Abstract
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the United States after cancers of the lung and the breast/prostate. While the incidence of CRC in the United States is among the highest in the world (approximately 52/100,000), its incidence in countries in India is among the lowest (approximately 7/100,000), suggesting that lifestyle factors may play a role in development of the disease. Whereas obesity, excessive alcohol consumption, a high-calorie diet, and a lack of physical activity promote this cancer, evidence indicates that foods containing folates, selenium, Vitamin D, dietary fiber, garlic, milk, calcium, spices, vegetables, and fruits are protective against CRC in humans. Numerous agents from “mother nature” (also called “nutraceuticals,”) that have potential to both prevent and treat CRC have been identified. The most significant discoveries relate to compounds such as cardamonin, celastrol, curcumin, deguelin, diosgenin, thymoquinone, tocotrienol, ursolic acid, and zerumbone. Unlike pharmaceutical drugs, these agents modulate multiple targets, including transcription factors, growth factors, tumor cell survival factors, inflammatory pathways, and invasion and angiogenesis linked closely to CRC. We describe the potential of these dietary agents to suppress the growth of human CRC cells in culture and to inhibit tumor growth in animal models. We also describe clinical trials in which these agents have been tested for efficacy in humans. Because of their safety and affordability, these nutraceuticals provide a novel opportunity for treatment of CRC, an “old age” disease with an “age old” solution.
Keywords: nutraceuticals, CRC, curcumin, gingerol, piperine
Introduction
Colorectal cancer (CRC), the third most commonly diagnosed cancer in the United States, develops through a multistep process in which normal mucosa first transitions to adenomatous polyps and then eventually to invasive carcinoma. CRC is a major cause of morbidity and mortality in the United States, with an estimated 143,460 new cases diagnosed and 51,690 deaths occurring in 2012[1]. It is estimated that 5-10% of all CRCs are due to inherited gene defects, but the great majority are sporadic and exhibit no heritable tendency. Life style has been shown to play a major role in the incidence of most cancers, especially CRC. Almost 70% incidence of CRC, has been linked to diet. Since certain type of diets accelerate CRC while others prevent CRC. We will focus in this review on the dietary agents that have been implicated in preventing this cancer.
Regardless of whether a cancer specifically results from a heritable mutation, extensive research within the last few years has indicated that most cancers are caused by dysregulation of as many as 500 gene products. These gene products include growth factors (e.g., EGF, VEGF, and IGF-1), growth factor receptors (e.g., EGF receptor), protein kinases (e.g., Src), inflammatory cytokines (e.g., TNF, IL-1, IL-6), inflammatory enzymes (e.g., COX-2, 5-LOX, PLA-2), proapoptotic proteins (e.g., TNF, Fas, TRAIL), antiapoptotic proteins (e.g., bcl-2, bcl-xL, cFLIP, IAP-1, IAP-2, survivin), tumor suppressors (e.g., p53, Rb), and transcription factors (e.g., NF-κB, AP-1, STAT3, HIF-1, PPARγ). Most of these gene products and the associated signaling pathways have been linked with CRCs. Perhaps one of the most important pathways in most CRCs is the pro-inflammatory pathway activated through the transcription factor NF-κB. This transcription factor has been shown to be activated by most risk factors linked to CRCs, including grilled meat, fried foods, saturated fatty acids, chemical and physical stress, and environmental pollutants [2]. Furthermore, constitutively active NF-κB has been encountered in most CRCs. Once activated, NF-κB regulates the expression of gene products that mediate survival (e.g., anti-apoptotic proteins bcl-2, bcl-xL, cFLIP, IAP-1, IAP-2, and survivin), proliferation (e.g., COX-2, c-myc, and cyclin D1), invasion (e.g., 5-LOX, MMP-9, ICAM-1, ELAM-1, and VCAM-1), and neo-angiogenesis (e.g., VEGF, IL-8, TNF, and IL-1) [3]. Most currently available agents that downregulate these pathways are highly specific for their targets (e.g., inhibitors of COX-2, VEGF, and EGFR). Such agents are unlikely to prevent diseases such as CRC which is caused by dysregulation of multiple gene products. Moreover, these agents are expensive and have numerous side effects [4-7]. A multi-targeted approach is therefore required for both treatment and prevention. It is worth noting that the same molecular targets are used for both prevention and treatment strategies [2, 8].
The incidence of CRC in the US (530 cases per million) is among the highest in the world. This contrasts with regions such as the Indian subcontinent (30 cases per million), in which CRC incidence is among the lowest in the world. Epidemiological and migration studies of Indians suggest that, to a large extent, these dramatic international differences in incidence rates can be explained by differences in environment, lifestyle, and diet. What is so unique about the Indian environment, lifestyle and/or diet that CRC risk in India is disproportionately lower than in the US? Given that food-derived compounds are constantly present in the intestine and may regulate the homeostasis of intestinal mucosa, one likely explanation is the nature of the Indian diet. Based on statistical and epidemiological data, Richard Doll and Richard Peto have postulated that 10–70% (average 35%) of human cancer mortality is attributable to diet [9]. Multiple lines of compelling evidence from epidemiological, clinical, and laboratory studies link cancer risk to nutritional factors. A feature of the Indian diet that distinguishes its from the Western diet is its higher proportion of dietary fiber, due largely to greater consumption of vegetarian food and lower intake of red meat than is the case with a typical Western diet. However, the preponderance of evidence suggests that, after accounting for other dietary risk factors, high dietary fiber intake is not associated with a reduced risk of CRC [10-12] and indeed, dietary fiber supplementation fails to reduce the risk of recurrence of colorectal adenomas in multiple randomized trials [13-16]. An alternative hypothesis is that distinctive spices routinely used in Indian cuisine confer protective effects against CRC. Evidence from multiple laboratories indicates that spices prevent CRC development by modulating proinflammatory pathways closely linked with tumorigenesis.
The Role of Nutraceuticals in Cancer Prevention
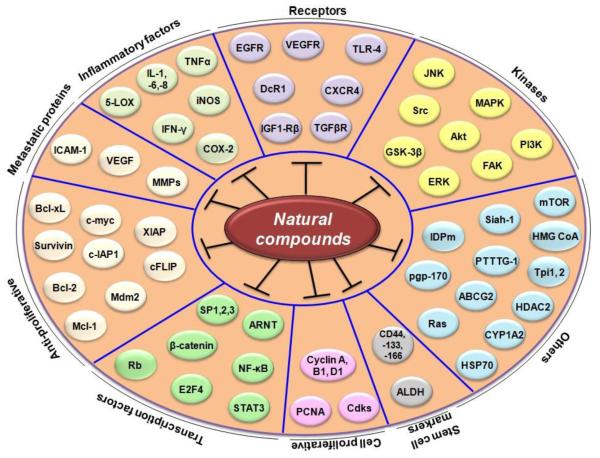
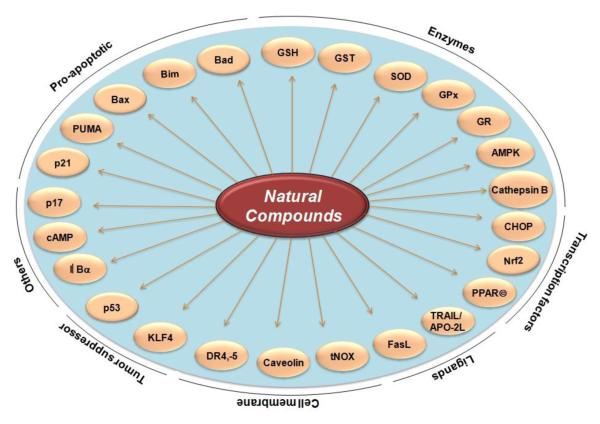
The use of plant-derived products (also called dietary botanicals) for the welfare of humankind is extends back as far as recorded history. The quest for plants, parts of plants, and components of parts of plants, that confer health benefits has perennially inspired human curiosity. At one time, the major source of medicine was the plants consumed by humans—prompting Hippocrates to preach almost 25 centuries ago, “let food be thy medicine and medicine be thy food.” This adage also calls to mind the common saying, “we are what we eat.” This philosophy is consistent with the position of both the National Cancer Institute and the American Cancer Society that eating more fruits and vegetables daily can reduce an individual’s risk of developing cancer. However, an accurate determination of the precise mechanisms by which the components of fruit and vegetables prevent cancer is needed before their inclusion in dietary supplements or evaluation in prospective clinical trials can be recommended. It is estimated that over 70% of cancers are preventable, with dietary agents making a 35% contribution. It remains unclear what specific dietary agents or nutraceuticals can prevent cancer. It is notable that more than half of all drugs approved by the Food and Drug Administration for cancer therapy within the last 4 decades have been either natural products, natural product derivatives, compounds based on natural products, or mimics of natural products [17]; indeed, cancer chemoprevention with botanicals is increasingly recognized as a promising research strategy [18]. In the current review, we show that that numerous nutraceuticals have potential as treatments for CRC (see table 1, 2 and 3). These include acetoxychavicol acetate, anacardic acid, berberine, betulinic acid, boswellic acid, butein, camptothecin, capsaicin, caffeic acid phenethyl ester, cardamonin, celastrol, chalcones, coronarin, curcumin, deguelin, diosgenin, elephantopin, emodine, embelin, escin, fisetin, flavopiridol, flavonoids, gambogic acid, garcinol, gossypol, gossypin, guggulsterone, indole-3-carbinol, morin, naphthoquinone, nimbolide, noscapine, oleandrin, piperine, piceatannol, pinitol, plumbagin, pomegranate, retnoids, honokiol, sanguinarine, sesamin, silymarin, simvastatin, terpenoid, thymoquinone, tocotrienol, triptolide, ursolic acid, withanolides, xanthohumol, and zerumbone. Various molecular targets modulated by these nutraceuticals are shown in Table 1. These targets include growth factors and their receptors, protein kinases, inflammatory biomarkers, and various transcription factors. Various biomarkers that are downregulated by nutraceuticals are shown in Figure 1. Those that are upregulated are shown in Figure 2. The use of these nutraceuticals for both prevention (Table 2) and treatment (Table 3) of CRC can be envisioned. The modulation of various biomarkers has been observed not only in colon cancer cells in culture but also in animal models of CRC (tables 2 and 3).
Table 1.
