Abstract
Enlarged adenotonsillar tissue (AT) is a major determinant of obstructive sleep apnea (OSA) severity in children; however, mechanisms of AT proliferation are poorly understood. We hypothesized that early exposure to respiratory syncytial virus (RSV) may modify AT proliferation through up-regulation of nerve growth factor (NGF)-neurokinin 1 (NK1) receptor dependent pathways. AT harvested from 34 children with OSA and 25 children with recurrent tonsillitis (RI) were examined for mRNA expression of multiple growth factors and their receptors. In addition, NK1 receptor expression and location, and substance P tissue concentrations were compared in AT from OSA and RI children. NGF mRNA and its high-affinity tyrosine kinase receptor (trkA) expression were selectively increased in OSA (p < 0.001). NK1 receptor mRNA and protein expression were also enhanced in OSA (p < 0.01), and substance P concentrations in OSA patients were higher than in RI (p < 0.0001). AT from OSA children exhibit distinct differences in the expression of NGF and trkA receptors, NK1 receptors, and substance P. The homology between these changes and those observed in the lower airways following RSV infection suggests that RSV may have induced neuro-immunomodulatory changes within AT, predisposing them to increased proliferation, and ultimately contribute to emergence of OSA.
Obstructive sleep apnea syndrome (OSA) is a common and highly prevalent disorder in the pediatric age range, affecting 2–3% of all children (1). Adenotonsillar hypertrophy is by far the major pathophysiological contributor to OSA in children (2,3), and adenotonsillectomy (T&A) currently remains the first line of treatment for children with OSA (4). If left untreated, OSA can result in serious morbid consequences that affect neurocognitive, behavioral and cardiovascular systems (5–9). However, the mechanisms underlying the regulation of benign follicular lymphoid proliferation and hyperplasia are so far extremely poorly understood, such that prediction of which children will develop adenotonsillar hypertrophy is impossible at this stage. It has now been recognized by several epidemiologic studies that factors such as environmental smoking, allergies, and intercurrent respiratory infections are all associated with either transient or persistent hypertrophy of lymphadenoid tissue in the upper airways of snoring children (10–12). Interestingly, all of these risk factors involve the generation of an inflammatory response, suggesting that the latter may promote the onset and maintenance of proliferative signals to lymphadenoid tissues.
Bronchial-associated lymphoid tissue in the lungs of RSV-infected rats express high levels of the neurokinin 1 (NK1) receptor for substance P that is released from subepithelial sensory nerve fibers, a part of the nonadrenergic noncholinergic system (13,14). Furthermore, nerve growth factor (NGF), a major controller of sensorineural development and immuno-inflammatory responses, and its tyrosine kinase A receptor (trkA) are both overexpressed in rat lungs after respiratory syncytial virus (RSV) inoculation (15). The distal airways, however, are not the sole location in which the late effects of RSV consist in up-regulation of the NGF-NK1-substance P cascade. For example, toluene diisocyanate (TDI) exposures were associated with rhinitis and nasal irritation, via increases in the synthesis and release of substance P from airway sensory nerves following an increase in NGF (interacting with trkA), the latter events preceding the neuronal and mucosal increases in substance P concentration (16). Virtually 100% of children are infected with RSV in the first few years of life, and about 66% of infants are infected during their first year of life (17,18). Since the 1950s, a number of studies have shown that RSV bronchiolitis is often followed by post bronchiolitic wheezing and the diagnosis of asthma later in life (19). In an attempt to elucidate potential mechanisms through which a self-limiting infection such as RSV infection leads to airway dysfunction that persists long after the virus is cleared, several studies were conducted using bronchoalveolar lavage specimens obtained from intubated children having a RSV infection, as well as in animal models of RSV infection. The cumulative evidence from these studies suggested that RSV infection in the lower respiratory tract induces a neuro-immunomodulatory response (i.e. priming of immune responses through neural networks) that ultimately predisposes to an exaggerated inflammatory lung response to multiple environmental stimuli during and after the infection, and that these processes involved NGF-trkA interactions followed by NK1-substance P increases (20–22).
Based on aforementioned considerations, we hypothesized that early exposure to viruses, such as RSV, could facilitate alterations in the sensory innervation of airway lymphoid tissues, such as the adenotonsillar tissue (AT). Such neuro-immunomodulatory changes would manifest as increased expression of NGF, trkA, NK1 receptors, and substance P, particularly in children with OSA. Such changes in turn could facilitate AT subsequent proliferative responses to environmental risk factors.
