Abstract
An acute but transient response to insulin is essential for glucose homeostasis in mammals. Chakraborty et al. (2010) uncover a new feedback mechanism regulating insulin signaling. They show that the inositol pyrophosphate IP7, which is produced in response to insulin, inhibits the Akt kinase, a primary effector of insulin signaling.
Pancreatic beta cells produce insulin in response to the rise in circulating glucose levels after a meal. Insulin restores basal blood glucose levels by eliciting a distinct metabolic responses in target tissues, including the stimulation of glucose uptake into skeletal muscle and adipose tissue and the inhibition of glucose output in the liver. The homeostatic response to insulin must occur rapidly, but transiently, following a spike in blood glucose. Thus, proper control over both stimulatory and inhibitory signals affecting the response to insulin is important for preventing metabolic imbalance and common metabolic diseases such as type-2 diabetes. Chakraborty et al. (2010) now identify a new feedback mechanism that attenuates insulin signaling. They show that the production of a specific inositol pyrophosphate, which is stimulated by insulin, inhibits canonical insulin signaling by preventing activation of the kinase Akt.
While the response to insulin varies among tissues, the signal transduction pathway triggered by insulin is conserved (Taniguchi et al., 2006; Figure 1A). Insulin binds to and activates cell surface insulin receptors, and these receptor tyrosine kinases phosphorylate the insulin receptor substrate (IRS) proteins on specific tyrosine residues. Phosphorylated IRS proteins serve as scaffolding adaptors for signaling proteins, the most important of which is the class IA phosphatidylinositol 3-kinase (PI3K). Engagement of PI3K by the IRS protein activates this lipid kinase at the plasma membrane, where its substrate phosphatidylinositol-4,5-bisphosphate (PIP2) is abundant, stimulating the production of the key lipid second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 then binds the pleckstrin homology (PH) domain of the serine/threonine kinase Akt, allowing two other kinases -the phosphoinositide-dependent kinase (PDK1) and the mammalian target of rapamycin (mTOR) complex 2 (mTORC2) – to phosphorylate and activate Akt. Akt is a major effector of the insulin response, and its downstream substrates directly mediate many of the metabolic effects of insulin (Manning and Cantley, 2007). Insulin resistance is a hallmark of type-2 diabetes and is characterized by an inability of insulin to signal to Akt (Whiteman et al., 2002).
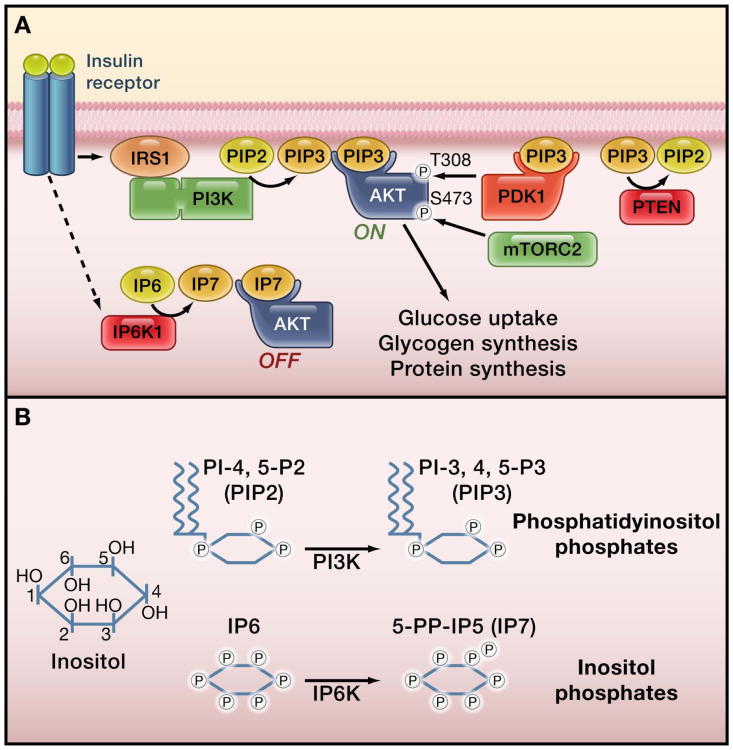
Figure 1. The insulin signaling pathway and inositol phosphates.
