Abstract
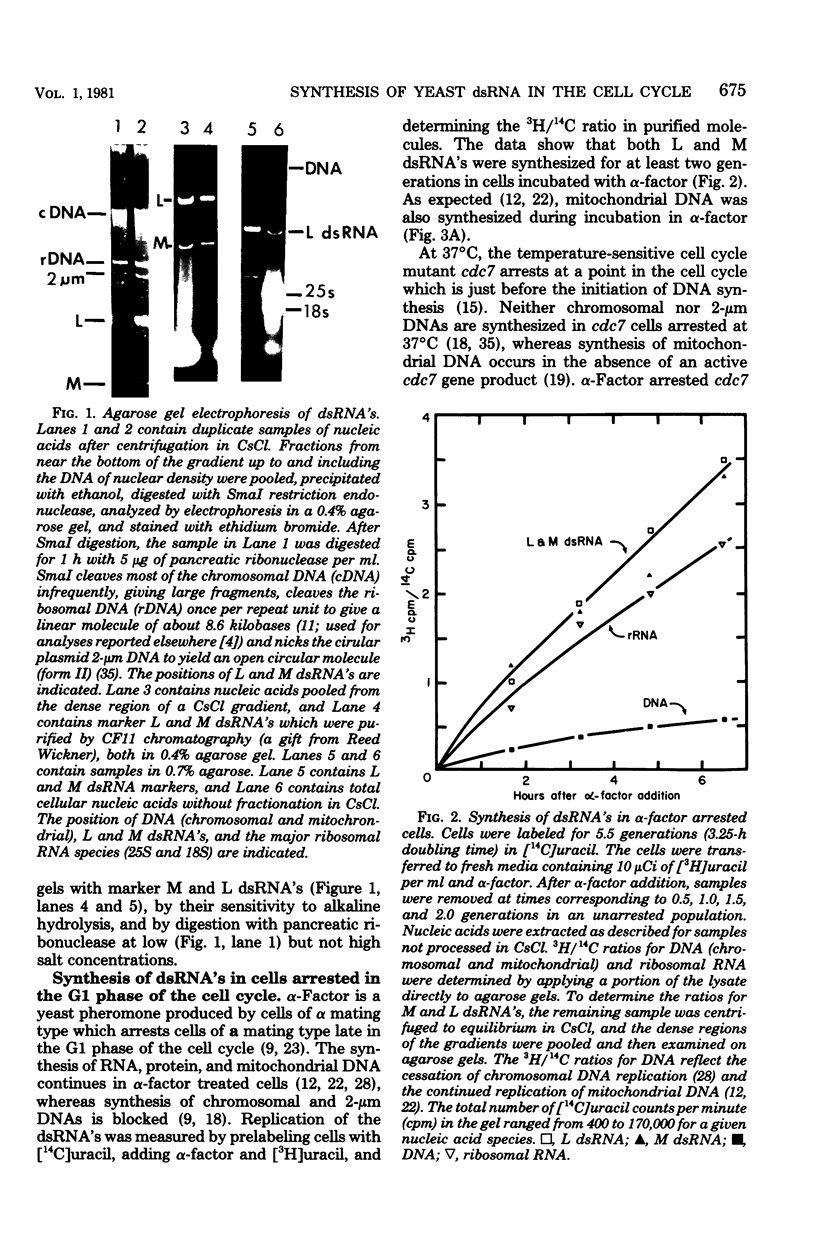
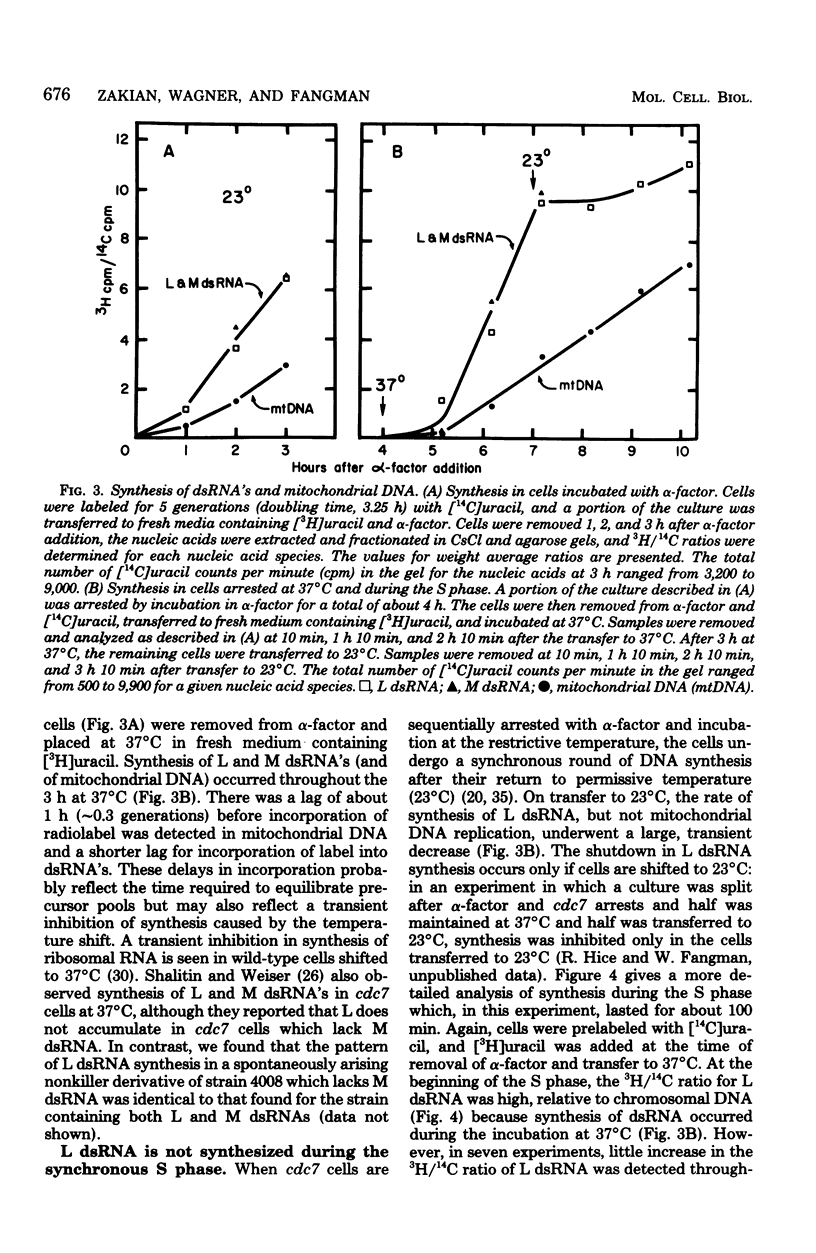
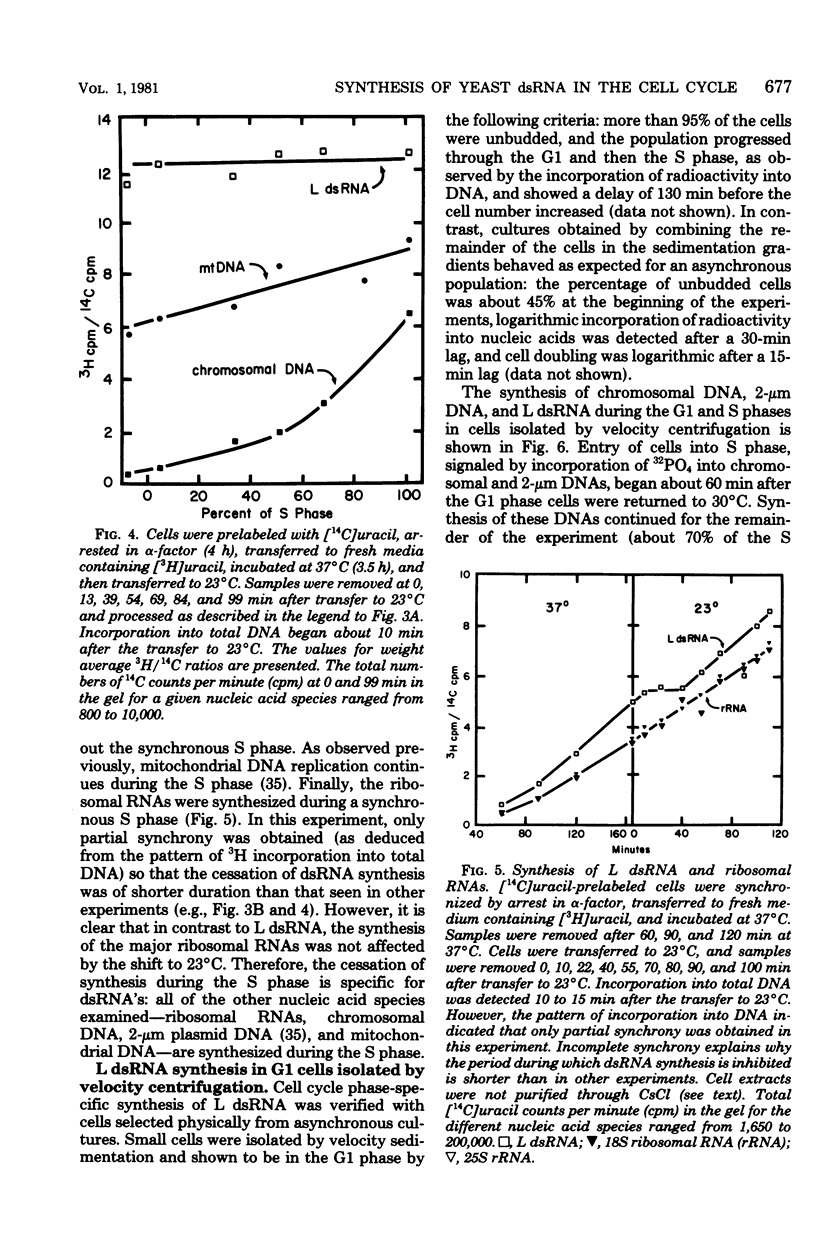
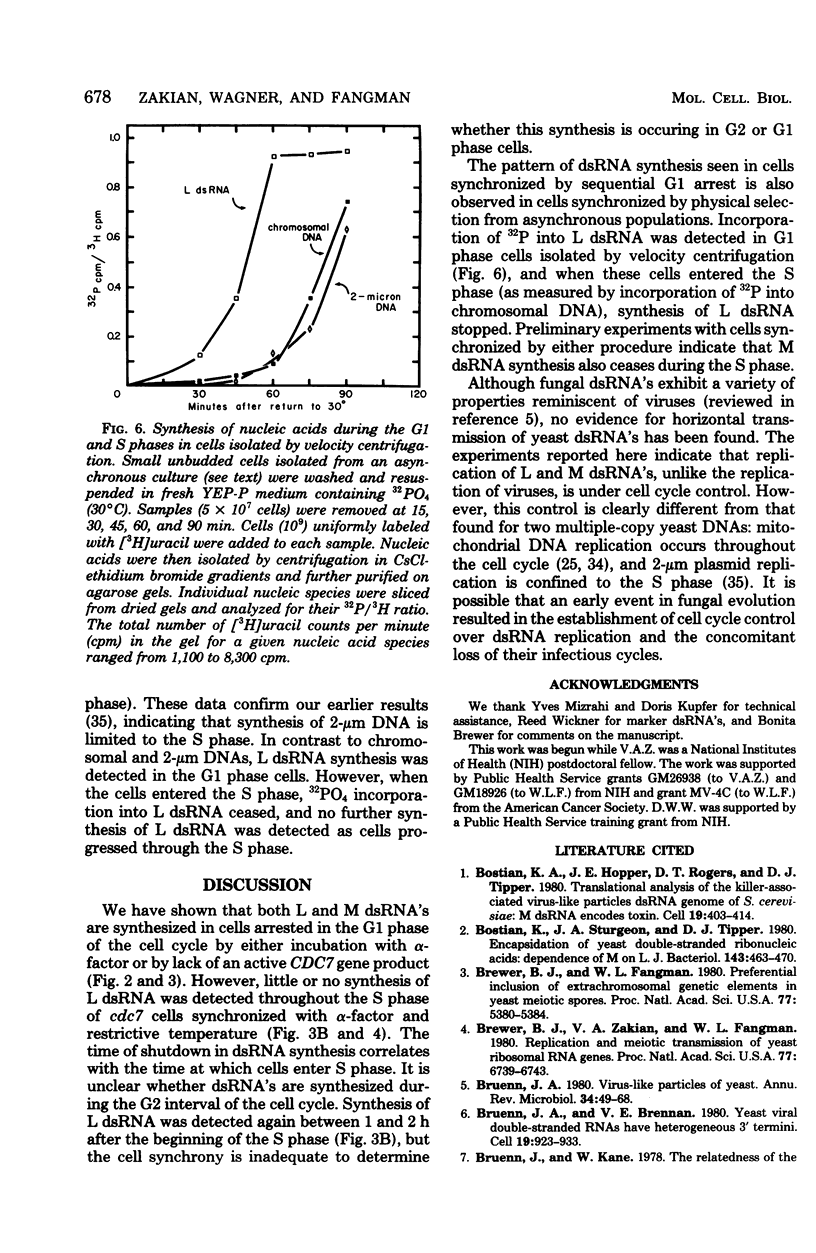
The cytoplasm of Saccharomyces cerevisiae contains two major classes of protein-encapsulated double-stranded ribonucleic acids (dsRNA's), L and M. Replication of L and M dsRNA's was examined in cells arrested in the G1 phase by either alpha-factor, a yeast mating pheromone, or the restrictive temperature for a cell cycle mutant (cdc7). [3H]uracil was added during the arrest periods to cells prelabeled with [14C]uracil, and replication was monitored by determining the ratio of 3H/14C for purified dsRNA's. Like mitochondrial deoxyribonucleic acid, both L and M dsRNA's were synthesized in the G1 arrested cells. The replication of L dsRNA was also examined during the S phase, using cells synchronized in two different ways. Cells containing the cdc7 mutation, treated sequentially with alpha-factor and then the restrictive temperature, enter a synchronous S phase when transferred to permissive temperature. When cells entered the S phase, synthesis of L dsRNA ceased, and little or no synthesis was detected throughout the S phase. Synthesis of L dsRNA was also observed in G1 phase cells isolated from asynchronous cultures by velocity centrifugation. Again, synthesis ceased when cells entered the S phase. These results indicate that L dsRNA replication is under cell cycle control. The