Abstract
Here we studied ability of two naphthoquinones to inhibit Leishmania growth (2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (TR 001) and 2,3-dibromo-1,4-naphthoquinone (TR 002). TR 001 was more efficient than TR 002 in inducing killing of promastigotes and intracellular amastigotes. These values compare well to those obtained with the standard first-line antileishmanial agent sodium stibogluconate (SSG). TR 001 also induced significantly more nitric oxide (NO) production than TR 002 or SSG. Taken together, these data show that TR 001 and TR 002 could be promising new drugs for treatment of visceral leishmaniasis.
Keywords: Leishmania, naphthoquinones, macrophages
Naphthoquinones are a class of chemical compounds exhibiting a variety of anti-carcinogenic, immunomodulatory and antimicrobial activities and several have shown activity against the protozoan parasite Leishmania.1–5) Infections caused by different species represent a wide range of clinical manifestations, including cutaneous (CL), mucocutaneous (MCL), diffuse (DCL) and visceral leishmaniasis (VL). This disease is transmitted by the bite of sandflies.6,7) The disease is mostly zoonotic in nature, but an anthroponotic mode of transmission also exists in some parts of Europe, Asia and other parts of the world.8,9) According to WHO, the worldwide prevalence of this disease is estimated at 12 million cases, with an annual mortality of about 60 000 and the population at risk is estimated to be about 350 million.9) The systemic pentavalent antimonials still remain the recommended drugs for treatment in most endemic countries, but these are toxic and have poor patient compliance because they require daily injections periods for 3 or more weeks. Treatment of CL or VL with antimonials is even more difficult in HIV-infected individuals and is associated with frequent relapses because these drugs require healthy immune system and CD4+ T cells for optimal anti-parasitic activity.10) In addition, parasite resistance to antimonial and other first line drugs is rapidly emerging in VL endemic regions of the world, particularly India.6,9–13) Therefore, there is an ongoing need for developing new drugs for CL and VL.6,8,14–16) Currently, there is not any commercial preparation to treat any form of human leishmaniasis based on the chemical structure of naphthoquinones or their chemical derivatives. However, several studies suggest that this class of compounds have potential for development as anti-leishmanial agents. A number of monomeric and dimeric naphthoquinones present antileishmanial activity in vitro.15) For example 8,8’-biplumbagine isolated from the plant Pera benensis showed activity comparable to that of the reference drug Glucantime™ towards L. amazonensis-infected mice,3) and 2-phenoxy-1,4-naphthoquinones showed activity against L. donovani in vitro.1) Our rationale for testing 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (TR 001) and 2,3-dibromo-1,4-naphthoquinone (TR 002) arose from the fact that these compounds are affordable and could be cheaply obtained in large quantities, which is critical for any drug developed against a neglected tropical disease.
MATERIAL AND METHODS
Parasites
We generated a line of L. donovani strain LV82 expressing firefly luciferase and a red fluorescent protein. Briefly, clonal transfectants were obtained by homologous integration of a LUC-DsRed2 cassette (SwaI fragment from plasmid pIR1SAT-LUC-DsRed2 (strain B5947) into the rRNA locus as described previously for L. major.17) Expression of DsRed2 was confirmed in both promastigotes and intracellular amastigotes by fluorescent microscopy. A number of clonal lines showing similar DsRed2 and LUC expression were obtained and used in these studies. This line will be referred to as DsRed2-L. donovani. Prior to each screening assay, fluorescence of parasites was verified by immunofluorescence microscopy. Direct counting of promastigotes co-cultured for 48 h with TR 001 or TR 002 was performed using a Neubauer chamber.
Leishmanicidal assay
For screening leishmanicidal activity of drugs, we incubated 106 DsRed2-L. donovani promastigotes with different concentrations of sodium stibogluconate, TR 001 or TR 002 at 28 °C for 48 hr. Parasites were centrifuged at 3000 RPM for 5 min, re-suspended in 1 ml of PBS and parasite killing was measured by flow cytometry quantifying proportion of dead parasites assessed by loss of the fluorescence. Untreated or sham treated (2% DMSO) parasites were used as live cell control and parasites treated with 100 µg/ml of saponin from Q. saponaria (Sigma-Aldrich) for 30 min were used as the dead/permeabilized cell control.
