhibitor | BP and pulse elevations | 2% to 10%; 12 wk | Discontinued in 1999 |
| Phenylpropanolamine | A1 adrenergic agonist | Increased risk of hemorrhagic stroke | 0% to 2.0%; 12 wk | Withdrawn from OTC market in 2000 |
| Orlistat | Pancreatic and gastric lipase inhibitor | None known | 2.9% to 3.4%; 1 y | FDA approved in 1999 for long-term use |
5HT indicates 5-hydroxytryptamine (serotonin); BP, blood pressure; MI, myocardial infarction; OTC, over-the-counter; and FDA, Food and Drug Administration.
Mean weight loss in excess of placebo given as percent of initial body weight.
Table 2.
Newer Agents for Obesity14
| Drug | Mechanism of Action | Cardiovascular Effects | Weight Loss* | Status |
|---|---|---|---|---|
| Rimonabant | Endocannabinoid receptor type 1 blocker | NA | 5.0% | Not approved 2007; psychiatric side effects cited |
| Topiramate/phentermine | GABA receptor modulation | BP and pulse elevations | 8.6% | Not approved 2010; cardiovascular effects and teratogenicity cited |
| Bupropion/naltrexone | Dopamine, norepinephrine reuptake inhibitor/opioid antagonists | BP elevation | 4.8% | Not approved 2010; FDA requesting preapproval long-term cardiovascular study |
| Lorcaserin | 5HT2C receptor agonist | Possible valvulopathy | 3.6% | Not approved 2010; breast tumors in animals cited |
| Bupropion/zonisamide | Dopamine norepinephrine reuptake inhibitor/sodium channel modulation | BP elevation | 6.1% | Phase IIB/III |
| Pramlintide/metreleptin | Incretin and adipose tissue hormone with satiety signal in hypothalamus | NA | 9.2%; 28 wk | Phase IIB |
| Liraglutide | GLP-1 agonist | NA | 4.5%; 20 wk | Phase IIB/III |
NA indicates not available; GABA, γ-aminobutyric acid; BP, blood pressure; FDA, Food and Drug Administration; 5HT2C, 5-hydroxytryptamine (serotonin) 2C receptor; and GLP-1, glucagon-like peptide 1.
Mean weight loss in excess of placebo give as percent of initial body weight; 1-year intention-to-treat analysis unless otherwise specified.
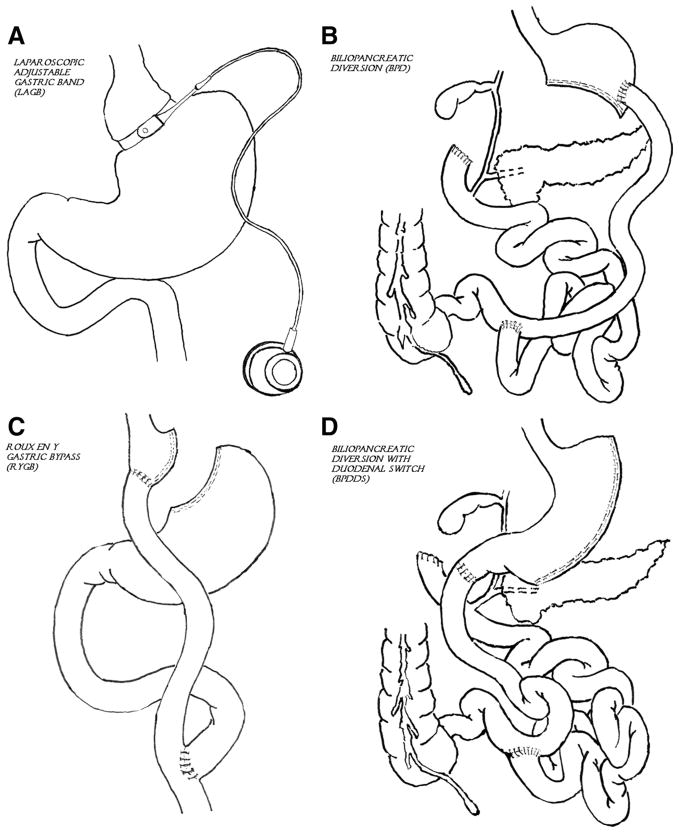
Given the limited efficacy of anti-obesity medications and behavioral interventions, bariatric operations have provided an alternative yet durable approach to combat obesity and its comorbidities. Bariatric surgery has increased exponentially in the United States and has evolved to become one of the safer surgical operations performed in the United States, with a 30-day mortality rate of 0.3% and an early complication rate of 4.1%.15 Bariatric surgery encompasses several different procedures: Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and the biliopancreatic diversion and biliopancreatic diversion with duodenal switch (Figure). The 2 most commonly performed in the United States are the Roux-en-Y gastric bypass and laparoscopic adjustable gastric band. These procedures are recommended for patients with BMI ≥40 kg/m2 or ≥35 kg/m2 with at least 1 serious comorbidity. One type of laparoscopic adjustable gastric band was recently approved by the FDA (2011) for a lower BMI indication, BMI ≥35 kg/m2 or ≥30 kg/m2 with a serious comorbidity.