control differs from that of mitochondrial deoxyribonucleic acid, which replicates in all phases of the cell cycle, and from that of 2-micron DNA, a multiple-copy plasmid whose replication is confined to the S phase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Sturgeon J. A., Tipper D. J. Encapsidation of yeast killer double-stranded ribonucleic acids: dependence of M on L. J Bacteriol. 1980 Jul;143(1):463–470. doi: 10.1128/jb.143.1.463-470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. Preferential inclusion of extrachromosomal genetic elements in yeast meiotic spores. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5380–5384. doi: 10.1073/pnas.77.9.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Zakian V. A., Fangman W. L. Replication and meiotic transmission of yeast ribosomal RNA genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6739–6743. doi: 10.1073/pnas.77.11.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Virus-like particles of yeast. Annu Rev Microbiol. 1980;34:49–68. doi: 10.1146/annurev.mi.34.100180.000405. [DOI] [PubMed] [Google Scholar]
- Bruenn J., Kane W. Relatedness of the double-stranded RNAs present in yeast virus-like particles. J Virol. 1978 Jun;26(3):762–772. doi: 10.1128/jvi.26.3.762-772.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Keitz B. The 5' ends of yeast killer factor RNAs are pppGp. Nucleic Acids Res. 1976 Oct;3(10):2427–2436. doi: 10.1093/nar/3.10.