Preparation of bone marrow derived macrophages (BMDM)
We isolated BMDM from long bones (femurs and tibias) of C57BL/6 mice, as described previously.18) More than 90% of differentiated cells were CD11b+ macrophages as determined by flow cytometry. 5 × 105 BMDM in 1 ml of complete RPMI 1640 were seeded into the wells of a 24 wells plate, each well containing a glass coverslip at the bottom. Macrophages were infected overnight with promastigotes of DsRed2-L. donovani at a 1:5 ratio (1 macrophage/5 promastigotes), and extracellular parasites were washed off with fresh warm media. Infected macrophages were treated with different concentrations (0 to 100 µM) of TR 001 or TR 002 at 37 °C in a CO2 incubator for 48 h. Supernatants were collected and adherent macrophages on the coverslips were fixed and stained by Giemsa to quantify infection rates as described previously.18) Sham-treated macrophages infected with DsRed2-L. donovani were used as controls.
Macrophage cytotoxicity
We cultured 5 × 105 BMDM in the presence of different concentrations (0–100 µM) of sodium stibogluconate (SSG, Pentostam™), TR 001, TR 002 or 2% DMSO (Control) for 48 h and then we tested viability by trypan blue exclusion test. Macrophage cytotoxicity (CC50) was calculated as drug concentration needed to kill 50% of macrophages. Therapeutic index (TI) for each drug was calculated by the formula: TI=CC50/IC50 for intracellular parasites.19,20)
NO determination
NO quantitation was performed by mixing the Griess reagent with samples and reading at 570 nm. Results so obtained were extrapolated from a standard curve prepared with Na NO2 at different concentrations (0 to 400 µM).18)