Figure.
Surgical treatments for obesity. A, Laparoscopic adjustable gastric band (LAGB). B, Biliopancreatic diversion (BPD). C, Roux-en-Y gastric bypass (RYGB). D, Biliopancreatic diversion with duodenal switch (BPDDS).
Cardiovascular Benefits of Weight Loss
Studies have shown the benefits of a modest 5% to 10% weight loss on cardiovascular risk factors, most notably the Diabetes Prevention Program, in which an average 7% weight loss with an intensive lifestyle intervention of diet and exercise delayed the progression to type 2 diabetes mellitus in those with prediabetes.16
Our patient underwent the Roux-en-Y gastric bypass, which resolved most of his comorbidities, including type 2 diabetes mellitus, hypertension, reflux disease, and osteoarthritis. The Roux-en-Y gastric bypass can produce on average a 25% weight loss by 9 to 12 months after the procedure. There is robust literature highlighting the immediate and consistent amelioration of metabolic abnormalities after bariatric surgery, in many cases much before major weight loss begins. This improvement, in the case of type 2 diabetes mellitus, may occur immediately and results from alterations in the secretion of several gut-derived hormones, notably GLP-1, GIP (gastric inhibitory polypeptide), PYY (peptide YY), and ghrelin, with endocrine functions that modulate insulin sensitivity and glucose utilization.17
Data suggest that gastric bypass has more profound effects on the gut milieu than restrictive operations such as laparoscopic adjustable gastric banding. The immediate change in insulin sensitivity also does not occur with medical weight loss alone, which suggests that gastric bypass surgery alters specific aspects of the gastrointestinal hormonal environment that are presently incompletely understood. Improvements in other cardiac risk factors, such as normalization of blood pressure and lipids, also occur in conjunction with laparoscopic adjustable gastric band and/or medical weight loss and tend to be apparent after a 5% to 10% change of initial body weight.
Although a favorable reduction in proatherogenic metabolic profiles occurs within weeks to months, long-term data from the Swedish Obese Subjects (SOS) study demonstrate substantial and sustained benefit, with >10-year rates of recovery from diabetes, sleep apnea, hypertension, hypertriglyceridemia, and hyperuricemia after bariatric surgery, which is remarkable. Importantly, these alterations are associated with reduced risk of myocardial infarction and prolonged cardiovascular survival.18 In addition to improving metabolic dysfunction, weight reduction is associated with decreases in circulating cytokines such as interleukin 6, tumor necrosis factor-α, and C-reactive protein and upregulation of vasculoprotective factors such as adiponectin. Beneficial physiological and structural changes also become manifest with weight loss. For example, our group has shown that vascular endothelial function improves significantly within 3 months after bariatric surgery and is sustained at 12 months.19,20 Cardiac geometry and myocardial performance also improve after weight intervention.
In the Utah Obesity Study, marked weight reduction 2 years after gastric bypass induced regression of left ventricular hypertrophy and ventricular enlargement and improved biventricular systolic function. These occurred in parallel with improved left ventricular diastolic relaxation and tissue Doppler parameters that correlated with improved exercise capacity.21 Additionally, electrophysiological properties may change, with several studies demonstrating correction of abnormal QTc intervals, heart rate recovery, and autonomic tone.