Molecular target of natural compound in in vitro models of colorectal cancer
| Cell lines/animals | Targets | References |
|---|---|---|
| Acetoxychavicol acetate | ||
| IEC6 | GST, NAD(P)H, NQO1, GSH, Nrf2, p21 |
[19] |
| Berberine | ||
| SW480 | NF-κB, COX-2, VEGF, p21, Bcl-2, survivin |
[20] |
| HT-29 | Survivin | [21] |
| SW620 | BID, c-IAP1, Bcl-2, Bcl-xL, JNK, p38 MAPK, FasL |
[22] |
| Colon 26 | IL-6 | [23] |
| HCT116 | Cyclin B1, cdc2 kinase | [24] |
| COLO 205,CT26 | pgp-170 | [25] |
| DLD-1 | COX-2 | [26] |
| Betulinic Acid | ||
| Colo-205 | Glyco-genes | [27] |
| RKO, SW480 | Sp-1,-2,-3, survivin, VEGF, EGFR, cyclin D1, PTTG-1, NF-κB |
[28] |
| SW948, HCT116 | Topoisomerase I and IIα | [29] |
| PTC | CYP1A2 | [30] |
| SNU-C5 | Bcl-2, Bad | [31] |
| SW480 | Caveolin-1 KLF4, PPARγ | [32] |
| HT29 | Bcl-2, cyclin D1, Bax | [33] |
| Colo-205 | p17 | [27] |
| AKBA | ||
| HT29 | Akt | [34] |
| HCT116 | Cyclin D1, cyclin E, CDK 2, CDK4, Rb, p21 |
[35] |
| Butein | ||
| CLL.220.1 | GSH | [36] |
| COLO 320HSR, CLL.220.1 |
GST | [37] |
| Capsaiscin | ||
| HCT116 | tNOX | [38] |
| Colo 205 | Bcl-2, Bax | [39] |
| HCT116 | p53, Mdm2, DR4, Fas (CD95), Bax, Bcl-2 |
[40] |
| HT29 | AMPK, ACC | [41, 42] |
| HT29 | PPARγ | [42] |
| CAPE | ||
| HCT116, SW480 | β-Catenin, cyclin D1, c-myc | [43] |
| CT26 | MMP-2, -9, VEGF | [44] |
| Cardamonin | ||
| HCT116 | DR-4, -5, DcR1, CHOP | [45] |
| CDDO | ||
| SW480 | PPARγ | [46] |
| Colon fibroblasts | IFN-γ, IL-1, TNFα, iNOS, COX-2 | [47] |
| Celastrol | ||
| HCT116 | CXCR4 | [48] |
| SW620 | TRAIL/APO-2L | [49] |
| Deguelin | ||
| COLO205, HCT116 | IL-8, IκBα, NF-κB, cFLIP, Bcl-2, Bcl-xL |
[50] |
| Diosgenin | ||
| HT29 | p38 MAPK, DR5, COX-2 | [51] |
| HT29, HCT116 | COX-2, 5-LOX | [52] |
| HCT116 | HMG-CoA reductase, p21 ras, β-catenin |
[53] |
| HT29 | Bcl-2 | [54] |
| HCT15 | Bcl-2, Bax | [55] |
| Emodin | ||
| LS1034 | Bcl-2, Bax | [56] |
| WiDr | MMP-2, -9, RhoB, NF-κB, VEGF |
[57] |
| DLD-1 | PRL-3, ezrin | [58] |
| DLD-1, HT2 | SOD, GST, tGPx, LDH | [59] |
| HCT116 | VEGFR-1, -2 | [60] |
| Escin | ||
| HT29 | p21 (WAF1/CIP1) | [61] |
| Fisetin | ||
| HCT116 | p53 | [62] |
| HCT116 | Bcl-xL, Bcl-2, Bak, Bim, FasL, DR5, TRAIL, p53 |
[63] |
| HT29 | COX2, EGFR, PGE2, β-catenin, NF-κB, Wnt, cyclin D1, MMP-7 |
[64] |
| HT29 | CDK-2, -4, CDC25C, cyclin E, cyclin D1, p21 (CIP1/WAF1) |
[65] |
| Flavopiridol | ||
| HCT116 | p53, Rad51, Cdk9 | [66] |
| HCT116 | Cdc2, survivin | [67] |
| HCT116 | p21 | [68] |
| HCT116 | p21, XIAP | [69] |
| HCT116 | Rb | [70] |
| T84 | cAMP | [71] |
| Garcinol | ||
| HCT116 | DR4, DR5, survivin, Bcl-2, XIAP, cFLIP, Bid, Bax |
[72] |
| HT29, HCT116 | ERK1/2, survivin | [73] |
| HT29 | FAK, Src, MAPK, ERK, PI3K, Akt, Bcl-2, Bax, MMP-7 |
[74] |
| Gossypol | ||
| HCT116 | DR5, Bcl-xL, Bcl-2, survivin, XIAP, cFLIP, CHOP, ERK1/2 |
[75] |
| CT26 | Bcl-2, Bcl-xL | [76] |
| HT29 | p21, cyclin D1, Bcl-2, Bcl-xL, Bag-1, Mcl-1, Bak |
[77] |
| HT29, LoVo | p53, Bcl-2, Bax | [78] |
| Guggulsterone | ||
| HT29 | p21, IGF1-Rβ | [79] |
| HT29 | CBR3 | [80] |
| HT29 | cIAP-1, cIAP-2, Bcl-2, Bid, Fas, p-JNK, c-Jun |
[81] |
| HT29 | VEGF, ARNT, STAT3, MMP-2, -9 |
[82] |
| Nimbolide | ||
| WiDr | NF-κB, ERK1/2, p38, JNK1/2, MMP-2, -9 |
[83] |
| HCT116 | DR4, DR5, ERK, p38 MAPK, I-FLICE, cIAP-1, cIAP-2, Bcl-2, Bcl-xL, survivin, XIAP, p53, Bax |
[84] |
| HT29 | p21, cyclin D2, Chk2, cyclin A, cyclin E, Cdk2, Rad17 |
[85] |
| Noscapine | ||
| LoVo | Survivin, Bcl-2, Bax | [86] |
| HCT116 | p53, p21, Bcl-2, Bax | [87] |
| Piperine | ||
| Caco-2 | P-glycoprotein, CYP3A4 | [88] |
| Gambogic acid | ||
| LOVO | PI3K, Akt, Bad | [89] |
| Curcumin | ||
| HuTu 80, Caco-2 | GST, UGT | [90] |
| HCT116 | p53, p21 | [91] |
| Caco2 | P-gp | [92] |
| HT29, HCT116 | CD133, CD44, CD166, ALDH | [93] |
| HCT116 | STAT3 | [94] |
| HCT116 | ERK1/2, p38 MAPK, JNK | [95] |
| HCT116 | ABCG2, EGFR, IGF-1R, NF-κB, β-catenin, COX-2, c-myc, Bcl-xL, Bax |
[96] |
| HCT116 | IDPm | [97] |
| HCT116 | E2F4, cyclin A, p21, p27 | [98] |
| HCT116, HT29 | p53, p21, PUMA | [99] |
| HCT116 | EGFR, HER-2, IGF-1R, | [100] |
| Caco2 | AKT, COX-2, cyclin D1 VDRE, GR, CYP3A4, CYP24, p21, TRPV6 |
[101] |
| HT29, HCT116 | CD44, CD166, EGFR | [102] |
| HCT116 | NF-κB, EGFR, IGF-1R | [103] |
| HCT116 | NF-κB, Akt, Bcl-2, Bcl-xL, IAP-2, COX-2, cyclin D1 |
[104] |
| HT29 | Akt, COX-2, AMPK | [105] |
| HCT116 | p53, p21 (CIP1/WAF1) | [106] |
| HCT116 | PCNA, CDK2, CDK4, cyclin B, p21, p27, p53, NF-B, Akt |
[107] |
| HCT116, SW480 | 20S & 26S proteasome | [108] |
| Plumbagin | ||
| HT29, HCT116 | EGFR, Akt, GSK-3β, PCNA, cyclin D1, COX-2 |
[109] |
| Reserpine | ||
| HCT116 | β-catenin, cyclin D1, c-myc, Siah-1 |
[110] |
| LS180 | CYP3A5 | [111] |
| Resveratrol | ||
| Caco-2, SW480 | iNOS, IκB, TLR-4 | [112] |
| HCT116 | JNK, p38 | [113] |
| HT29, SW480 | AKT, STAT3 | [114] |
| HCT116 | p53, Bax, Bcl-2 | [115] |
| SW480, HT29 | ERK, JNK, Akt, FAK, Fyn, Grb2, Ras, SOS |
[116] |
| Caco-2 | CYP1A1 | [117] |
| SW480 | PDCD4, PTEN, TGFβR, SMADs | [118] |
| HT29 | p27, cyclin D1, p53, IGF-1R, Akt, Wnt |
[119] |
| HT29 | CHOP, GRP-78, XBP1 | [120] |
| HCT116 | DR4, Fas (CD95), p53, Bax, Mdm2 |
[40] |
| HCT116 | NF-κB, EGFR, IGF-1R | [103] |
| Caco-2 | Bak, FADD | [121] |
| Lovo | VEGF, MMP-9, HIF-1 | [122] |
| RKO | β-catenin | [123] |
| HT29 | COX-2, PGE2 | [124] |
| Sanguinarine | ||
| HT29 | Bax, Bcl-2 | [125] |
| Silibinin | ||
| SW480 | DR4, DR5, Mcl-1, XIAP | [126] |
| CSLC | AKT, mTOR, PP2Ac β-catenin, IGF-1Rβ, ILGBP-1, GSK-3β, PKB/Akt |
[127] |
| SW480 | β-catenin, GSK3, cyclin D1, VEGF, iNOS, c-myc, survivin |
[128] |
| HCT116 | Cyclin B1, -D1, CDK2, p21, p27, COX-2 |
[129] |
| LoVo | Flt-1 | [130] |
| Tocotrienol | ||
| SW620 | β-catenin, Wnt-1, cyclin D1, c-jun, MMP-7 |
[131] |
| HCT116 | DR-4, DR5, ERK1, Bax, c-IAP2, Bcl-xL |
[132] |
| HT29, HCT116 | HMG-CoA reductase, RhoA | [133] |
| HT29 | NF-κB, Bcl-2, Bax | [134] |
| RKO | p53, WAF1/p21, Bcl-2 | [135] |
| Theaflavin | ||
| Caco-2 | COX-2, TNFα, ICAM-1, NF-κB | [136] |
| Thymoquinone | ||
| HT29 | HDAC2 | [137] |
| HCT116 | p53, CHEK1 | [138] |
| HCT116 | p53, p21WAF1, Bcl-2 | [139] |
| Ursolic acid | ||
| HCT116 | Bcl-xL, Bcl-2, cFLIP, survivin, cyclin D1, MMP-9, VEGF, ICAM-1 |
[140] |
| HCT116 | Sphingomyelinase | [141] |
| SW480 | Bcl-2, Bcl-xL, survivin | [142] |
| HT29 | EGFR, ERK1/2, p38 MAPK, JNK, Bcl-2, Bcl-xL |
[143] |
| HCT116 | DR4, DR5, DcR2, JNK | [144] |
| Withanolide | ||
| HCT116 | NF-κB, COX-2 | [145] |
| Xanthohumol | ||
| HCT116 | CXCR4 | [146] |
| HCT15 | DNA topoisomerase I, MDR1 | [147] |
| HCT116 | Bcl-2 | [148] |
| Zerumbone | ||
| HCT116 | DR4, DR5, cFLIP, ERK1/2, p38 MAPK, p53, Bax, p21 |
[149] |
| Caco-2, Colo320 | IL-1, IL-1, IL-6, TNFα | [150] |