Statistical analysis
Student t test was performed to determine level of significance. IC50 values for promastigotes and amastigotes inside of BMDM were determined with the use of a computer program LdP Line® and Prism 5®.
RESULTS
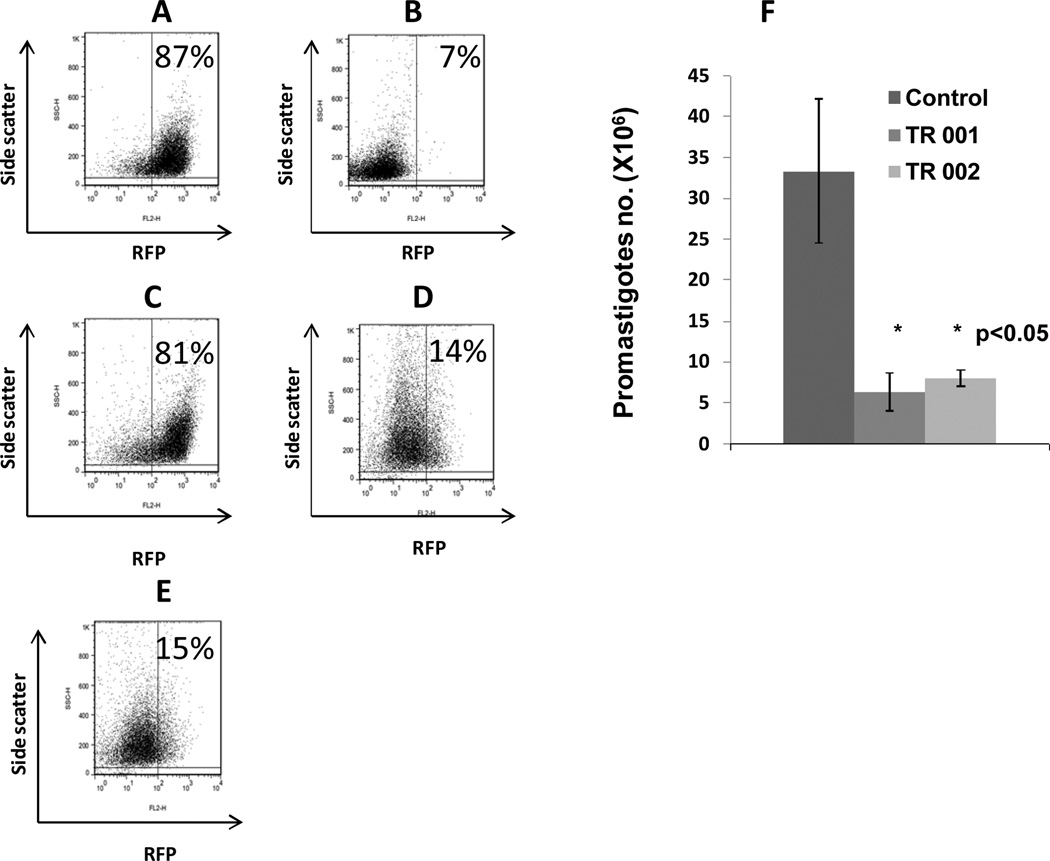
The chemical structure of TR 001 and TR 002 are presented in (Fig. 1). In Figs. 2A and 2B we show fluorescent promastigotes and amastigotes inside of BMDM, respectively. Promastigote death correlated with loss of fluorescence measured by flow cytometry. Figs. 3A and 3B correspond to negative and positive controls, Figs. 3C, 3D and 3E correspond to promastigotes treated with SSG, TR 001 or TR 002 respectively and Fig. 3F corresponds to parasite numbers after treatment with TR 001or TR002 for 48 hr. We found that TR 001 or TR 002 presented IC50 = 5.4 ± 2.4 and 7.04 ± 0.15 µM respectively and SSG displayed antileishmanial activity of IC50 = 306.85 ± 5.06 µM against L. donovani promastigotes (Table 1). Direct counting of promastigotes after 48 hr co-culture with TR 001 or TR 002 showed a significant reduction in their number (Fig. 3F). Furthermore, leishmanicidal activity of SSG was much lower for extracellular parasites than TR 001 or TR 002 (Table 1).
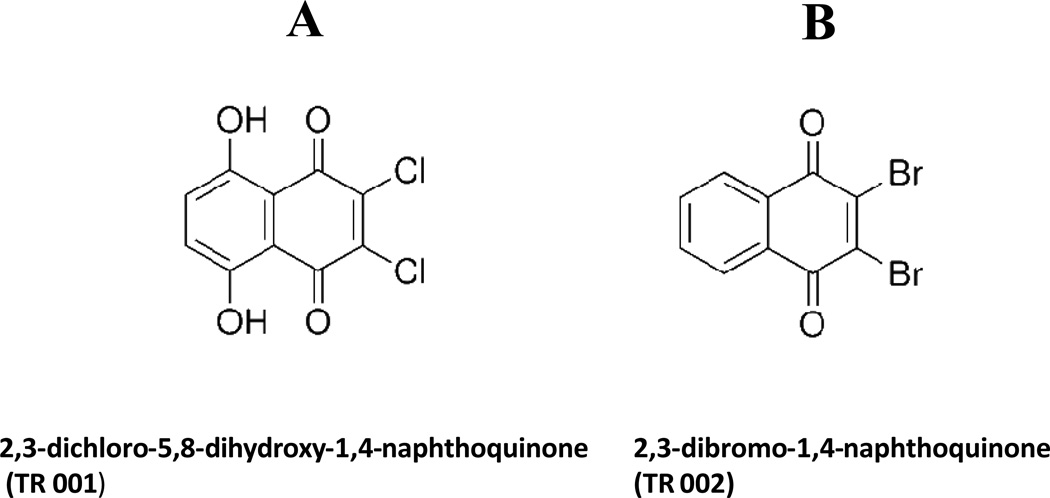
Fig. 1.
Chemical structure of two naphthoquinones tested for leishmanicidal activity: 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (TR 001, A) and 2,3-dibromo-1,4-naphthoquinone (TR 002, B).