(A) The figure shows the canonical insulin signaling pathway leading to activation of the serine/threonine kinase Akt. Chakraborty et al. (2010) show that insulin also stimulates the inositol phosphate kinase IP6K1 to produce IP7 (5-diphosphoinositolpentakisphosphate) , which in turn inhibits Akt. The authors' results suggest a model for the inhibition of Akt by IP7. In this model, IP7 binding to the PH domain of Akt prevents the tranlsocation of Akt to the membrane, and also prevents the binding of PIP3 (phosphatidylinositol-3,4,5-trisphosphate) to the same domain, thus blocking insulin signaling to Akt. (B) Inositol-derivatives serve as signaling molecules when phosphorylated on distinct hydroxyl groups on the inositol ring. The figure shows the reactions catalyzed by phosphatidylinositol 3-kinase (PI3K) and IP6K1. PI3K phosphorylates the 3 position of PIP2 (phosphatidylinositol-4,5-bisphosphate) to make PIP3. IP6K1 phosphorylates the phosphate group at the 5 position of IP6 (inositol hexakisphosphate) to generate IP7.
Insulin signaling can be inhibited at multiple steps between the insulin receptor and Akt activation. The best-characterized inhibitors include lipid phosphatases such as PTEN and SHIP2, which hydrolyze lipids produced by PI3K. In addition, insulin induces signaling pathways that can promote inhibitory phosphorylation of the IRS proteins, preventing the activation of PI3K and Akt. For instance, Akt signaling activates mTOR complex 1 (mTORC1) and its downstream target S6K1, and these ser/thr kinases can directly phosphorylate serine residues on IRS1, leading to its inhibition (Harrington et al., 2005). In this manner, the stimulation of mTORC1 activity in response to insulin creates an inhibitory feedback mechanism that decreases insulin signaling. Chakraborty et al. now report that production of a specific inositol pyrophosphate represents another mechanism by which an insulin-stimulated pathway leads to attenuation of insulin signaling.
Inositol phosphates are a diverse group of signaling molecules in which hydroxyl groups positioned around an inositol ring are phosphorylated in different combinations by an array of inositol phosphate kinases. One such kinase, inositol hexakisphosphate (IP6) kinase 1 (IP6K1), produces a pyrophosphate group at the 5 position of IP6 to generate 5-diphosphoinositolpentakisphosphate (5-PP-IP5 or IP7; Figure 1B). Studies on IP6K demonstrate a role for the IP7 product in promoting insulin production by pancreatic beta cells (Illies et al., 2007). Interestingly, despite low blood insulin levels in the Ip6k1 knockout mice due to defects in insulin secretion, the levels of blood glucose in these mice are normal, suggesting that these mice have enhanced peripheral insulin sensitivity (Bhandari et al., 2008).
Chakraborty et al. examine the molecular mechanism and physiological consequences of the increased responsiveness to insulin suggested by the IP6K1 knockout mouse phenotype. Using insulin and insulin-like growth factor 1 (IGF-1) to stimulate heptocytes and mouse embryo fibroblasts, the authors demonstrate enhanced Akt activation in Ip6k1 knockout cells relative to wild-type. Interestingly, the authors also find that insulin and IGF-1 stimulate a gradual increase in the levels of the IP6K1 product IP7 in wild-type cells, and this inositol pyrophosphate inhibits Akt translocation to the plasma membrane and its subsequent phosphorylation by PDK1. Taken together with a previous study by this group demonstrating that IP7 can bind directly to the PH domain of Akt (Luo et al., 2003), the data suggest that IP7 competes with PIP3 for binding to Akt, thereby blocking Akt activation (Figure 1A). Thus, insulin and IGF-1 stimulate the production of two phosphoinositol species, PIP3 through PI3K and IP7 through IP6K1 (Figure 1B), which have reciprocal effects on Akt activation.