ABCCG2, ATP-binding cassette sub-family G member 2; ACC, acetyl-CoA carboxylase; AKBA, acetyl-keto-beta-boswellic acid; AMPK, AMP-activated protein kinase; AR, aldose reductase; ARNT, aryl hydrocarbon receptor nuclear translocator; Bag-1, Bcl-2-binding protein; CAPE, Caffeic acid phenethyl ester; CBR3, Carbonyl reductase 3; CDDO, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid; CDK, cyclin dependent kinase; CHOP, CCAAT/enhancer-binding protein-homologous protein; COX-2, cyclooxygenase-2; CXCR4, Cysteine X Cysteine (CXC) chemokine receptor 4; DcR, decoy receptor; DR, death Receptor; EGFR, Epidermal growth factor receptor; ERK, extracelluar signal- regulated kinases; FAK, focal adhesion kinase; GPx, glutathione peroxidase; GR, glucocorticoid receptor; GRP, glucose-regulated protein; GSH, glutathione; GSK-3β, Glycogen synthase kinase-3β; GST, glutathione S-transferase; HO-1, hemoxygenase-1; ICAM, intracellular cell adhesion molecule; IDPm, NADP(+)-dependent isocitrate dehydrogenase; IFN, interferon; IGF, insulin-like growth factor; IL, interleukin; iNOS, inducible nitric oxide synthase; IκBα, inhibitor of kappaB alpha; KLF, Krüppel-like factor; KLF4, Krüppel-like factor 4; LC3, microtubule-associated protein 1 light chain 3; MAPK, Mitogen-activated protein kinase; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappaB; NQO1, quinone oxidoreductase 1; Nrf2, NF-E2-related factor 2; PCNA, proliferating cell nuclear antigen; PGE2, prostaglandin2; Pgp, phosphoglycoprotein; PI3K, phosphatidylinositol-3-kinase; PP2Ac, protein phosphatase 2Ac subunit; PPARgamma, peroxisome proliferator- activated receptor gamma; PRL-3, phosphatase of regenerating liver-3; PTC, Primary tumor cells of colon adenocarcinoma; PTTG, pituitary tumor transforming gene; QR, quinone reductase; SOD, superoxide dismutase; STAT3, signal transducers and activators of transcription 3; TNF, Tumor necrotic factor; tNOX, tumor-associated NADH oxidase; TRAIL, TNF related apoptosis-inducing ligand; VDRE, vitamin D responsive element; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; XIAP, X-linked inhibitor of apoptosis.
Table 2.
Prevention of colorectal cancer with natural compounds
| Targets | References |
|---|---|
| Rat Model | |
| Acetoxychavicol acetate | |
| GST, QR | [151] |
| Berberine | |
| β-Catenin | [152] |
| SOD, catalase, GST, GPx | [153] |
| COX-2 | [154] |
| Capsaiscin | |
| GST, QR | [155] |
| HMG CoA reductase | [156] |
| Morin | |
| HMG CoA reductase | [157] |
| SOD, catalase, GST, GPx, GR | [158] |
| Curcumin | |
| HSP70 | [159] |
| Resveratrol | |
| SOD, catalase, GPx, GST | [160] |
| Silibinin | |
| Bcl-2, Bax, IL-1β, TNFα, MMP7 | [161] |
| CytochromeP450, GST | [162] |
| Theaflavin | |
| COX-2, iNOS | [163] |
| Thymoquinone | |
| Catalase, GPx, SOD | [164] |
| Zerumbone | |
| COX-2, prostaglandins | [165] |
| Mouse Model | |
| Curcumin | |
| TNFα, IL-6, COX-2, AMPK, NF-κB |
[166] |
| iNOS, COX-2, ERK1/2, Wnt-1, β-catenin |
[167] |
| TNFα, IFNγ, COX-2, β-catenin, p53 |
[168] |
| Resveratrol | |
| NF-κB, PKC-2, iNOS, COX-2, AR, HO-1, Nrf2 |
[169] |
| NF-κB, STAT3, iNOS, ERK | [170] |
| Silibinin | |
| PCNA, cyclin D1, Cip1/p21, iNOS, COX-2, VEGF |
[171] |
| Zerumbone | |
| NF-αB, HO-1 | [40] |
| Diosgenin | |
| IL-1β, lipoprotein lipase, triglyceride, HO-1, SOD-3 |
[172] |
| Noscapine | |
| β-Catenin, cyclin D1, c-myc, p21 | [173] |
AMPK, AMP-activated protein kinase; AR, aldose reductase; COX-2, cyclooxygenase-2; GPx, glutathione peroxidase; GST, glutathione S-transferase; ERK, extracelluar signal-regulated kinases; HO-1, hemoxygenase-1; HMG-CoA reductase, 3-hydroxy-3-methyl-glutaryl-CoA reductase; HSP, heat shock proteins; NF-κB, nuclear factor-kappaB; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; MMP, matrix metalloproteinase; Nrf2, NF-E2-related factor 2; PCNA, proliferating cell nuclear antigen; QR, quinone reductase; SOD, superoxide dismutase; STAT3, signal transducers and activators of transcription 3; TNF, Tumor necrotic factor; VEGF, vascular endothelial growth factor
Table 3.
Therapeutic approach to colorectal cancer using natural compounds
| Agent | Cells | Target | Reference |
|---|---|---|---|
| Curcumin | SW480 | NF-κB, c-myc, cyclin D1, Bcl-2, CD31 | [174] |
| Deguelin | COLO 205 | Ki-67, NF-κB, VEGF | [50] |
| Guggulsterone | HT-29 | Bcl-2 | [81] |
| Plumbagin | HCT116 | von Willebrand Factor | [175] |
| Silibinin | SW480 | β-Catenin, GSK3β, cyclin D1, c-myc, survivin, VEGF, iNOS |
[128] |
| Thymoquinone | HCT116 | Ki-67 | [139] |
| Ursolic acid | HCT116 | NF-κB, STAT3, β-catenin, EGFR, CD31, p53, p21, Ki-67, Bcl-xL, Bcl-2, cFLIP, survivin, cyclin D1, MMP-9, VEGF, ICAM1 |
[140] |
| Resveratrol | HT-29 | p21, PCNA | [176] |
COX-2, cyclooxygenase-2; EGFR, Epidermal growth factor receptor; ERK, extracelluar signal-regulated kinases; GSK-3β, Glycogen synthase kinase-3β; NF-κB, nuclear factor-kappaB; ICAM, intracellular cell adhesion molecule; iNOS, inducible nitric oxide synthase; MMP, matrix metalloproteinase; PCNA, proliferating cell nuclear antigen; VEGF, vascular endothelial growth factor
Fig. 1.
Molecular targets in colorectal cancer that are downregulated by natural compounds.
Fig. 2.
Molecular targets in colorectal cancer that are upregulated by natural compounds
Role of nutraceuticals in CRC prevention
There are numerous reasons to conclude that most cancers, and CRCs in particular, are preventable. First, CRCs are more common in developed countries than in developing countries The causes of this disparity are not fully understood but numerous studies have indicated that lifestyle contributes as much as 95% to the incidence of all cancers. What is so unique about the Indian subcontinent lifestyle is uncertain. There is evidence to suggest that first, vegetarianism and certain spices unique to the diets of Indian people may contribute to a lower incidence of CRC. Second, grilled meat, fried foods, environmental pollutants, and certain viruses have been linked to colorectal tumorigenesis in rodent models. Third, dietary components derived from fruits and vegetables have been shown to suppress colorectal carcinogenesis in animals. Fourth, epidemiological studies and limited clinical trials in humans suggest that increased consumption of fruits and vegetables reduces the risk of developing CRCs and improves clinical outcomes in those diagnosed with CRC. The foods and active agents from Indian spices that have been linked with prevention of CRCs include curcumin from Curcuma longa; piperine from Piper nigrum; [6]-gingerol from Zingiber officinale; resveratrol from grapes, peanuts, and berries; catechins from tea; genistein from soybeans; caffeic acid from mustard seeds and olive oil; quercetin from onions; ellagic acid from pomegranate; diallyl disulfide from garlic; sulforaphane from broccoli; lycopene from tomatoes; and indole-3-carbinol from cruciferous vegetables. Extensive studies have provided “proof of concept” that these agents have potent anticancer and chemopreventive effects against CRC and that they mediate their effects by targeting multiple molecular targets. Only a select few agents that have been examined extensively are described below.
Curcumin
The active principle of Curcuma longa, curcumin, is perhaps the dietary agent about which most is known with respect to gastrointestinal cancers. This agent gives curry powder (turmeric) its yellow color; its active ingredient has been identified as diferuloylmethane. Curcumin has been shown to protect animals from a wide variety of carcinogens that cause gastrointestinal cancers. The protective effects of curcumin have also been reported in patients with Crohn’s disease, ulcerative colitis, familial adenomatous polyposis (FAP), and tropical pancreatitis. For instance, in one clinical trial of five FAP patients, polyps decreased by approximately 60% in number and 50% in polyp size between baseline and after treatment with curcumin [177]. A similar study involving 77 patients treated with celecoxib showed reductions of only 28 and 30% in polyp number and burden, respectively [178].