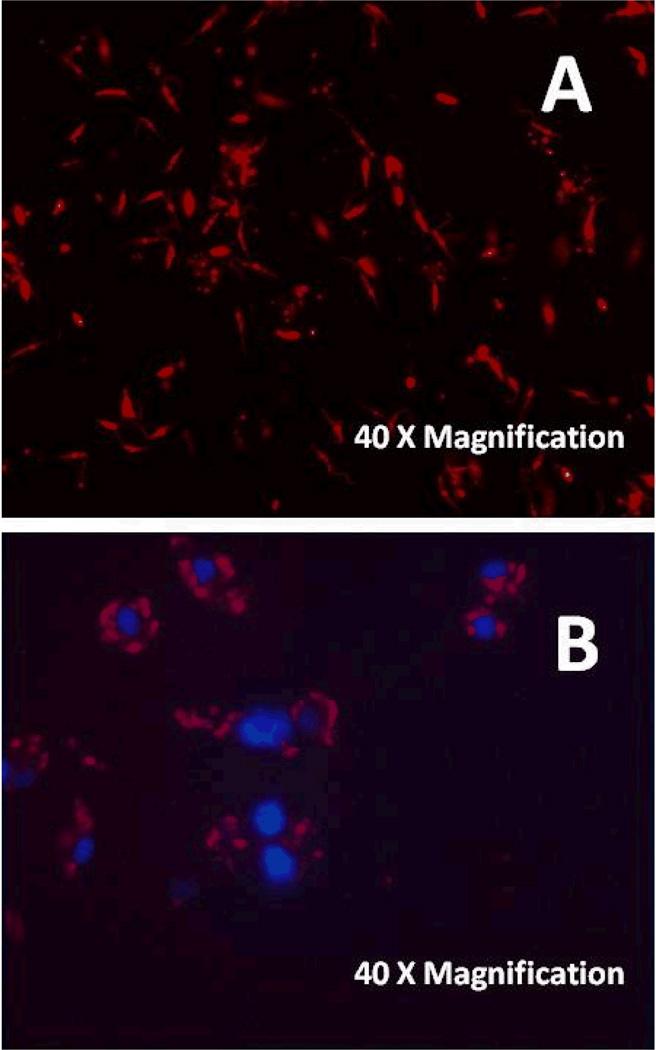
Fig. 2.
Axenic DsRed2-L. donovani promastigotes (A) and DsRed2-L. donovani amastigotes inside of bone marrow derived macrophages (BMDM) from C57BL/6 mice and counterstained with DAPI (B).
Fig. 3.
Flow cytometry analysis of promastigotes of DsRed2-L. donovani promastigotes treated with 100 µM of sodium stibogluconate (reference drug, C), 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (TR 001, D) or 2,3-dibromo-1,4-naphthoquinone (TR 002, E). Negative control (treated with DMSO 2%, A) and positive control (treated with 100 µg/ml of saponin, B) were included. Numbers of promastigotes co-cultured with TR 001 or TR 002 were included (F). Data shown are representative of two different experiments performed in triplicate.
Table I.
| Compound | Promastigote Killing (IC50) |
Amastigote Killing** (IC50) |
Macrophage cytotoxicity§ (CC50) |
In vitro Therapeutic Index§§ |
|---|---|---|---|---|
| SSG | 306.8 + 5.06 | 13.32 + 5.14 | 53.4 + 1 | 4 |
| TR 001 | 5.4 + 2.4Π | 0.069 + 0.02Π | 46.5 + 2 | 659 |
| TR 002 | 7.04 + 0.15Π | 0.26 + 0.18Π | 49.2 + 2.7 | 189.2 |
IC50 is the concentration of drug (µM) to achieve 50% killing of parasites per 106 axenic promastigotes or 2.5 × 106 amastigotes inside of 0.5 × 106 BMDM after 48 h of culture.
Drug concentration (µM) per 0.5 × 106 BMDM to achieve 50% killing of macrophages.
Therapeutic index = CC50/IC50**.
significantly different (p<0.05) as compared to SSG.