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- Clare J. J., Oliver S. G. The regulation of RNA synthesis in yeast IV. Synthesis of double-stranded RNA. Mol Gen Genet. 1979 Mar 20;171(2):161–166. doi: 10.1007/BF00270002. [DOI] [PubMed] [Google Scholar]
- Cramer J. H., Farrelly F. W., Rownd R. H. Restriction endonuclease analysis of ribosomal DNA from Saccharomyces cerevisiae. Mol Gen Genet. 1976 Nov 17;148(3):233–241. doi: 10.1007/BF00332897. [DOI] [PubMed] [Google Scholar]
- Cryer D. R., Goldthwaite C. D., Zinker S., Lam K. B., Storm E., Hirschberg R., Blamire J., Finkelstein D. B., Marmur J. Studies on nuclear and mitochondrial DNA of Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1974;38:17–29. doi: 10.1101/sqb.1974.038.01.005. [DOI] [PubMed] [Google Scholar]
- Forte M. A., Fangman W. L. Naturally occurring cross-links in yeast chromosomal DNA. Cell. 1976 Jul;8(3):425–431. doi: 10.1016/0092-8674(76)90155-0. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Three additional genes required for deoxyribonucleic acid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1973 Sep;115(3):966–974. doi: 10.1128/jb.115.3.966-974.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Lemke P. A. Viruses of eucaryotic microorganisms. Annu Rev Microbiol. 1976;30:105–145. doi: 10.1146/annurev.mi.30.100176.000541. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Kupfer D. M. Control of Saccharomyces cerevisiae 2microN DNA replication by cell division cycle genes that control nuclear DNA replication. J Mol Biol. 1977 Oct 25;116(2):249–260. doi: 10.1016/0022-2836(77)90215-7. [DOI] [PubMed] [Google Scholar]