METHODS
Patients
The study was approved by the University of Louisville Human Research Committee. Informed consent was obtained from the legal caretaker of each participant, and assent was obtained from the child when appropriate.
Overnight polysomnography
For determination of which children had OSA, sleep studies were performed in a dedicated, quiet, dark room. No sleep deprivation or sedation was used. Children were studied for at least 8 h in a quiet, darkened room with an ambient temperature of 24°C in the company of one of their parents. The following parameters were measured: chest and abdominal wall movement by respiratory impedance or inductance plethysmography, heart rate by ECG, air flow was monitored with a side stream end-tidal capnograph (Petco2; Pryon ), as well as a nasal pressure transducer (Braebon, Ogdensburg, NY) and a oronasal thermistor (Miami, FL). Arterial oxygen saturation (SaO2) was assessed by pulse oximetry (Nellcor N 100; Nellcor Inc., Hayward, CA), with simultaneous recording of the pulse wave form. The bilateral electrooculogram (EOG), 8 channels of EEG, chin and bilateral anterior tibial and forearm electromyograms (EMG), and analog output from a body position sensor were also monitored. All measures were digitized (REMBrandt, Medcare, Amsterdam, The Netherlands). Tracheal sounds were monitored with a microphone sensor and a digital time-synchronized video recording was performed.
Sleep staging was assessed using standard criteria (23). The obstructive apnea/hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of total sleep time (TST) (24,25). Arousals were defined as recommended (26) and included respiratory-related (occurring immediately following an apnea, hypopnea, or snore), technician-induced, and spontaneous arousals. The diagnostic criteria for OSA included an obstructive apnea index greater than 1/h TST, and/or an obstructive apnea-hypopnea index greater than 5/h TST with a nadir oxygen saturation value of at least <92%. Children with recurrent tonsillar infection (RI) were selected based on a history of at least five tonsillar infections requiring administration of antibiotic courses over a period of less than 6 mo, as well as the absence of any symptoms suggestive of OSA using a previously validated questionnaire (27). When polysomnography was available, absence of OSA was defined as an obstructive AHI ≤1/h TST.
Adenotonsillar tissue collection
Since AT cannot be obtained from normal children for obvious ethical reasons, consecutive children undergoing T&A at Kosair Children’s Hospital for either OSA or RI were identified before surgery and recruited to the study. OSA and RI children were also required to have received their last dose of antibiotic therapy at least 6 wk before the day of the surgery. Children with OSA were excluded if they suffered from RI (based on aforementioned criteria). Children with known asthma, allergic rhinitis, history of allergies, and/or having received corticosteroid or leukotriene modifier therapy within 12 mo from surgery were excluded (for both groups). Both palatine tonsils and adenoids were removed by a pediatric ENT specialist, and a portion of each tonsil was snap frozen in liquid nitrogen and stored at −80°C. Another portion of the tonsils was fixed in 4% formalin, cryoprotected with 30% sucrose, and kept at 4°C. Adenoids were randomly assigned to be kept either in formalin or snap frozen and kept at −80°C.
Growth factors gene expression
Total RNA was prepared using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Isolated total RNA was quantified using a spectrophotometer (Beckman DU-530, Beckman Coulter, Inc., Fullerton, CA). Aliquots of total RNA (1 μg) were reverse transcribed using random primers and Superscript II-Reverse Transcriptase (Invitrogen) according to the manufacturer’s protocol. The cDNA equivalent to 20 ng of total RNA were subjected to real-time PCR analysis (MX4000, Stratagene, La Jolla, CA). PCR Primers (Invitrogen) and Taqman probes (Biosearch Technologies, Novato, CA) for NGF, BDNF (brain-derived neurotrophic factor), trk-A, NT-3 (neurotrophin-3), and GAPDH. The cycling conditions consisted of 1 cycle at 95°C for 10 min and 40 two-segment cycles (95°C for 30 s and 55°C for 60 s). Standard curves for target gene and the housekeeping gene (GAPDH) were performed for each assay, through10-fold serial dilutions of control cDNA. CT Value (initial amplification cycle) of each standard dilution was plotted against standard cDNA copy numbers. Based on the standard curves for each gene, the sample cDNA copy number was calculated according to the sample CT value. Finally, each of the calculated copy numbers for each of the genes of interest was normalized against the corresponding (GAPDH) copy numbers. Standard curves and PCR results were analyzed using MX4000 software (Stratagene).