In summary, targeted weight loss reverses many aspects of abnormal cardiovascular function, and clinical data demonstrate long-term survival benefit, in particular with bariatric surgical intervention. The treatment decision for a given obese individual must be tailored on the basis of patient preference and eligibility, comorbidity burden, and the risk-benefit ratio of potential medical and surgical options. Future research may unravel additional beneficial mechanisms of different weight loss strategies and potentially pave the way for safer and more effective dietary or medical options for obesity treatment. The goal of future research into antiobesity agents is to induce a “medical gastric bypass” with perhaps a combination of incretin hormones and appetite suppressants.
Acknowledgments
Sources of Funding
Dr Apovian is supported by National Institutes of Health grants 5P30DK046200-19, R21 HD061311-01A1, R01 AG037547-01, R01 HL084213, and P01 HL081587; Dr Gokce is supported by National Institutes of Health grants R01 HL084213 and P01 HL081587.
Footnotes
Disclosures
Dr Apovian participates on advisory boards for Allergan, Amylin, Orexigen, Merck, Johnson and Johnson, Arena, and Sanofiaventis. Dr Gokce reports no conflicts.
References
- 1.Berrington de Gonzalez A, Hartge P, Cerhan JF, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jaquotte A, Willett WC, Thun MJ. Body mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Haley J, Qizlbash N, Collins R, Peto R. Body mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirier P, Cornier MA, Mazzone T, Stiles S, Cummings S, Klein S, McCullough PA, Ren Fielding C, Franklin BA. Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation. 2011;123:1683–1701. doi: 10.1161/CIR.0b013e3182149099. [DOI] [PubMed] [Google Scholar]
- 4.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial function in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D’Agostino RB, Sr, O’Donnell CJ, Fox CS. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity. 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pou KM, Massaro JM, Hoffmann U, Lieb K, Vasan RS, O’Donnell CJ, Fox CS. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care. 2009;32:481–485. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaodhiar L, Apovian CM. Current perspectives of obesity and its treatment. Manag Care Interface. 2007;20:24–31. [PubMed] [Google Scholar]
- 8.James WPT, Caterson ID, Coutinho W, Finer N, VanGaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, Renz CL. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 9.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 10.Glazer G. Long term pharmacotherapy of obesity 2000: a review of safety and efficacy. Arch Intern Med. 2001;161:1814–1824. doi: 10.1001/archinte.161.15.1814. [DOI] [PubMed] [Google Scholar]
- 11.Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannides-Demos L, Piccenna L, McNeil JJ. Pharmacotherapies for obesity: past, current, and future therapies. J Obes. 2011;2011:179674. doi: 10.1155/2011/179674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute (NHLBI) and North American Association for the Study of Obesity (NAASO) The Practical Guide to the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Institutes of Health; 2000. Publication No. 00-4084. [Google Scholar]
- 14.Powell A, Apovian CM, Aronne LJ. New targets for the treatment of obesity. Clin Pharmacol Ther. 2011;90:40–51. doi: 10.1038/clpt.2011.82. [DOI] [PubMed] [Google Scholar]
- 15.Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, Pories W, Courcoulas A, McCloskey C, Mitchell J, Patterson E, Pomp A, Staten MA, Yanovski SZ, Thirlby R, Wolfe B Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in incidence of type 2 diabetes with lifestyle intervention or met-formin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubino F, Kaplan LM, Schauer PR, Cummings DE. Diabetes Surgery Summit Delegates. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399–405. doi: 10.1097/SLA.0b013e3181be34e7. [DOI] [PubMed] [Google Scholar]
- 18.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 19.Gokce N, Vita JA, McDonnell M, Forse AR, Istfan N, Stoeckl M, Lipinska I, Keaney JF, Jr, Apovian CM. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95:266–268. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Bigornia SJ, Mott MM, Hess DT, Apovian CM, McDonnell ME, Duess MA, Kluge MA, Fiscale AJ, Vita JA, Gokce N. Long-term successful weight loss improves vascular endothelial function in severely obese individuals. Obesity. 2010;18:754–759. doi: 10.1038/oby.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owan T, Avelar E, Morley K, Jifi R, Hall N, Krezowski J, Gallagher J, Williams Z, Preece K, Gundersen N, Strong MB, Pendleton RC, Segerson N, Cloward TV, Walker JM, Farney RJ, Gress RE, Adams TD, Hunt SC, Litwin SE. Favorable changes in cardiac geometry and function following gastric bypass surgery. J Am Coll Cardiol. 2011;57:732–739. doi: 10.1016/j.jacc.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]