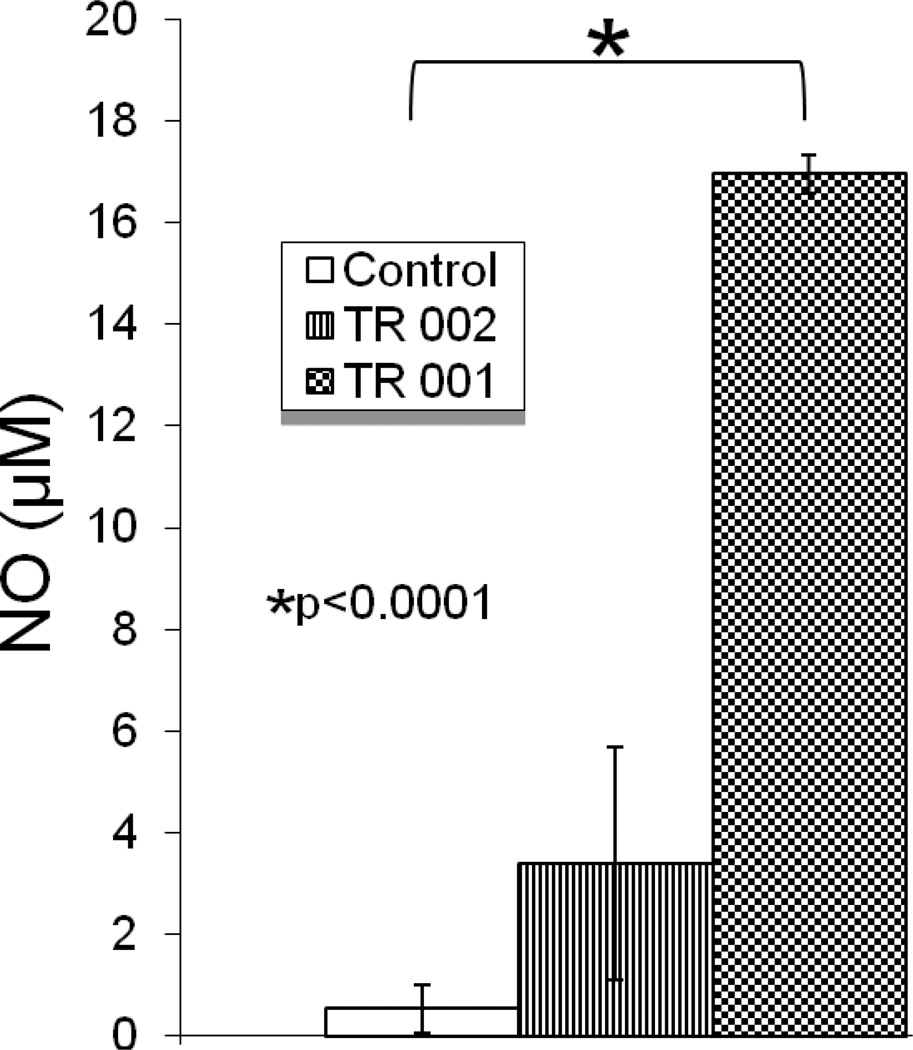
Next, we examined the efficacy of TR 001 and TR 002 against intracellular amastigotes grown in BMDM as described previously. These compounds presented IC50 for amastigotes inside of BMDM of 0.069 ± 0.02 µM for TR 001 and IC50 = 0.26 ± 0.18 µM for TR 002. The reference drug (SSG) presented IC50 = 13.32 ± 5.14 µM (Table 1). Both TR 001 and TR 002 displayed significantly higher antileishmanial activity against intracellular amastigotes than the reference drug SSG and TR 001 was more efficient than TR 002 to kill internalized parasites. We also recorded the TI of SSG, TR 001 and TR 002. As can be observed in Table 1 both naphthoquinones presented higher TI than SSG. As killing intracellular Leishmania is often mediated by NO, NO quantification in supernatants from macrophages cultures (48 h) was performed. Both TR 001 and TR 002 induced nitric oxide (NO) production in BMDMs, 32-fold and 7-fold respectively (16.7 vs 0.5 µM; p<0.0001 for TR 001 and 3.3 vs 0.5 µM; p = 0.2 for TR 002), compared to control BMDMs treated with 2% DMSO (Fig. 4).
Fig. 4.
Nitric oxide (NO) concentration (µM) in cultures of bone marrow derived macrophages (BMDM) infected with DsRed2-L. donovani promastigotes; excess parasites were washed off and then treated with 100 µM of 2,3-dichloro-5,8-dihydroxy-1,4-naphthoquinone (TR 001), 2,3-dibromo-1,4-naphthoquinone (TR 002) for 48 hr. Control group (sham) were treated only with 2% DMSO. Data shown are representative of two different experiments ran in triplicate.