NK1 receptor and substance P assays
For NK1 receptor assays, adenoids and tonsils were homogenized in a lysis buffer (50 mM Tris, pH 7.5, 0.4% NP-40, 10% glycerol, 150 mM NaCl, 10 mg/mL aprotinin, 20 mg/mL leupeptin, 10 mM EDTA, 1 mM sodium orthovanadate, 100 mM sodium fluoride), and the protein concentration was determined, using the Bradford method (Bio-Rad DC). A custom-designed ELISA was prepared using an unconjugated mouse anti-NK1 receptor MAb (Invitrogen, clone PADZN003). This assay has a detection limit of 2.2 pg/mL when using the immunogenic peptide as the calibration curve. The intra-assay and inter-assay variability of the assay were 8.5% and 11.7%, respectively.
For substance P concentrations, adenoid and tonsillar tissue specimens were processed as described by Bachert and colleagues (28). In brief, tissues were weighed, and 1 mL of 0.9% NaCl solution was added per 0.1 g of tissue. Tissues were then homogenized with a mechanical homogenizer at 5000 rpm for 5 min (Tissue-Tearor, Biospec Products, Bartlesville, OK). After homogenization, suspensions were centrifuged at 3000 rpm for 10 min at 4°C, and supernatants were separated and stored at −80°C. All supernatants were assayed for substance P with a commercially available ELISA kit (cat # 900-018, Assay Designs, Ann Arbor, MI). All samples were loaded in duplicates and assayed at two dilutions, and plate reader absorbance results were analyzed with a four-parameter logistic curve fit. The assay has a detection limit of 4.98 pg/mL. The intra-assay and inter-assay variability of the assay were 6% and 8.7%, respectively.
Immunohistochemistry for NK1 receptor
Coronal sections (30 μm) of both tonsils and adenoids were initially incubated in 0.3% H2O2 for 30 min, washed several times in PBS, and blocked with a PBS/0.4% Triton X-100/0.5%TSA (Tyramide Signal Amplification, Perkin Elmer Life Sciences, Boston, MA) blocking reagent/10% normal goat serum (Vector Laboratories, Burlingame CA) for 1 h. Sections were then serially incubated with anti-NK1 antibody (1:100; Chemicon, Temecula, CA) at 4°C for 24 h, and then washed in PBS six times for 5 min each wash. Sections were incubated at room temperature for 1 h in biotinylated anti-rabbit antibody (Vectastain Elite ABC Kit, Vector Laboratories; 1:600) in a PBS/0.5% TSA blocking reagent/10% goat serum solution, and then with streptavidin-horseradish peroxidase diluted 1:100 in PBS/0.5% TSA blocking reagent. Subsequently, the sections were incubated with tetramethyl rhodamine tyramide (red) diluted 1:50 in amplification diluent (Perkin Elmer Life Sciences) for 2 min. Sections were then washed and mounted onto glass slides. Negative controls were prepared by either omitting the primary or the secondary antibody. Sections were prepared from five sets of tonsils and of adenoids from either OSA or RI groups, and were visualized using a fluorescent microscope by an investigator who was blinded to the sample source.
Statistical analysis
Results are presented as means ± SD, unless stated otherwise. All numerical data were subjected to statistical analyses using either t tests, or ANOVA procedures followed by Newman-Keuls post hoc tests, as appropriate. A two-tailed p value of <0.05 was considered as statistically significant.
RESULTS
Adenoid and tonsillar tissues were obtained from 59 consecutive children (34 OSA and 25 RI). Mean demographic and polysomnographic characteristics of the OSA and RI groups are shown in Table 1. The mean obstructive AHI for the OSA group was 7.2 ± 1.6/h TST, nadir SpO2 was 83.3 ± 2.5%, and total arousal index was 14.8 ± 0.7/h TST. Overnight polysomnographic recordings were available in 14 of 25 children with RI and showed a mean AHI of 0.8 ± 0.1/h TST (p < 0.001), with mean nadir SpO2 of 93.3 ± 0.7% (p < 0.001), and total arousal index of 8.2 ± 0.4/h TST (p < 0.005).
Table 1.