These cell intrinsic effects of IP6K1 and its product IP7 suggest a mechanistic basis for the enhanced insulin sensitivity implied from previous studies on the Ip6k1 knockout mice (Bhandari et al., 2008). Measuring systemic responses to insulin, Chakraborty et al. (2010) find that Ip6k1 knockout mice display enhanced activation of Akt in response to insulin in both skeletal muscle and adipose tissue, accompanied by increased glucose uptake into these tissues. Importantly, the Ip6k1 knockout mice are lean and resistant to both age and diet-induced obesity, showing greatly diminished white adipose depots. As it is well known that increased adiposity is closely associated with the development of systemic insulin resistance (Guilherme et al., 2008), the lean phenotype of the Ip6k1 knockout mice confounds the interpretation of their enhanced insulin sensitivity. Indeed, the improved insulin sensitivity of the knockout mice is more pronounced on a high fat-diet, where the control mice develop obesity and insulin resistance. Therefore, the beneficial effects of IP6K1 loss on global insulin action reflect both increased cellular insulin signaling and the systemic effects of decreased adiposity. The lean nature of the Ip6k1 knockout mice appears to be due to an increase in lean muscle mass and in the breakdown of fatty acids by beta-oxidation. However, the authors also demonstrate that Ip6k1 plays an important role in promoting adipocyte differentiation.
This study raises some interesting questions regarding control of the insulin response at both the cellular and organismal levels. The findings by Chakraborty et al. that the same signals that increase the levels of PIP3 also increase the levels of IP7, which appears to compete with PIP3 for binding the Akt-PH domain, suggest a rheostat-like control over Akt activation. Although these inositol derivatives bind with different affinities to the Akt-PH domain, this model suggests that the relative localized concentrations of PIP3 and IP7 directly influence the spatial and temporal status of Akt activation. Further studies are needed to determine how the ratios of PIP3 to IP7 change in metabolic tissues following feeding and whether the relative levels of these opposing molecules change under different conditions of insulin resistance. It will also be important to understand the mechanism by which insulin and IGF-1, and perhaps other growth factors, stimulate the production of IP7 by IP6K1. It remains possible that this stimulation is downstream of Akt, making this a classic negative-feedback mechanism analogous to that mediated by mTORC1 and S6K1 (Harrington et al., 2005). Interestingly, the major metabolic features of the Ip6k1 knockout phenotype - defects in beta-cell insulin production, resistance to obesity, and improved peripheral insulin sensitivity - are the same as those reported for the S6k1 knockout mice (Um et al., 2004), perhaps suggesting a mechanistic link between the IP6K and mTORC1 pathways. Finally, IP6K1 could represent a new therapeutic target to improve insulin sensitivity in type-2 diabetics. However, a major consideration in the development of such inhibitors is the involvement of IP6K1 in pancreatic insulin output (Illies et al., 2007; Bhandari et al., 2008). While it is clear that there are many new avenues to explore, the findings reported by Chakraborty et al. add another key element to the complex regulation of the insulin response.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhandari R, Juluri KR, Resnick AC, Snyder SH. Proc Natl Acad Sci U S A. 2008;105:2349–2353. doi: 10.1073/pnas.0712227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty, et al. 2010 …. [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Lamb RF. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Illies C, Gromada J, Fiume R, Leibiger B, Yu J, Juhl K, Yang SN, Barma DK, Falck JR, Saiardi A, et al. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K, Huang Y, Nagata E, Devreotes P, Snyder SH. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Whiteman EL, Cho H, Birnbaum MJ. Trends in endocrinology and metabolism: TEM. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]