- Newlon C. S., Fangman W. L. Mitochondrial DNA synthesis in cell cycle mutants of Saccharomyces cerevisiae. Cell. 1975 Aug;5(4):423–428. doi: 10.1016/0092-8674(75)90061-6. [DOI] [PubMed] [Google Scholar]
- Newlon C. S., Petes T. D., Hereford L. M., Fangman W. L. Replication of yeast chromosomal DNA. Nature. 1974 Jan 4;247(5435):32–35. doi: 10.1038/247032a0. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Preferential synthesis of yeast mitochondrial DNA in alpha factor-arrested cells. Biochem Biophys Res Commun. 1973 Dec 10;55(3):603–609. doi: 10.1016/0006-291x(73)91186-8. [DOI] [PubMed] [Google Scholar]
- Rivin C. J., Fangman W. L. Cell cycle phase expansion in nitrogen-limited cultures of Saccharomyces cerevisiae. J Cell Biol. 1980 Apr;85(1):96–107. doi: 10.1083/jcb.85.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Sena E. P., Welch J. W., Halvorson H. O., Fogel S. Nuclear and mitochondrial deoxyribonucleic acid replication during mitosis in Saccharomyces cerevisiae. J Bacteriol. 1975 Aug;123(2):497–504. doi: 10.1128/jb.123.2.497-504.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin C., Weiser I. Killer double-stranded ribonucleic acid synthesis in cell division cycle mutants of Saccharomyces cerevisiae. J Bacteriol. 1977 Sep;131(3):735–740. doi: 10.1128/jb.131.3.735-740.