Demographic and polysomnographic characteristics of children with obstructive sleep apnea and children with recurrent tonsillar infection undergoing surgical removal of hypertrophic tonsils and adenoids
| OSA (n = 34) | RI (n = 25) | p Value | |
|---|---|---|---|
| Age (y) | 5.7 ± 3.2 | 5.2 ± 2.8 | NS |
| Gender (F:M) | 14:11 | 19:15 | NS |
| African American, no. (%) | 7 (28%) | 10 (29%) | NS |
| BMI (kg/m2) | 17.2 ± 0.4 | 17.4 ± 0.5 | NS |
| AHI (/h TST) | 7.2 ± 1.6 (4.6 –17.8) | 0.8 ± 0.1 (0.0 –1.7) | <0.001 |
| SpO2 nadir | 83.3 ± 2.5 (73.2–91.7) | 93.3 ± 0.7 (91.9 –95.4) | <0.001 |
| Total arousal index | 14.8 ± 0.7 (11.6 –17.7) | 8.2 ± 0.4 (6.4 –11.3) | <0.005 |
Polysomnographic data was obtained in 14 children with RI.
AHI, obstructive apnea-hypopnea index.
Growth Factors and NK1 Receptor Expression
NGF mRNA and its high-affinity trkA expression showed higher levels of expression in OSA compared with RI (Fig. 1; p < 0.001 for both target genes). In contrast, expression of BDNF and NT-3 mRNA was similar in both patient groups (Fig. 1; p > 0.1). Similarly, expression of NK1 receptor mRNA and protein expression showed enhanced levels in OSA when compared with RI (Fig. 2; p < 0.001 for both mRNA and protein). NK1 receptor immunoreactivity was abundantly expressed within the germinal centers of the tonsillar tissues harvested from children with OSA (n = 5). NK1 receptor staining was not present within blood vessels, or in the epithelial layers and within the extra follicular area in the tonsillar parenchyma (Fig. 3). In contrast, only a restricted number of cells primarily constrained to the germinal centers expressed NK1 receptor in the tonsils obtained from RI patients (Fig. 3).
Figure 1.
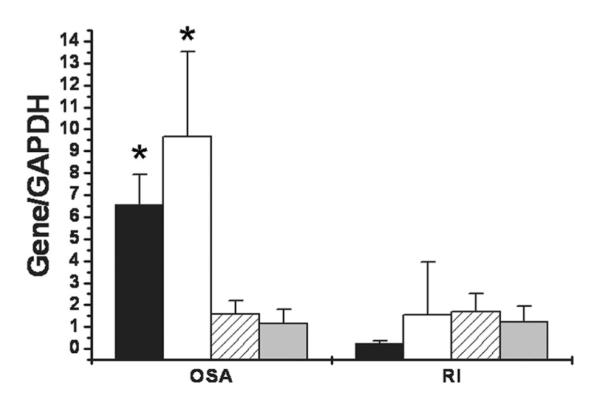
Expression of mRNA for neurotrophic factors NGF, BDNF, NT3, and for TrkA in adenotonsillar tissues harvested from patients with either RI or OSA, and shown as ratios with GAPDH as housekeeping gene. Significantly higher NGF (■, *p < 0.001) and trkA (□; *p = 0.001) expression occurred in OSA patients (n = 32) compared with RI patients (n = 25), with similar expression of BDNF  and NT3
and NT3 
Figure 2.
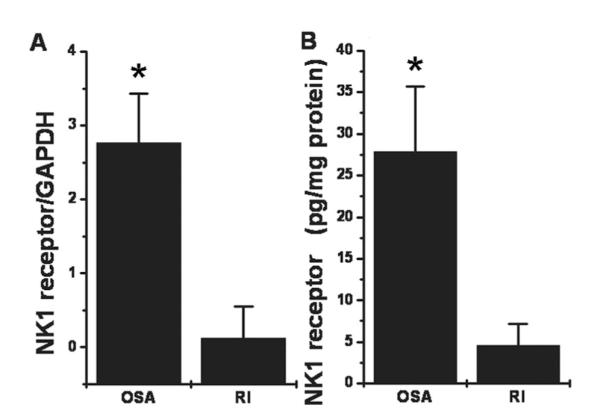
(A) Mean NK1 receptor mRNA expression in adenotonsillar tissues obtained from children with obstructive sleep apnea (OSA; n = 32) and recurrent infections shown as ratios with GAPDH as housekeeping gene (RI; n = 25; OSA vs. RI: *p < 0.002). (B) Mean NK1 receptor protein expression in adenotonsillar tissues obtained from children with OSA (n = 20) and recurrent infections (RI; n = 20) (*p < 0.001).
Figure 3.