Curcumin’s mechanism of action has been studied extensively [179]. Our group showed that curcumin downregulated the activation of NF-κB [180], thus leading to the downregulation of the expression of anti-apoptotic, cell-proliferative, invasive, and angiogenic gene products [181]. In addition to downregulating NF-κB activation, curcumin can suppress activation of STAT3 [182], HIF-1 [183], and PPAR-[184]. Curcumin also downregulates the activity and expression of both cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) [185] as well as the expression of TNF, IL-1, and IL-6. It additionally inhibits both the anti-apoptotic activating transcription factor [186] and EGF receptor signaling [187]. In spite of interfering with all of these targets, curcumin has been found to be relatively safe pharmacologically at very high doses [188]. Moreover, no dose-limiting toxicity has been established in previously published clinical trials.
Although curcumin does exhibit activity against CRC in various preclinical models, it has several limitations. First and foremost, its bioavailability and tissue distribution are very poor [189], although piperine, a component of Piper nigrum that is often consumed with turmeric, has been shown to enhance the bioavailability of curcumin [190]. Second, it is not curcumin but turmeric that is consumed routinely by people in the Indian subcontinent where the incidence of CRC is relatively low. Third, people consuming turmeric do not consume turmeric alone but rather in combination with other spices such as Piper nigrum (black pepper) and Zingiber officinale (ginger). Fourth, turmeric exhibits activities that are different from those of curcumin [191, 192]. Fifth, the activity of curcumin is potentiated by turmerones and other minor components of turmeric [193]. For all of these reasons, further in-depth exploration of turmeric is warranted.
[6]-Gingerol
Ginger, the rhizome of Zingiber officinale, is as reputed for its medicinal properties as is turmeric. Ginger has traditionally been used in different parts of the world for various human ailments and in particular to aid digestion and treat nausea and vomiting in pregnancy [194]. Some pungent constituents present in ginger and other zingiberaceous plants have potent antioxidant, anti-inflammatory, antiemetic, antiulcer, cardiotonic, antihypertensive, hypoglycemic, antihyperlipidemic, and immuno-stimulant properties. Additionally, some of these constituents exhibit cancer-preventive activity in experimental studies and clinical trials [195, 196]. These properties of ginger are attributed to the presence of certain pungent vallinoids, specifically [6]-gingerol and [6]-paradol, as well as other constituents such as shogaols and zingerone. Experimental studies have also revealed that ginger and its most active constituent, [6]-gingerol, regulate the molecules in cellular signal transduction pathways, including NF-κB, AP-1, growth factors, chemokines, MAPK, p53, cyclin D1, VEGF, COX-2 and iNOS pathways [197-203]. By modulating multiple cell signaling pathways, these components inhibit cancer development and/or progression. Both [6]-gingerol and [6]-paradol have been found to induce cancer cell apoptosis [204]. [6]-gingerol has also been shown to inhibit phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity, and skin tumor promotion in mice [205]. Specifically in CRC, [6]-gingerol has been shown to reduce the incidence of CRCs in a rat azoxymethane (AOM) model [206], and to inhibit CRC cell proliferation, and endothelial cell tube formation [207], and G1 cell cycle arrest. [6]-gingerol inhibits these processes through downregulation of cyclin D1 via inhibition of -catenin translocation [201]. [6]-shogaol has been shown to induce apoptosis via reactive oxygen species (ROS) production, caspase activation, and GADD153 expression [208]. The chemopreventive properties of both ginger and turmeric have been linked in part to the upregulation of MAP kinase phosphatase-5 [209]. Ginger thus appears to contain a variety of constituents that may ultimately be of use in cancer prevention and treatment.
Piperine
Black pepper (Piper nigrum), a native Indian botanical, has been used in Indian cooking for centuries. It is valued for its distinctive sharp, stinging quality, which has been attributed to its active ingredient, the alkaloid piperine. Since the discovery of Piper nigrum’s active piperine, the use of black pepper has captivated the interest of modern medical researchers. Many physiological effects of Piper nigrum, its extracts, or its bioactive compound piperine, have been reported in recent decades. By stimulating the digestive enzymes of the pancreas, piperine enhances digestive capacity and significantly reduces gastrointestinal transit time for food [210]. Piperine has been shown to enhance the bioavailability of a number of therapeutic drugs as well as phytochemicals through its inhibitory influence on enzymatic drug biotransforming reactions in the liver and intestine [211]. Piperine strongly inhibits the activity of hepatic and intestinal aryl hydrocarbon hydroxylase and uridine dinucleotide phosphate-glucuronyl transferase [211]. Most clinical studies on piperine have focused on its effect on drug metabolism as a means of improving the bioavailability of other botanicals [189, 212, 213]. Piperine’s bioavailability-enhancing property is also partly attributed to increased absorption caused by its effect on the ultrastructure of the intestinal brush border [214, 215]. Piperine has been demonstrated, in in vitro studies, to protect against oxidative damage by inhibiting or quenching ROS. Piper nigrum or piperine treatment has also been found to lower lipid peroxidation in vivo and beneficially influence antioxidant status in several experiments involving oxidative stress [215, 216].
1′-Acetoxychavicol Acetate
1′-Acetoxychavicol acetate (ACA), which is obtained from the rhizomes of Alpinia galanga, is a component of traditional Asian condiments. It has both chemopreventive and chemotherapeutic potential in animals as well as in vitro models of CRC. ACA has been shown to induce apoptosis in CRC cell lines. It has also been reported that ACA inhibits DNA synthesis, thereby inhibiting cell proliferation [217]. In rat intestine epithelial cells (IEC6), ACA induced glutathione S-transferase (GST) and NAD(P)H: quinone oxidoreductase 1 (NQO1) activities, increased intracellular glutathione levels, and upregulated intranuclear Nrf2 and cytosolic p21 [19]. It also has the ability to inhibit azoxymethane (AOM)-induced colon tumorigenesis in rats [151]. Feeding these rats with ACA significantly reduced the incidence of colon carcinoma by suppressing proliferation biomarkers such as ornithine decarboxylase activity and colonic mucosal polyamine contents. Such inhibition was also associated with elevated levels of activity of phase II enzymes, including GST and QR, in the rat colon [151].
Berberine
Berberine is an isoquinoline natural alkaloid found in the roots, rhizomes, stem, and bark of a wide variety of traditional herbs, including goldenseal, barberry and Oregon grape. Numerous studies have shown that it can prevent and treat CRC. In in vitro assay, berberine inhibited proliferation and induced apoptosis of various CRC cells. It induced cell cycle arrest at the G2/M phase and caused apoptosis as evidenced by the loss of mitochondrial membrane potential, release of cytochrome c, suppression of c-IAP1, Bcl-2, Bcl-xL, induction of p21 expression, activation of caspases, and cleavage of PARP [22, 20]. In addition, berberine-induced apoptosis was accompanied by phosphorylation of JNK and p38 MAPK, increases in FasL and t-BID levels, and ROS generation [22]. Berberine’s anti-proliferative and apoptotic activity was found to be associated with the inhibition of NF-κB and Wnt/β-catenin signaling pathways [152, 20]. Moreover, berberine modulated pgp-170 expression in cancer cells, which was associated with changes in drug resistance [25] and inhibited arylamine N-acetyltransferase (NAT) activity in a human colon tumor cell line [218]. In an AOM-induced colon carcinogenesis rat model, berberine significantly inhibited the increases in lipid peroxidation, protein bound carbohydrates, and enhanced antioxidative status [153]. Oral administration of berberine was also found to inhibit COX-2 activities without inhibiting COX-1 activity in AOM-induced rat colon [154]. Thus, berberine inhibits neoplastic transformation in rat colon.
Whole botanical is better than a single active principle
Whether genistein, lycopenes, statins, catechins, or resveratrol; evidence indicates that the original sources of these nutraceuticals (soy, tomato, red yeast rice, green tea, and red grapes, respectively) may exhibit activity in vivo superior to that of their isolated active components. Indeed, numerous lines of evidence suggest that the whole botanical may be better than its active principle. For instance, Curcuma longa contains curcumin, demethoxy curcumin (DMC), bisdemethoxy curcumin (BDMC), turmeric oil (also known as turmerones), cyclocurcumin, and other constituents. Although curcumin is a major constituent (2-6%) of Curcuma longa, other components exist in minor but significant amounts. Curcuma longa oil consists of aromatic turmerone (ar-turmerone), -turmerone, and -turmerone. Curcuma longa oil has been linked with antifungal [219, 220], antibacterial [221], insecticidal [222], mosquitocidal [223], antioxidant [224], antimutagenic [224], and anticancer [225] activities. This oil has also been found to inhibit oral submucous fibrosis, a precancerous condition for oral cancer in healthy volunteers [225]. Additionally, turmeric oil has been shown to enhance the bioavailability of curcumin in human volunteers [193, 226].
A synergy has been observed between curcumin, DMC, and BDMC. Specifically, it has been shown that the ability of curcumin to inhibit peroxidation of linoleic acid by 15-lipoxygenase is synergistically enhanced by DMC and BDMC. Furthermore, we showed that the anti-inflammatory activity of curcumin is enhanced when curcumin is combined with DMC and BDMC [227]. Similarly, nematocidal activity levels have been reported to be higher in turmeric than in curcumin alone [228]. Curcumin-free aqueous turmeric extracts have been shown to suppress 7,12-dimethylbenz[α]anthracene (DMBA)-induced rat mammary tumor formation [192] and to inhibit benzo(a)pyrene-induced forestomach papillomas in mice [191]. Consistent with evidence that a whole botanical may potentially be more effective than its active principle, there is also an evolving body of evidence to suggest that whole botanicals exhibits potent antitumor activity at clinically meaningful doses. Various reports indicate that turmeric alone blocks glucuronidation and sulfation in Caco-2 cells in vitro [229], inhibits early activation of the Epstein-Barr-virus antigen [230], inhibits benzo(a)pyrene-derived DNA adduct formation [231], and suppresses the growth of Helicobacter pylori [232]. In rodents, 1% dietary turmeric was found to inhibit DMBA-induced carcinogenesis [233], abrogate croton oil-induced skin tumor formation [234], and suppress nitrosodiethylamine-induced hepatocarcinogenesis [235]. In addition, turmeric was found to prevent benzo[a]pyrene-induced forestomach tumors in Swiss mice and methyl-(acetoxymethyl)-nitrosamine-induced oral mucosal tumors in Syrian golden hamsters [236]. In a study of DMBA-induced mammary tumorigenesis in C3H (Jax) mice and Wistar rats, dietary turmeric suppressed mammary tumor virus-related reverse transcriptase activity, abrogated preneoplastic changes in the mammary glands, and decreased tumor incidence and tumor burden [237].