DISCUSSION
In summary, we have demonstrated that two naphthoquinones, TR 001 and TR 002 display better antileishmanial activity against extracellular as well as intracellular L. donovani in macrophages as compared to SSG. Furthermore, we show that TR 001 displays significantly better leishmanicidal activity against intracellular parasites as compared to TR 002. This antiparasitic activity correlates with an increase in NO production. Our findings also suggest that antileishmanial activity of TR 002 against intracellular parasites is likely to be mediated via NO-dependent mechanism. Cytotoxicity of the two compounds was similar to SSG, but their therapeutic indexes were much higher. Additional in vivo work is necessary using animal models of experimental VL exploring different routes of administration (oral, intravenous or intramuscular injections) in order to perform lead optimization. Selection of TR 001 and TR 002 was considered because they have similar structure to plumbagin which is a well known antileishmanial compound. Naphthoquinones have been tested for their anti-parasite potential,21) and in most cases tested compounds correspond to dimeric and monomeric naphthoquinones such as plumbagin originally isolated from Pera benensis.5) Interestingly, TR 001 and TR 002 (two related monomeric naphthoquinones) displayed significantly better anti-Leishmania activity against intracellular amastigotes of L. donovani as compared to extracellular promastigotes. Similar compounds have been used for in vivo treatment of certain types of cancer in mice with not signs of unacceptable toxicity.4) Taken together, our findings suggest that these compounds could be further explored as therapeutic agents for VL.
ACKNOWLEDGEMENTS
This work was developed with funding from NIH grants A1 076309, AT 004160, A1 090 803 and RC4 AI 092624 to ARS, AI 21903 and AI 29646 to SMB and CONACYT (Fondo Sectorial Salud 140091) grants to APIM and CMLD. TR 001 and TR 002 were kindly provided by GJK and purchased at Sigma-Aldrich.
Footnotes
Authors declare they do not have conflict of interest.
REFERENCES
- 1.Bolognesi ML, Lizzi F, Perozzo R, Brun R, Cavalli A. Synthesis of a small library of 2-phenoxy-1,4-naphthoquinone and 2-phenoxy-1,4-anthraquinone derivatives bearing anti-trypanosomal and anti-leishmanial activity. Bioorg. Med. Chem. Lett. 2008;18:2272–2276. doi: 10.1016/j.bmcl.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Checker R, Sharma D, Sandur SK, Subrahmanyam G, Krishnan S, Poduval TB, Sainis KB. Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols. J. Cell Biochem. 2010;110:1082–1093. doi: 10.1002/jcb.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fournet A, Angelo A, Muñoz V, Roblot F, Hocquemiller R, Cavé A. Biological and chemical studies of Pera benensis, a Bolivian plant used in folk medicine as a treatment of cutaneous leishmaniasis. J. Ethnopharmacol. 1992;37:159–164. doi: 10.1016/0378-8741(92)90074-2. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia GJ, Balasubramanian V, Tokuda H, Konoshima T, Takasaki M, Koyama J, Tagahaya K. Nishino H Anti-tumor promoting effects of naphthoquinone derivatives on short term Epstein-Barr early antigen activation assay and in mouse skin carcinogenesis. Cancer Lett. 1997;113:47–53. doi: 10.1016/s0304-3835(96)04582-x. [DOI] [PubMed] [Google Scholar]
- 5.Parimala R, Sachdanandam P. Effect of plumbagin on some glucose metabolizing enzymes studied in rats in experimental hepatoma. Mol. Cell Biochem. 1993;125:59–63. doi: 10.1007/BF00926835. [DOI] [PubMed] [Google Scholar]
- 6.Isaac-Márquez AP, Lezama-Dávila CM, Satoskar AR. Immunobiology of visceral leishmaniasis. Current status and future perspectives. In: Thakur HP, editor. Kala Azar Emerging perspectives and prospects in South Asia. Chapter 17. India: Mittal publications; 2011. pp. 289–310. [Google Scholar]