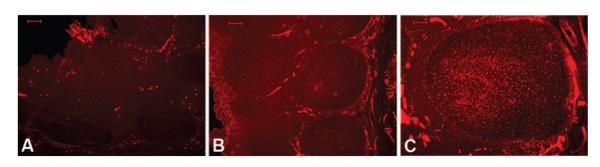
Illustrative examples of immunoreactivity patterns of the NK1 receptor in tonsils obtained from patients with either RI (A) or OSA (B); n = 5/group. Enhanced immunoreactivity of NK1 receptor is apparent in OSA, and is mainly observed within tonsillar germinal centers (C). No staining was observed within germinal centers of patients with RI. Scale bar shown on left upper corner of each image. Scale bar = 100 μm, all panels.
Substance P
Substance P concentrations in the tonsils of OSA patients were significantly higher than those of RI children (Fig. 4; p < 0.0001).
Figure 4.
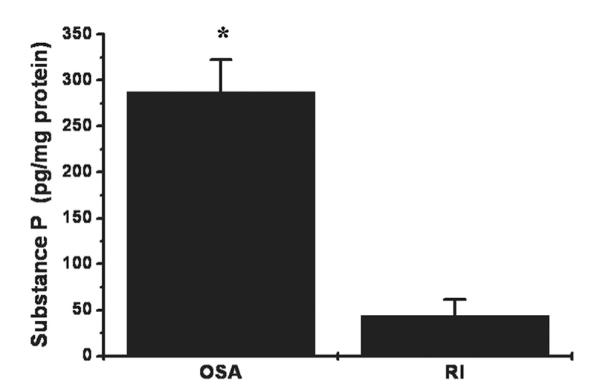
Mean substance P concentrations in adenotonsillar tissues obtained from children with obstructive sleep apnea (OSA; n = 32) or recurrent tonsillitis (n = 23). (*p < 0.003).
DISCUSSION
This study shows that NGF and its cognate receptor TrkA are expressed in AT of children, particularly in those with a diagnosis of OSA. Furthermore, elevated concentrations of substance P and increased expression of its receptor, NK1 receptor, were detected in the upper airway lymphoid tissues of pediatric patients with OSA.
The patterns described herein are remarkably analogous to the late neuro-immunomodulatory responses in the lung associated with RSV infection as well as other viral infections during early development. Neurogenic inflammation within AT tissues, as suggested in our study, has been previously studied in an animal model of asthma. Neurogenic inflammation was markedly potentiated in the lower airways of rats that were infected with RSV long after the virus was cleared from the airway (22). Thus, the presence of a RSV-initiated inflammatory process appeared to be mediated by the high affinity receptor for substance P (NK1 receptor), the expression of which is greatly increased following inoculation with RSV (21). Furthermore, RSV-induced NGF release leads to both short- and long-term changes in the distribution and reactivity of sensory nerves across the respiratory tract, and such changes are integral components of the exaggerated inflammatory reactions during and after the infection (29). Of note, human infants ventilated due to RSV infection express increased levels of neurotrophic factors such as NGF, and of receptors like NK1 in bronchoalveolar lavage samples, suggesting that neurotrophic factors may contribute to induction and perpetuation of inflammatory process in the lower airways following RSV infection (20).
An increasing body evidence supports the existence of local and systemic inflammation in the upper airway of both adults and children afflicted with OSA (30,31). Indeed, increased levels of NO, IL-6, and 8-isopentane can be detected in exhaled condensates from the upper airway of adults with OSA (32–34), and increased numbers of neutrophils, as well as increased concentrations of bradykinin and vasointestinal peptide have been measured in nasal lavage fluid from such patients (35). Furthermore, adenotonsillar tissues of children with OSA display increased expression levels of leukotriene receptors along with increased concentrations of leukotrienes (36,37). Mechanoreceptors and sensory nerves are crucial for tonic and dynamic maintenance of upper airway patency (38), such that the abnormal varicose nerves that are found in the soft palate tissues of OSA patients suggest that alterations in innervation of the upper airway may lead to changes in upper airway protective function (39). More recently, reports on selective impairments in the detection of mechanical stimuli in the upper airway, and the presence of inflammatory cell infiltrates, as well as denervation changes in the upper airway mucosa and muscle of OSA patients (40,41), suggest that inflammation and altered innervation of the airway may be linked to the pathophysiology of upper airway dysfunction in OSA.