Combination of botanicals is expected to be superior to a single botanical alone
There is considerable interest in evaluating the likelihood that a combination of key botanicals might exhibit synergistic protective activity against CRC. A wide variety of botanicals and their phenolic compounds and flavonoids possesses potent antioxidant, antimutagenic, and anticarcinogenic activities. Multiple studies have suggested that a combination of botanicals and/or their active principles might be more efficacious than any one botanical alone. Piperine has been shown to enhance the bioavailability of curcumin in rodents and humans in part through the inhibition of glucuronidation [190]. We have also confirmed that piperine enhances the bioavailability of curcumin in human subjects [189]. In one study testing this combination, oral curcumin with piperine reversed lipid peroxidation in patients with tropical pancreatitis [212]. In another study, the combination of piperine plus curcumin significantly enhanced anti-immobility, neurotransmitter-enhancing (serotonin and dopamine), and monoamine oxidase inhibitory effects compared with curcumin alone [213]. When 5-lipoxygenase, the key enzyme involved in biosynthesis of leukotrienes, was evaluated in human polymorphonuclear leucocytes, the order of inhibitory activity was noted to be quercetin > eugenol > curcumin > cinnamaldehyde > piperine > capsaicin > allyl sulfide [238]. Furthermore, the inhibitory potency of aqueous extracts of these botanicals correlated with the active principles of their respective botanicals, with the combination of active principles or extracts synergistically inhibiting 5-LOX activity [238].
Human Studies
Clinical trials of nutraceuticals for CRC prevention and treatment in humans have established a larger body of knowledge about curcumin than about all other nutraceuticals. This agent has been examined in patients with ulcerative colitis (UC) and Crohn’s disease (CD) [239]. In an open-label study, curcumin was administered to five patients with ulcerative proctitis and five with CD. All proctitis patients improved, with reductions in concomitant medications in four, and four of five CD patients had lowered Crohn’s Disease Activity Index scores and sedimentation rates. Curcumin was also examined in a randomized, multicenter, double-blind, placebo-controlled trial of a maintenance therapy for UC [240]. Eighty-nine patients with quiescent UC were recruited. Forty-five patients received curcumin (1g after breakfast and 1g after the evening meal) plus sulfasalazine or mesalamine for 6 months, and 44 patients received placebo plus sulfasalazine or mesalamine for 6 months. Clinical activity index and endoscopic index were determined at entry, then every 2 months, at the conclusion of the 6-month trial, and at the end of 6-month follow-up. Of 43 patients who received curcumin, two relapsed during 6 months of therapy, whereas eight of 39 patients in the placebo group relapsed. These results indicate a significant difference between curcumin and placebo. Furthermore, curcumin improved both the clinical activity index and endoscopic index levels in patients, thus suppressing the morbidity associated with UC. A 6-month follow-up was conducted during which patients in both groups were on sulfasalazine or mesalamine. Eight additional patients in the curcumin group and six patients in the placebo group relapsed.
Familial adenomatous polyposis is an autosomal-dominant disorder characterized by the development of hundreds of colorectal adenomas and eventual colorectal cancer. Regression of adenomas in this syndrome occurs with the administration of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors, both of which can have considerable side effects. Curcumin was examined in combination with quercetin in FAP patients [177]. Five FAP patients with prior colectomy received curcumin 480 mg and quercetin 20 mg orally 3 times a day. All 5 patients’ polyps had decreased in number and size from baseline after a mean of 6 months of treatment with curcumin and quercetin. The mean percentage decreases in the number and size of polyps from baseline were 60.4% and 50.9%, respectively.
Recently, Carroll and colleagues trialed the use of curcumin for the prevention of colorectal neoplasia [241]. The group examined the effects of oral curcumin (2g or 4g per day for 30 days on prostaglandin E2 (PGE2) within aberrant crypt foci (ACF; primary endpoint), 5-HETE, ACF number, and proliferation in a non-randomized, open-label clinical trial of 44 eligible smokers with eight or more ACF on screening colonoscopy. Forty-one subjects completed the study. A significant reduction (40%) in patients’ ACF numbers occurred in the 4g dose group, whereas ACF numbers were not reduced in the 2g dose group. Interestingly, neither dose of curcumin reduced PGE2 or 5-HETE within ACF or normal mucosa or reduced Ki-67 in normal mucosa.
Numerous studies have also considered the use of curcumin for the treatment of CRC. A dose escalation study conducted in healthy volunteers showed insignificant serum levels of curcumin even when as much as 12,000 mg of curcumin was consumed daily [188]. Sharma and colleagues have examined both pharmacodynamic and pharmacokinetic properties with oral curcumin in patients with CRC [242]. Interestingly, Sharma and colleagues showed that daily ingestion of 440mg of curcumin for 29 days (with 15 patients) was accompanied by a 59% decrease in lymphocytic glutathione S-transferase activity, but this was not the case at a higher dose (2200mg). Another study [243] showed that a daily dose of 3.6g curcumin on days 1 and 29 caused 62% and 57% decreases, respectively, in inducible PGE2 production in blood samples taken 1 hour after dose administration compared with levels observed immediately before predose. Garcea and colleagues [244] found that administration of curcumin (3,600mg) decreased DNA adduct 3-(2-deoxy-beta-di-erythro-pentafuranosyl)-pyr[1,2-alpha]-purin-10(3H)one M(1)G levels in malignant colorectal tissue from 4.8 +/- 2.9 adducts per 107 nucleotides to 2.0 +/- 1.8 adducts per 107 nucleotides. COX-2 protein levels in malignant colorectal tissue were not affected by curcumin. In another study, 106 colorectal cancer patients were given 360mg of curcumin orally 3 times/day and then monitored for cancer-induced weight loss, serum TNF levels, tumor cell apoptosis, and other biomarkers on days 10, 20, and 30 after treatment [245]. The authors showed that curcumin administration increased body weight, decreased serum TNF-levels, increased numbers of apoptotic tumor cells, enhanced the expression of p53 molecules in tumor tissue, and modulated tumor cell apoptotic pathways, as indicated by upregulation of bax and downregulation of bcl-2. They concluded that curcumin treatment improves the general health of patients. All these studies therefore indicate that curcumin is quite safe when consumed in large quantities. Paradoxically, however, its effect in patients is unrelated to serum levels. Lower doses also appear to be more effective than higher doses in modulating biomarkers in human subjects.
Conclusion
Thus, this review clearly demonstrates that various nutraceuticals provided by the Mother Nature have a huge potential for both prevention and treatment of CRC. However, more clinical trials are required to prove neutraceuticals’ potential against this highly lethal disease. Since these agents can be administered chronically without any concern for safety and are highly affordable, their use has been the wave of the past and is likely to continue as the wave of the future.
Acknowledgement
The authors thank Amelia Scholtz, Diane S. Hackett, and the MD Anderson Department of Scientific Publications for carefully editing the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. doi:10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71(10):1397–421. doi: 10.1016/j.bcp.2006.02.009. doi:S0006-2952(06)00095-5 [pii] 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Maeda K, Chung Y, Kang S, Ogawa M, Onoda N, Nishiguchi Y, et al. Cyclin D1 overexpression and prognosis in colorectal adenocarcinoma. Oncology. 1998;55(2):145–51. doi: 10.1159/000011849. doi:ocl55145 [pii]. [DOI] [PubMed] [Google Scholar]
- 4•.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102(3):580–92. doi: 10.1002/jcb.21500. doi:10.1002/jcb.21500. This report deals drug discovery from natural sources using cell signaling pathways as the targets. Also indicates that our current models for drug development are not optimum.