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throm E., Duntze W. Mating-Type-Dependent Inhibition of Deoxyribonucleic Acid Synthesis in Saccharomyces cerevisiae. J Bacteriol. 1970 Dec;104(3):1388–1390. doi: 10.1128/jb.104.3.1388-1390.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Udem S. A. Temperature sensitive mutations affecting ribosome synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):243–257. doi: 10.1016/0022-2836(72)90280-x. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Killer of Saccharomyces cerevisiae: a double-stranded ribonucleic acid plasmid. Bacteriol Rev. 1976 Sep;40(3):757–773. doi: 10.1128/br.40.3.757-773.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Mak mutants of yeast: mapping and characterization. J Bacteriol. 1979 Oct;140(1):154–160. doi: 10.1128/jb.140.1.154-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. The killer double-stranded RNA plasmids of yeast. Plasmid. 1979 Jul;2(3):303–322. doi: 10.1016/0147-619x(79)90015-5. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Moustacchi E. The synthesis of mitochondrial DNA during the cell cycle in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1971 Jan 22;42(2):195–201. doi: 10.1016/0006-291x(71)90087-8. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Brewer B. J., Fangman W. L. Replication of each copy of the yeast 2 micron DNA plasmid occurs during the S phase. Cell. 1979 Aug;17(4):923–934. doi: 10.1016/0092-8674(79)90332-5. [DOI] [PubMed] [Google Scholar]