- 7.Ronet C, Beverley SM, Fasel N. Muco-cutaneous leishmaniasis in the New World: the ultimate subversion. Virulence. 2011;2:547–552. doi: 10.4161/viru.2.6.17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lezama-Dávila CM, Isaac-Márquez AP, Zamora-Crescencio P, Uc-Encalada M del R, Justiniano-Apolinar SY, del Angel-Robles L, Satoskar A, Hernández-Rivero L. Leishmanicidal activity of Pentalinon andrieuxii. Fitoterapia. 2007;78:255–257. doi: 10.1016/j.fitote.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Croft S, Coombs GH. Leishmaniasis current chemotherapy and recent advances in the search for novel drugs. Trends in Parasitol. 2003;19:502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Prasad N, Ghiya BC, Bumb RA, Kaushal H, Saboskar AA, Lezama-Davila CM, Salotra P, Satoskar AR. Heat, Oriental sore, and HIV. Lancet. 2011;377:610. doi: 10.1016/S0140-6736(10)61495-X. [DOI] [PubMed] [Google Scholar]
- 11.Croft SL, Sundar S, Fairlamb AH. Drug Resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luque-Ortega JR, de la Torre BG, Hornillos V, Bart JM, Rueda C, Navarro M, Amat-Guerri F, Acuña AU, Andreu D, Rivas L. Defeating Leishmania resistance to Miltefosine (hexadecylphosphocholine) by peptide-mediated drug smuggling: A proof of mechanism for trypanosomatid chemotherapy. J. Control Release. 2012 May 18; doi: 10.1016/j.jconrel.2012.05.023. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Nishi SG. Visceral leishmaniasis: Experimental models for drug discovery. Indian J. Med. Res. 2011;133:27–39. [PMC free article] [PubMed] [Google Scholar]
- 14.Isaac-Márquez AP, McChesney JD, Nanayakara NP, Satoskar AR, Lezama-Dávila CM. Leishmanicidal activity of racemic +/− 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline. Nat. Prod. Comm. 2010;5:387–390. [PubMed] [Google Scholar]
- 15.Kayser O, Kiderlen AF, Laatsch H, Croft SL. In vitro leishmanicidal activity of monomeric and dimeric naphthoquinones. Acta Tropica. 2000;76:131–138. doi: 10.1016/s0001-706x(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 16.Lezama-Dávila CM, Satoskar AR, Úc-Encalada M, Isaac-Márquez R, Isaac-Márquez AP. Leishmanicidal Activity of Artemisinin, Deoxoartemisinin, Artemether and Arteether. Nat. Prod. Comm. 2007;1:1–4. [Google Scholar]
- 17.Ng LG, Hsu A, Mandell MA, Roediger B, Hoeller C, Mrass P, Iparraguirre A, Cavanagh LL, Triccas JA, Beverley SM, Scott P, Weninger W. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4:e1000222. doi: 10.1371/journal.ppat.1000222. Epub Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lezama-Dávila CM, Isaac-Márquez AP, Barbi J, Cummings HE, Lu B, Satoskar AR. Role of phosphatidylinositol-3-kinase-gamma (PI3Kgamma)-mediated pathway in 17β-estradiol-induced killing of L. mexicana in macrophages from C57BL/6 mice. Immunol. Cell Biol. 2008;86:539–543. doi: 10.1038/icb.2008.39. [DOI] [PubMed] [Google Scholar]
- 19.Osorio Y, Travi BL, Renslo AR, Peniche AG, Melby PC. Identification of small molecule lead compounds for visceral leishmaniasis using a novel ex vivo splenic explant model system. PLoS Negl. Trop. Dis. 2011;5:e962. doi: 10.1371/journal.pntd.0000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poorrajab F, Ardestani SK, Foroumadi A, Emami S, Kariminia A, Behrouzi-Fardmoghadam M, Shafiee A. Selective leishmanicidal effect of 1,3,4-thiadiazole derivatives and possible mechanism of action against Leishmania species. Exp Parasitol. 2009;121:323–330. doi: 10.1016/j.exppara.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Weiss CR, Moideen SVK, Croft SL, Houghton PJ. Activity of Extracts and Isolated Naphthoquinones from Kigelia pinnata against Plasmodium falciparum. J. Nat. Prod. 2000;63:1306–1309. doi: 10.1021/np000029g. [DOI] [PubMed] [Google Scholar]