- 5.Berenson A. A cancer drug shows promise, at a price that many can’t pay. NY Times (Print) 2006:A1, C2. [PubMed] [Google Scholar]
- 6.Goffin JR, Talavera JR. Overstated conclusions of a pooled analysis of bevacizumab in colon cancer. J Clin Oncol. 2006;24(3):528–9. doi: 10.1200/JCO.2005.04.3570. author reply 9-30. doi:24/3/528 [pii] 10.1200/JCO.2005.04.3570. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz N, Fernandez-Martos C, Romero I, Pla A, Maiquez J, Calatrava A, et al. Invasive fungal infection and nasal septum perforation with bevacizumab-based therapy in advanced colon cancer. J Clin Oncol. 2007;25(22):3376–7. doi: 10.1200/JCO.2007.12.0006. doi:25/22/3376 [pii] 10.1200/JCO.2007.12.0006. [DOI] [PubMed] [Google Scholar]
- 8.Abbruzzese JL, Lippman SM. The convergence of cancer prevention and therapy in early-phase clinical drug development. Cancer Cell. 2004;6(4):321–6. doi: 10.1016/j.ccr.2004.09.021. doi:S1535610804002788 [pii] 10.1016/j.ccr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191–308. [PubMed] [Google Scholar]
- 10.Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, et al. Dietary fiber intake and risk of colorectal cancer: a pooled analysis of prospective cohort studies. Jama. 2005;294(22):2849–57. doi: 10.1001/jama.294.22.2849. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Stampfer MJ, Rosner B, et al. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med. 1999;340(3):169–76. doi: 10.1056/NEJM199901213400301. [DOI] [PubMed] [Google Scholar]
- 12.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93(7):525–33. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 13.McKeown-Eyssen GE, Bright-See E, Bruce WR, Jazmaji V, Cohen LB, Pappas SC, et al. A randomized trial of a low fat high fibre diet in the recurrence of colorectal polyps. Toronto Polyp Prevention Group. J Clin Epidemiol. 1994;47(5):525–36. doi: 10.1016/0895-4356(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 14.MacLennan R, Macrae F, Bain C, Battistutta D, Chapuis P, Gratten H, et al. Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas. J Natl Cancer Inst. 1995;87(23):1760–6. doi: 10.1093/jnci/87.23.1760. [DOI] [PubMed] [Google Scholar]
- 15.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians’ Network. N Engl J Med. 2000;342(16):1156–62. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 16.Schatzkin A, Lanza E, Corle D, Lance P, Iber F, Caan B, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N Engl J Med. 2000;342(16):1149–55. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 17.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2(2):143–8. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 18.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 19.Yaku K, Matsui-Yuasa I, Azuma H, Kojima-Yuasa A. 1′-Acetoxychavicol acetate enhances the phase II enzyme activities via the increase in intranuclear Nrf2 level and cytosolic p21 level. Am J Chin Med. 2011;39(4):789–802. doi: 10.1142/S0192415X11009196. doi:S0192415X11009196 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Chidambara Murthy KN, Jayaprakasha GK, Patil BS. The natural alkaloid berberine targets multiple pathways to induce cell death in cultured human colon cancer cells. Eur J Pharmacol. 2012;688(1-3):14–21. doi: 10.1016/j.ejphar.2012.05.004. doi:S0014-2999(12)00429-3 [pii] 10.1016/j.ejphar.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Hu W, Yu L, Wang MH. Antioxidant and antiproliferative properties of water extract from Mahonia bealei (Fort.) Carr. leaves. Food Chem Toxicol. 2011;49(4):799–806. doi: 10.1016/j.fct.2010.12.001. doi:S0278-6915(10)00705-2 [pii] 10.1016/j.fct.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang HI, Wang CJ, et al. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol. 2007;81(10):719–28. doi: 10.1007/s00204-006-0169-y. doi:10.1007/s00204-006-0169-y. [DOI] [PubMed] [Google Scholar]
- 23.Iizuka N, Hazama S, Yoshimura K, Yoshino S, Tangoku A, Miyamoto K, et al. Anticachectic effects of the natural herb Coptidis rhizoma and berberine on mice bearing colon 26/clone 20 adenocarcinoma. Int J Cancer. 2002;99(2):286–91. doi: 10.1002/ijc.10338. doi:10.1002/ijc.10338. [DOI] [PubMed] [Google Scholar]
- 24.Li XK, Motwani M, Tong W, Bornmann W, Schwartz GK. Huanglian, A chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol Pharmacol. 2000;58(6):1287–93. doi: 10.1124/mol.58.6.1287. [DOI] [PubMed] [Google Scholar]
- 25.Lin HL, Liu TY, Wu CW, Chi CW. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. Br J Cancer. 1999;81(3):416–22. doi: 10.1038/sj.bjc.6690710. doi:10.1038/sj.bjc.6690710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;66(2):227–33. doi: 10.1016/s0378-8741(98)00162-7. doi:S0378-8741(98)00162-7 [pii]. [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Ma R, Boyle PJ, Mikulla B, Bradley M, Smith B, et al. Apoptosis of human carcinoma cells in the presence of potential anti-cancer drugs: III. Treatment of Colo-205 and SKBR3 cells with: cis -platin, Tamoxifen, Melphalan, Betulinic acid, L-PDMP, L-PPMP, and GD3 ganglioside. Glycoconj J. 2004;20(9):563–77. doi: 10.1023/B:GLYC.0000043293.46845.07. doi:10.1023/B:GLYC.0000043293.46845.07 5277288 [pii]. [DOI] [PubMed] [Google Scholar]
- 28.Chintharlapalli S, Papineni S, Lei P, Pathi S, Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome-dependent and -independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11:371. doi: 10.1186/1471-2407-11-371. doi:1471-2407-11-371 [pii] 10.1186/1471-2407-11-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar FM, Khanfar MA, Elnagar AY, Liu H, Zaghloul AM, Badria FA, et al. Rational design and semisynthesis of betulinic acid analogues as potent topoisomerase inhibitors. J Nat Prod. 2009;72(9):1643–50. doi: 10.1021/np900312u. doi:10.1021/np900312u. [DOI] [PubMed] [Google Scholar]
- 30.Rajendran P, Jaggi M, Singh MK, Mukherjee R, Burman AC. Pharmacological evaluation of C-3 modified Betulinic acid derivatives with potent anticancer activity. Invest New Drugs. 2008;26(1):25–34. doi: 10.1007/s10637-007-9081-4. doi:10.1007/s10637-007-9081-4. [DOI] [PubMed] [Google Scholar]
- 31.Jung GR, Kim KJ, Choi CH, Lee TB, Han SI, Han HK, et al. Effect of betulinic acid on anticancer drug-resistant colon cancer cells. Basic Clin Pharmacol Toxicol. 2007;101(4):277–85. doi: 10.1111/j.1742-7843.2007.00115.x. doi:PTO115 [pii] 10.1111/j.1742-7843.2007.00115.x. [DOI] [PubMed] [Google Scholar]
- 32.Chintharlapalli S, Papineni S, Liu S, Jutooru I, Chadalapaka G, Cho SD, et al. 2-cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor gamma in colon and pancreatic cancer cells. Carcinogenesis. 2007;28(11):2337–46. doi: 10.1093/carcin/bgm189. doi:bgm189 [pii] 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]
- 33.Rzeski W, Stepulak A, Szymanski M, Sifringer M, Kaczor J, Wejksza K, et al. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn Schmiedebergs Arch Pharmacol. 2006;374(1):11–20. doi: 10.1007/s00210-006-0090-1. doi:10.1007/s00210-006-0090-1. [DOI] [PubMed] [Google Scholar]
- 34.Liu JJ, Duan RD. LY294002 enhances boswellic acid-induced apoptosis in colon cancer cells. Anticancer Res. 2009;29(8):2987–91. doi:29/8/2987 [pii]. [PubMed] [Google Scholar]
- 35.Liu JJ, Huang B, Hooi SC. Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br J Pharmacol. 2006;148(8):1099–107. doi: 10.1038/sj.bjp.0706817. doi:0706817 [pii] 10.1038/sj.bjp.0706817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K, Wong KP, Chow P. Conjugation of chlorambucil with GSH by GST purified from human colon adenocarcinoma cells and its inhibition by plant polyphenols. Life Sci. 2003;72(23):2629–40. doi: 10.1016/s0024-3205(03)00173-5. doi:S0024320503001735 [pii]. [DOI] [PubMed] [Google Scholar]
- 37.Zhang K, Wong KP. Glutathione conjugation of chlorambucil: measurement and modulation by plant polyphenols. Biochem J. 1997;325(Pt 2):417–22. doi: 10.1042/bj3250417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu NC, Hsieh PF, Hsieh MK, Zeng ZM, Cheng HL, Liao JW, et al. Capsaicin-mediated tNOX (ENOX2) up-regulation enhances cell proliferation and migration in vitro and in vivo. J Agric Food Chem. 2012;60(10):2758–65. doi: 10.1021/jf204869w. doi:10.1021/jf204869w. [DOI] [PubMed] [Google Scholar]
- 39.Lu HF, Chen YL, Yang JS, Yang YY, Liu JY, Hsu SC, et al. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J Agric Food Chem. 2010;58(24):12999–3005. doi: 10.1021/jf103335w. doi:10.1021/jf103335w. [DOI] [PubMed] [Google Scholar]
- 40.Kim MY, Trudel LJ, Wogan GN. Apoptosis induced by capsaicin and resveratrol in colon carcinoma cells requires nitric oxide production and caspase activation. Anticancer Res. 2009;29(10):3733–40. doi:29/10/3733 [pii]. [PubMed] [Google Scholar]