Considering the increased inflammation present in the AT of children with OSA and the current findings indicating heightened expression of NGF and its cognate receptor TrKA along with increased expression of NK1 receptor and its agonist substance P, the possibility exists that these a priori unrelated findings may indeed be pathophysiologically linked. The most immediate relationship between increased inflammation in AT tissues and the current findings involves the potential role of early viral infection in the induction of these AT biologic properties. Based on this highly speculative assumption, RSV infection may have triggered the induction of the pathway that we now characterize in this study as being present in AT tissues. In addition, this pathway may promote the increased presence of inflammatory markers within the tissues, along with an increased susceptibility to proliferative responses when exposed to the stimuli that operate as risk factors in pediatric OSA (see Fig. 5). The present study cannot rule out the possibility that children with RI will also develop changes in the NGF axis when compared with healthy children. However, since obtaining tissues in true, healthy controls would be unethical, we compared the differences in the expression of NGF-pathway associated elements between children with RI and those with OSA. Of note, a minor potential limitation of the interpretation of the data in the RI group involves the fact that polysomnographic studies were obtained in only 14 of the 25 children with RI. Obviously, this study cannot confirm the veracity of our hypothetical framework. The latter will require extensive epidemiologic studies involving children with and without clinically apparent specific viral infections such as RSV during infancy and the prevalence of OSA in such populations. Furthermore, the role NGF, NK1 receptors, and SP in the proliferative responses of AT tissues will need to be investigated in vitro. Notwithstanding such limitations, it is intriguing to conceptualize the potential therapeutic benefits that might be derived from disruption of the pathway studied herein. Indeed, anti-NGF antibody inhibited NK1 receptor up-regulation and neurogenic inflammation in RSV-infected lungs (15). Similarly, a specific NK1 receptor antagonist abolished the recruitment of both lymphocytes and monocytes to infected airways thereby reducing the local inflammatory response (14). Recently a study done in rats showed that administration of palivizumab 24 h before virus inoculation prevented the development of abnormal neurogenic inflammatory responses (22). Taken together, such interventions may prove beneficial in children with OSA in the future, and may be applicable to other highly prevalent respiratory viruses. Indeed, conditions such as upper airway fluttering (due to snore-associated vibration) and upper airway collapse, as well as chronic nasopharyngeal infection causing nasal drip or even recurring hypoxia and re-oxygenation could all contribute to the development or maintenance of the adenotonsillar inflammatory process (42). The known biologic association between RSV infection and subsequent neuroimmune changes in the airway (29) and our current findings are supportive of a novel mechanism underlying lymphadenoid tissue proliferative properties and responses.
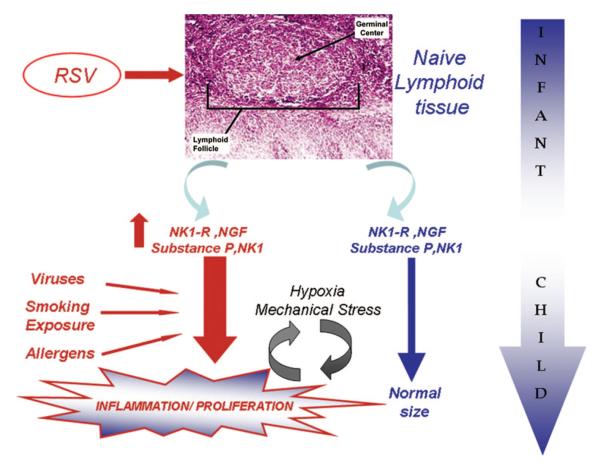
Figure 5.
Schematic illustration of putative neurotrophic factor-neurokinin pathways and their effects on the developing human upper airway lymphoid tissues. Early life exposures to viruses may lead in a genetically predisposed population to exaggerated tissue specific inflammation/proliferation following subsequent environmental/viral exposures.
The rather selective location of NK1 in the germinal centers within the tonsils is suggestive of the possibility that NK1 expression may have occurred during early life, particularly considering that maturation of lymphoid tissue occurs in a similar time frame as that of the increased susceptibility to infection by RSV and other respiratory viruses. Notwithstanding such considerations, the overarching concept emanating from this study supports the hypothesis on the existence of a chronic, neuro-immunomodulatory process in the lymphoid tissues of the upper airways of children with obstructive sleep apnea that may have been triggered by early RSV infection. As indicated above, a significant limitation in this study involves the inability at this late stage of development to identify evidence for previous infection by specific viruses such as RSV among the recruited subjects from both groups. Furthermore, not all children sustaining such infections ultimately develop asthma, and similarly not all such children would be expected to develop hypertrophy of AT tissues following RSV infection, with even fewer ultimately developing OSA. Thus, other factors, which were not analyzed in the present work, may also play an important role (e.g. age and severity of RSV infection, number of RSV infections, presence of other risk factors for OSA, etc.), further suggesting that the linkage between infection and OSA may not only require the induction of NGF-mediated pathways, but that the latter will occur only in genetically susceptible individuals (43). The use of GAPDH, a hypoxia sensitive gene, as the housekeeping gene could potentially influence the interpretation of our findings. However, since only NGF increased (and not BDNF), and since intermittent hypoxia would be expected to increase GAPDH (which would diminish, rather than enhance differences between OSA and RI children), we further contend that the emerging differences indeed represent true differences among the two study groups.