- 41.Kim YM, Hwang JT, Kwak DW, Lee YK, Park OJ. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci. 2007;1095:496–503. doi: 10.1196/annals.1397.053. doi:1095/1/496 [pii] 10.1196/annals.1397.053. [DOI] [PubMed] [Google Scholar]
- 42.Kim CS, Park WH, Park JY, Kang JH, Kim MO, Kawada T, et al. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food. 2004;7(3):267–73. doi: 10.1089/jmf.2004.7.267. doi:10.1089/1096620041938713. [DOI] [PubMed] [Google Scholar]
- 43.Xiang D, Wang D, He Y, Xie J, Zhong Z, Li Z. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the beta-catenin/T-cell factor signaling. Anticancer Drugs. 2006;17(7):753–62. doi: 10.1097/01.cad.0000224441.01082.bb. doi:10.1097/01.cad.0000224441.01082.bb 00001813-200608000-00003 [pii]. [DOI] [PubMed] [Google Scholar]
- 44.Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, et al. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem. 2003;51(27):7907–12. doi: 10.1021/jf034729d. doi:10.1021/jf034729d. [DOI] [PubMed] [Google Scholar]
- 45.Yadav VR, Prasad S, Aggarwal BB. Cardamonin sensitizes tumour cells to TRAIL through ROS- and CHOP-mediated up-regulation of death receptors and down-regulation of survival proteins. Br J Pharmacol. 2012;165(3):741–53. doi: 10.1111/j.1476-5381.2011.01603.x. doi:10.1111/j.1476-5381.2011.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chintharlapalli S, Papineni S, Konopleva M, Andreef M, Samudio I, Safe S. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol Pharmacol. 2005;68(1):119–28. doi: 10.1124/mol.105.011437. doi:mol.105.011437 [pii] 10.1124/mol.105.011437. [DOI] [PubMed] [Google Scholar]
- 47.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, et al. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Res. 1999;59(2):336–41. [PubMed] [Google Scholar]
- 48.Yadav VR, Sung B, Prasad S, Kannappan R, Cho SG, Liu M, et al. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med (Berl) 2010;88(12):1243–53. doi: 10.1007/s00109-010-0669-3. doi:10.1007/s00109-010-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu H, Ding WJ, Wu R, Weng QJ, Lou JS, Jin RJ, et al. Synergistic anti-cancer activity by the combination of TRAIL/APO-2L and celastrol. Cancer Invest. 2010;28(1):23–32. doi: 10.3109/07357900903095664. doi:10.3109/07357900903095664 [pii] 10.3109/07357900903095664. [DOI] [PubMed] [Google Scholar]
- 50.Kang HW, Kim JM, Cha MY, Jung HC, Song IS, Kim JS. Deguelin, an Akt Inhibitor, Down-Regulates NF-kappaB Signaling and Induces Apoptosis in Colon Cancer Cells and Inhibits Tumor Growth in Mice. Dig Dis Sci. 2012 doi: 10.1007/s10620-012-2237-x. doi:10.1007/s10620-012-2237-x. [DOI] [PubMed] [Google Scholar]
- 51.Lepage C, Leger DY, Bertrand J, Martin F, Beneytout JL, Liagre B. Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett. 2011;301(2):193–202. doi: 10.1016/j.canlet.2010.12.003. doi:S0304-3835(10)00553-7 [pii] 10.1016/j.canlet.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Lepage C, Liagre B, Cook-Moreau J, Pinon A, Beneytout JL. Cyclooxygenase-2 and 5-lipoxygenase pathways in diosgenin-induced apoptosis in HT-29 and HCT-116 colon cancer cells. Int J Oncol. 2010;36(5):1183–91. doi: 10.3892/ijo_00000601. [DOI] [PubMed] [Google Scholar]
- 53.Raju J, Bird RP. Diosgenin, a naturally occurring steroid [corrected] saponin suppresses 3-hydroxy-3-methylglutaryl CoA reductase expression and induces apoptosis in HCT-116 human colon carcinoma cells. Cancer Lett. 2007;255(2):194–204. doi: 10.1016/j.canlet.2007.04.011. doi:S0304-3835(07)00197-8 [pii] 10.1016/j.canlet.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1392–8. doi:13/8/1392 [pii]. [PubMed] [Google Scholar]
- 55.Wang SL, Cai B, Cui CB, Liu HW, Wu CF, Yao XS. Diosgenin-3-O-alpha-L-rhamnopyranosyl-(1 --> 4)-beta-D-glucopyranoside obtained as a new anticancer agent from Dioscorea futschauensis induces apoptosis on human colon carcinoma HCT-15 cells via mitochondria-controlled apoptotic pathway. J Asian Nat Prod Res. 2004;6(2):115–25. doi: 10.1080/1028602031000147357. doi:10.1080/1028602031000147357. [DOI] [PubMed] [Google Scholar]
- 56.Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH, Yang JS, et al. Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem Toxicol. 2012;50(5):1271–8. doi: 10.1016/j.fct.2012.01.033. doi:S0278-6915(12)00060-9 [pii] 10.1016/j.fct.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 57.Suboj P, Babykutty S, Valiyaparambil Gopi DR, Nair RS, Srinivas P, Gopala S. Aloe emodin inhibits colon cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-kappaB. Eur J Pharm Sci. 2012;45(5):581–91. doi: 10.1016/j.ejps.2011.12.012. doi:S0928-0987(11)00467-2 [pii] 10.1016/j.ejps.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Han YM, Lee SK, Jeong DG, Ryu SE, Han DC, Kim DK, et al. Emodin inhibits migration and invasion of DLD-1 (PRL-3) cells via inhibition of PRL-3 phosphatase activity. Bioorg Med Chem Lett. 2012;22(1):323–6. doi: 10.1016/j.bmcl.2011.11.008. doi:S0960-894X(11)01540-X [pii] 10.1016/j.bmcl.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 59.El-Shemy HA, Aboul-Soud MA, Nassr-Allah AA, Aboul-Enein KM, Kabash A, Yagi A. Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr Med Chem. 2010;17(2):129–38. doi: 10.2174/092986710790112620. doi:CMC - AbsEpub/2010 - 009 [pii]. [DOI] [PubMed] [Google Scholar]
- 60.Lu Y, Zhang J, Qian J. The effect of emodin on VEGF receptors in human colon cancer cells. Cancer Biother Radiopharm. 2008;23(2):222–8. doi: 10.1089/cbr.2007.0425. doi:10.1089/cbr.2007.0425. [DOI] [PubMed] [Google Scholar]
- 61.Patlolla JM, Raju J, Swamy MV, Rao CV. Beta-escin inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growth by inducing p21(waf1/cip1) in colon cancer cells. Mol Cancer Ther. 2006;5(6):1459–66. doi: 10.1158/1535-7163.MCT-05-0495. doi:5/6/1459 [pii] 10.1158/1535-7163.MCT-05-0495. [DOI] [PubMed] [Google Scholar]
- 62.Yu SH, Yang PM, Peng CW, Yu YC, Chiu SJ. Securin depletion sensitizes human colon cancer cells to fisetin-induced apoptosis. Cancer Lett. 2011;300(1):96–104. doi: 10.1016/j.canlet.2010.09.015. doi:S0304-3835(10)00460-X [pii] 10.1016/j.canlet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Lim do Y, Park JH. Induction of p53 contributes to apoptosis of HCT-116 human colon cancer cells induced by the dietary compound fisetin. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1060–8. doi: 10.1152/ajpgi.90490.2008. doi:90490.2008 [pii] 10.1152/ajpgi.90490.2008. [DOI] [PubMed] [Google Scholar]
- 64.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30(2):300–7. doi: 10.1093/carcin/bgn269. doi:bgn269 [pii] 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu X, Jung J, Cho HJ, Lim DY, Lee HS, Chun HS, et al. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135(12):2884–90. doi: 10.1093/jn/135.12.2884. doi:135/12/2884 [pii]. [DOI] [PubMed] [Google Scholar]
- 66.Ambrosini G, Seelman SL, Qin LX, Schwartz GK. The cyclin-dependent kinase inhibitor flavopiridol potentiates the effects of topoisomerase I poisons by suppressing Rad51 expression in a p53-dependent manner. Cancer Res. 2008;68(7):2312–20. doi: 10.1158/0008-5472.CAN-07-2395. doi:68/7/2312 [pii] 10.1158/0008-5472.CAN-07-2395. [DOI] [PubMed] [Google Scholar]
- 67.Nawrocki ST, Carew JS, Douglas L, Cleveland JL, Humphreys R, Houghton JA. Histone deacetylase inhibitors enhance lexatumumab-induced apoptosis via a p21Cip1-dependent decrease in survivin levels. Cancer Res. 2007;67(14):6987–94. doi: 10.1158/0008-5472.CAN-07-0812. doi:67/14/6987 [pii] 10.1158/0008-5472.CAN-07-0812. [DOI] [PubMed] [Google Scholar]
- 68.Jung C, Motwani M, Kortmansky J, Sirotnak FM, She Y, Gonen M, et al. The cyclin-dependent kinase inhibitor flavopiridol potentiates gamma-irradiation-induced apoptosis in colon and gastric cancer cells. Clin Cancer Res. 2003;9(16 Pt 1):6052–61. [PubMed] [Google Scholar]
- 69.Motwani M, Jung C, Sirotnak FM, She Y, Shah MA, Gonen M, et al. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in Hct116 colon cancer monolayers and xenografts. Clin Cancer Res. 2001;7(12):4209–19. [PubMed] [Google Scholar]
- 70.Smith V, Raynaud F, Workman P, Kelland LR. Characterization of a human colorectal carcinoma cell line with acquired resistance to flavopiridol. Mol Pharmacol. 2001;60(5):885–93. doi: 10.1124/mol.60.5.885. [DOI] [PubMed] [Google Scholar]
- 71.Kahn ME, Senderowicz A, Sausville EA, Barrett KE. Possible mechanisms of diarrheal side effects associated with the use of a novel chemotherapeutic agent, flavopiridol. Clin Cancer Res. 2001;7(2):343–9. [PubMed] [Google Scholar]
- 72•.Prasad S, Ravindran J, Sung B, Pandey MK, Aggarwal BB. Garcinol potentiates TRAIL-induced apoptosis through modulation of death receptors and antiapoptotic proteins. Mol Cancer Ther. 2010;9(4):856–68. doi: 10.1158/1535-7163.MCT-09-1113. doi:1535-7163.MCT-09-1113 [pii]. This manuscript deals with novel targets for colorectal cancer and its modulation by natural product. 10.1158/1535-7163.MCT-09-1113.