In summary, we have delineated the expression of NGF and NK1 as well as substance-P in the upper airway lymphoid tissue of children with OSA. We postulate that early RSV infection may lead to long-term alterations in the neuro-immunomodulatory pathways of AT, and may predispose such tissues to accelerated proliferative responses when exposed to stimuli, such as viruses, allergens, etc., that are associated with increased prevalence of OSA in pediatric populations.
Acknowledgments
The authors thank Kenneth R. Brittian for technical assistance with the immunohistochemical procedures.
DG is supported by grants from the National Institutes of Health HL65270 and HL69932, The Children’s Foundation Endowment for Sleep Research, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund.
Abbreviations
- AT
adenotonsillar tissue
- NGF
nerve growth factor
- NK1
neurokinin 1
- OSA
obstructive sleep apnea syndrome
- RI
recurrent tonsillar infection
- RSV
respiratory syncytial virus
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Arens R, McDonough JM, Corbin AM, Rubin NK, Carroll ME, Pack AI, Liu J, Udupa JK. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2003;167:65–70. doi: 10.1164/rccm.200206-613OC. [DOI] [PubMed] [Google Scholar]
- 3.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 4.Schechter MS. Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome 2002 Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 5.Tal A, Leiberman A, Margulis G, Sofer S. Ventricular dysfunction in children with obstructive sleep apnea: radionuclide assessment. Pediatr Pulmonol. 1988;4:139–143. doi: 10.1002/ppul.1950040304. [DOI] [PubMed] [Google Scholar]
- 6.Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–1103. doi: 10.1164/ajrccm.157.4.9704080. [DOI] [PubMed] [Google Scholar]
- 7.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 8.Owens J, Opipari L, Nobile C, Spirito A. Sleep and daytime behavioral sleep disorders. Pediatrics. 1998;102:1178–1184. doi: 10.1542/peds.102.5.1178. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien LM, Gozal D. Behavioral and neurocognitive implications of snoring and obstructive sleep apnea in children: facts and theory. Paediatr Respir Rev. 2002;3:3–9. doi: 10.1053/prrv.2002.0177. [DOI] [PubMed] [Google Scholar]
- 10.Kaditis AG, Finder J, Alexopoulos EI, Starantzis K, Tanou K, Gampeta S, Agorogiannis E, Christodoulou S, Pantazidou A, Gourgoulianis K, Molyvdas PA. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol. 2004;37:499–509. doi: 10.1002/ppul.20002. [DOI] [PubMed] [Google Scholar]
- 11.Teculescu DB, Caillier I, Perrin P, Rebstock E, Rauch A. Snoring in French preschool children. Pediatr Pulmonol. 1992;13:239–244. doi: 10.1002/ppul.1950130412. [DOI] [PubMed] [Google Scholar]
- 12.Ersu R, Arman AR, Save D, Karadag B, Karakoc F, Berkem M, Dagli E. Prevalence of snoring and symptoms of sleep-disordered breathing in primary school children in Istanbul. Chest. 2004;126:19–24. doi: 10.1378/chest.126.1.19. [DOI] [PubMed] [Google Scholar]
- 13.King KA, Hu C, Rodriguez MM, Romaguera R, Jiang X, Piedimonte G. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am J Respir Cell Mol Biol. 2001;24:101–107. doi: 10.1165/ajrcmb.24.2.4264. [DOI] [PubMed] [Google Scholar]
- 14.Auais A, Adkins B, Napchan G, Piedimonte G. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L105–L113. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283:L494–L502. doi: 10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- 16.Wilfong ER, Dey RD. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am J Respir Cell Mol Biol. 2004;30:793–800. doi: 10.1165/rcmb.2003-0303OC. [DOI] [PubMed] [Google Scholar]
- 17.Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussig LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in the first year of life. Am J Epidemiol. 1991;133:1135–1151. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 18.Ruuskanen O, Ogra PL. Respiratory syncytial virus. Curr Probl Pediatr. 1993;23:50–79. doi: 10.1016/0045-9380(93)90003-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 20.Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172:233–237. doi: 10.1164/rccm.200412-1693OC. [DOI] [PubMed] [Google Scholar]
- 21.Piedimonte G, Rodriguez MM, King KA, McLean S, Jiang X. Respiratory syncytial virus up-regulates expression of the substance P receptor in rat lungs. Am J Physiol. 1999;277:L831–L840. doi: 10.1152/ajplung.1999.277.4.L831. [DOI] [PubMed] [Google Scholar]
- 22.Piedimonte G, Hegele RG, Auais A. Persistent airway inflammation after resolution of respiratory syncytial virus infection in rats. Pediatr Res. 2004;55:657–665. doi: 10.1203/01.PDR.0000112244.72924.26. [DOI] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subject. U.S. Department of Health, Education, and Welfare; National Institutes of Health; Washington, DC: 1968. [Google Scholar]
- 24.Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Resp Crit Care Med. 1996;153:866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-age children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 26.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 27.Montgomery-Downs HE, O’Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep. 2004;27:87–94. doi: 10.1093/sleep/27.1.87. [DOI] [PubMed] [Google Scholar]
- 28.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–614. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 29.Piedimonte G. Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr Infect Dis J. 2003;22:S66–S74. doi: 10.1097/01.inf.0000053888.67311.1d. discussion S74–S75. [DOI] [PubMed] [Google Scholar]
- 30.Bergeron C, Kimoff J, Hamid Q. Obstructive sleep apnea syndrome and inflammation. J Allergy Clin Immunol. 2005;116:1393–1396. doi: 10.1016/j.jaci.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Gozal D, Kheirandish L. Oxidant stress and inflammation in the snoring child: confluent pathways to upper airway pathogenesis and end-organ morbidity. Sleep Med Rev. 2006;10:83–96. doi: 10.1016/j.smrv.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Olopade CO, Christon JA, Zakkar M, Hua C, Swedler WI, Scheff PA, Rubinstein I. Exhaled pentane and nitric oxide levels in patients with obstructive sleep apnea. Chest. 1997;111:1500–1504. doi: 10.1378/chest.111.6.1500. [DOI] [PubMed] [Google Scholar]
- 33.Sekosan M, Zakkar M, Wenig B, Olopade CO, Rubinstein I. Inflammation in the uvula mucosa with obstructive sleep apnea. Laryngoscope. 1996;106:1018–1020. doi: 10.1097/00005537-199608000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–1167. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 35.Salerno FG, Carpagnano E, Guido P, Bonsignore MR, Roberti A, Aliani M, Vignola AM, Spanevello A. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med. 2004;98:25–28. doi: 10.1016/j.rmed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Goldbart AD, Goldman GL, Li RC, Brittian KR, Tauman R, Gozal D. Differential expression of cysteinyl leukotriene l receptors 1 and 2 in tonsils of children with obstructive sleep apnea and recurrent infection. Chest. 2004;126:13–18. doi: 10.1378/chest.126.1.13. [DOI] [PubMed] [Google Scholar]
- 37.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172:364–370. doi: 10.1164/rccm.200408-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gozal D, Burnside MM. Increased upper airway collapsibility in children with obstructive sleep apnea during wakefulness. Am J Respir Crit Care Med. 2004;169:163–167. doi: 10.1164/rccm.200304-590OC. [DOI] [PubMed] [Google Scholar]
- 39.Friberg D, Gazelius B, Hokfelt T, Nordlander B. Abnormal afferent nerve endings in the soft palatal mucosa of sleep apneics and habitual snorers. Regul Pept. 1997;71:29–36. doi: 10.1016/s0167-0115(97)01016-1. [DOI] [PubMed] [Google Scholar]
- 40.Kimoff RJ, Sforza E, Champagne V, Ofiara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164:250–255. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 41.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:541–546. doi: 10.1164/rccm.200308-1100OC. [DOI] [PubMed] [Google Scholar]
- 42.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 43.Schwab RJ. Genetic determinants of upper airway structures that predispose to obstructive sleep apnea. Respir Physiol Neurobiol. 2005;147:289–298. doi: 10.1016/j.resp.2005.06.006. [DOI] [PubMed] [Google Scholar]