- 73.Hong J, Kwon SJ, Sang S, Ju J, Zhou JN, Ho CT, et al. Effects of garcinol and its derivatives on intestinal cell growth: Inhibitory effects and autoxidation-dependent growth-stimulatory effects. Free Radic Biol Med. 2007;42(8):1211–21. doi: 10.1016/j.freeradbiomed.2007.01.016. doi:S0891-5849(07)00043-3 [pii] 10.1016/j.freeradbiomed.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 74.Liao CH, Sang S, Ho CT, Lin JK. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J Cell Biochem. 2005;96(1):155–69. doi: 10.1002/jcb.20540. doi:10.1002/jcb.20540. [DOI] [PubMed] [Google Scholar]
- 75.Sung B, Ravindran J, Prasad S, Pandey MK, Aggarwal BB. Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J Biol Chem. 2010;285(46):35418–27. doi: 10.1074/jbc.M110.172767. doi:M110.172767 [pii] 10.1074/jbc.M110.172767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Yan F, Cao XX, Jiang HX, Zhao XL, Wang JY, Lin YH, et al. A novel water-soluble gossypol derivative increases chemotherapeutic sensitivity and promotes growth inhibition in colon cancer. J Med Chem. 2010;53(15):5502–10. doi: 10.1021/jm1001698. doi:10.1021/jm1001698. [DOI] [PubMed] [Google Scholar]
- 77.Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, et al. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. 2003;66(1):93–103. doi: 10.1016/s0006-2952(03)00248-x. doi:S000629520300248X [pii]. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Wang J, Wong SC, Chow LS, Nicholls JM, Wong YC, et al. Cytotoxic effect of gossypol on colon carcinoma cells. Life Sci. 2000;67(22):2663–71. doi: 10.1016/s0024-3205(00)00857-2. doi:S0024320500008572 [pii]. [DOI] [PubMed] [Google Scholar]
- 79.Choudhuri R, Degraff W, Gamson J, Mitchell JB, Cook JA. Guggulsterone-mediated enhancement of radiosensitivity in human tumor cell lines. Front Oncol. 2011;1:19. doi: 10.3389/fonc.2011.00019. doi:10.3389/fonc.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebert B, Kisiela M, Malatkova P, El-Hawari Y, Maser E. Regulation of human carbonyl reductase 3 (CBR3; SDR21C2) expression by Nrf2 in cultured cancer cells. Biochemistry. 2010;49(39):8499–511. doi: 10.1021/bi100814d. doi:10.1021/bi100814d. [DOI] [PubMed] [Google Scholar]
- 81.An MJ, Cheon JH, Kim SW, Kim ES, Kim TI, Kim WH. Guggulsterone induces apoptosis in colon cancer cells and inhibits tumor growth in murine colorectal cancer xenografts. Cancer Lett. 2009;279(1):93–100. doi: 10.1016/j.canlet.2009.01.026. doi:S0304-3835(09)00060-3 [pii] 10.1016/j.canlet.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 82.Kim ES, Hong SY, Lee HK, Kim SW, An MJ, Kim TI, et al. Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol Rep. 2008;20(6):1321–7. [PubMed] [Google Scholar]
- 83.Babykutty S, S PP, J NR, Kumar MA, Nair MS, Srinivas P, et al. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP-2/9 expression via inhibiting ERK1/2 and reducing DNA-binding activity of NF-kappaB in colon cancer cells. Mol Carcinog. 2010;51(6):475–90. doi: 10.1002/mc.20812. doi:10.1002/mc.20812. [DOI] [PubMed] [Google Scholar]
- 84.Gupta SC, Reuter S, Phromnoi K, Park B, Hema PS, Nair M, et al. Nimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and Bax. J Biol Chem. 2011;286(2):1134–46. doi: 10.1074/jbc.M110.191379. doi:M110.191379 [pii] 10.1074/jbc.M110.191379. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Tsushida T. Inhibition of colon cancer (HT-29) cell proliferation by a triterpenoid isolated from Azadirachta indica is accompanied by cell cycle arrest and up-regulation of p21. Planta Med. 2006;72(10):917–23. doi: 10.1055/s-2006-946694. doi:10.1055/s-2006-946694. [DOI] [PubMed] [Google Scholar]
- 86.Yang ZR, Liu M, Peng XL, Lei XF, Zhang JX, Dong WG. Noscapine induces mitochondria-mediated apoptosis in human colon cancer cells in vivo and in vitro. Biochem Biophys Res Commun. 2012;421(3):627–33. doi: 10.1016/j.bbrc.2012.04.079. doi:S0006-291X(12)00746-2 [pii] 10.1016/j.bbrc.2012.04.079. [DOI] [PubMed] [Google Scholar]
- 87.Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007;67(8):3862–70. doi: 10.1158/0008-5472.CAN-06-4282. doi:67/8/3862 [pii] 10.1158/0008-5472.CAN-06-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302(2):645–50. doi: 10.1124/jpet.102.034728. doi:10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 89.Fang L, Chen B, Liu S, Wang R, Hu S, Xia G, et al. Synergistic effect of a combination of nanoparticulate Fe3O4 and gambogic acid on phosphatidylinositol 3-kinase/Akt/Bad pathway of LOVO cells. Int J Nanomedicine. 2012;7:4109–18. doi: 10.2147/IJN.S32475. doi:10.2147/IJN.S32475 ijn-7-4109 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Odenthal J, van Heumen BW, Roelofs HM, te Morsche RH, Marian B, Nagengast FM, et al. The influence of curcumin, quercetin, and eicosapentaenoic acid on the expression of phase II detoxification enzymes in the intestinal cell lines HT-29, Caco-2, HuTu 80, and LT97. Nutr Cancer. 2012;64(6):856–63. doi: 10.1080/01635581.2012.700994. doi:10.1080/01635581.2012.700994. [DOI] [PubMed] [Google Scholar]
- 91.Mosieniak G, Adamowicz M, Alster O, Jaskowiak H, Szczepankiewicz AA, Wilczynski GM, et al. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech Ageing Dev. 2012;133(6):444–55. doi: 10.1016/j.mad.2012.05.004. doi:S0047-6374(12)00095-4 [pii] 10.1016/j.mad.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 92.Yue GG, Cheng SW, Yu H, Xu ZS, Lee JK, Hon PM, et al. The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells. J Med Food. 2012;15(3):242–52. doi: 10.1089/jmf.2011.1845. doi:10.1089/jmf.2011.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 2011;6:7. doi: 10.1186/1750-2187-6-7. doi:1750-2187-6-7 [pii] 10 1186/1750-2187-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94••.Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer. 2011;105(2):212–20. doi: 10.1038/bjc.2011.200. doi:bjc2011200 [pii]. Colon cancer stem cells has become an attractive target for cancer prevention. This article is about modulation of stem cell by curcumin. 10.1038/bjc.2011.200.
- 95.Lee YJ, Kim NY, Suh YA, Lee C. Involvement of ROS in Curcumin-induced Autophagic Cell Death. Korean J Physiol Pharmacol. 2011;15(1):1–7. doi: 10.4196/kjpp.2011.15.1.1. doi:10.4196/kjpp.2011.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Padhye S, Sarkar FH, et al. Difluorinated-curcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res. 2011;28(4):827–38. doi: 10.1007/s11095-010-0336-y. doi:10.1007/s11095-010-0336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jung KH, Park JW. Suppression of mitochondrial NADP(+)-dependent isocitrate dehydrogenase activity enhances curcumin-induced apoptosis in HCT116 cells. Free Radic Res. 2011;45(4):431–8. doi: 10.3109/10715762.2010.540574. doi:10.3109/10715762.2010.540574. [DOI] [PubMed] [Google Scholar]
- 98.Kim KC, Lee C. Curcumin Induces Downregulation of E2F4 Expression and Apoptotic Cell Death in HCT116 Human Colon Cancer Cells; Involvement of Reactive Oxygen Species. Korean J Physiol Pharmacol. 2010;14(6):391–7. doi: 10.4196/kjpp.2010.14.6.391. doi:10.4196/kjpp.2010.14.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watson JL, Hill R, Yaffe PB, Greenshields A, Walsh M, Lee PW, et al. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett. 2010;297(1):1–8. doi: 10.1016/j.canlet.2010.04.018. doi:S0304-3835(10)00233-8 [pii] 10.1016/j.canlet.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 100.Patel BB, Gupta D, Elliott AA, Sengupta V, Yu Y, Majumdar AP. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res. 2010;30(2):319–25. doi:30/2/319 [pii]. [PMC free article] [PubMed] [Google Scholar]
- 101.Bartik L, Whitfield GK, Kaczmarska M, Lowmiller CL, Moffet EW, Furmick JK, et al. Curcumin: a novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J Nutr Biochem. 2010;21(12):1153–61. doi: 10.1016/j.jnutbio.2009.09.012. doi:S0955-2863(09)00212-5 [pii] 10.1016/j.jnutbio.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of Colon Cancer Stem-Like Cells by the Combination of Curcumin and FOLFOX. Transl Oncol. 2009;2(4):321–8. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Majumdar AP, Banerjee S, Nautiyal J, Patel BB, Patel V, Du J, et al. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr Cancer. 2009;61(4):544–53. doi: 10.1080/01635580902752262. doi:912716829 [pii] 10.1080/01635580902752262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sandur SK, Deorukhkar A, Pandey MK, Pabon AM, Shentu S, Guha S, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75(2):534–42. doi: 10.1016/j.ijrobp.2009.06.034. doi:S0360-3016(09)00946-8 [pii] 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee YK, Park SY, Kim YM, Park OJ. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann N Y Acad Sci. 2009;1171:489–94. doi: 10.1111/j.1749-6632.2009.04699.x. doi:NYAS4699 [pii] 10.1111/j.1749-6632.2009.04699.x. [DOI] [PubMed] [Google Scholar]
- 106.Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78(10):1305–15. doi: 10.1016/j.bcp.2009.06.105. doi:S0006-2952(09)00595-4 [pii] 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 107.Giri B, Gomes A, Sengupta R, Banerjee S, Nautiyal J, Sarkar FH, et al. Curcumin synergizes the growth inhibitory properties of Indian toad (Bufo melanostictus Schneider) skin-derived factor (BM-ANF1) in HCT-116 colon cancer cells. Anticancer Res. 2009;29(1):395–401. [PubMed] [Google Scholar]
- 108.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68(18):7283–92. doi: 10.1158/0008-5472.CAN-07-6246. doi:68/18/7283 [pii] 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Subramaniya BR, Srinivasan G, Sadullah SS, Davis N, Subhadara LB, Halagowder D, et al. Apoptosis inducing effect of plumbagin on colonic cancer cells depends on expression of COX-2. PLoS One. 2011;6(4):e18695. doi: 10.1371/journal.pone.0018695. doi:10.1371/journal.pone.0018695 PONE-D-11-00240 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gwak J, Song T, Song JY, Yun YS, Choi IW, Jeong Y, et al. Isoreserpine promotes beta-catenin degradation via Siah-1 up-regulation in HCT116 colon cancer cells. Biochem Biophys Res Commun. 2009;387(3):444–9. doi: 10.1016/j.bbrc.2009.07.027. doi:S0006-291X(09)01366-7 [pii] 10.1016/j.bbrc.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 111.Schuetz EG, Beck WT, Schuetz JD. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol Pharmacol. 1996;49(2):311–8. [PubMed] [Google Scholar]
- 112.Panaro MA, Carofiglio V, Acquafredda A, Cavallo P, Cianciulli A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-kappaB activation in Caco-2 and SW480 human colon cancer cells. Br J Nutr. 2012:1–10. doi: 10.1017/S0007114511007227. doi:S0007114511007227 [pii] 10.1017/S0007114511007227. [DOI] [PubMed] [Google Scholar]
- 113.Mohapatra P, Preet R, Choudhuri M, Choudhuri T, Kundu CN. 5-fluorouracil increases the chemopreventive potentials of resveratrol through DNA damage and MAPK signaling pathway in human colorectal cancer cells. Oncol Res. 2011;19(7):311–21. doi: 10.3727/096504011x13079697132844. [DOI] [PubMed] [Google Scholar]
- 114.Santandreu FM, Valle A, Oliver J, Roca P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell Physiol Biochem. 2011;28(2):219–28. doi: 10.1159/000331733. doi:000331733 [pii] 10.1159/000331733. [DOI] [PubMed] [Google Scholar]
- 115.Radhakrishnan S, Reddivari L, Sclafani R, Das UN, Vanamala J. Resveratrol potentiates grape seed extract induced human colon cancer cell apoptosis. Front Biosci (Elite Ed) 2011;3:1509–23. doi: 10.2741/e352. doi:352 [pii]. [DOI] [PubMed] [Google Scholar]
- 116.Colin D, Limagne E, Jeanningros S, Jacquel A, Lizard G, Athias A, et al. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev Res (Phila) 2011;4(7):1095–106. doi: 10.1158/1940-6207.CAPR-10-0274. doi:1940-6207.CAPR-10-0274 [pii] 10.1158/1940-6207.CAPR-10-0274. [DOI] [PubMed] [Google Scholar]
- 117.Perdew GH, Hollingshead BD, Dinatale BC, Morales JL, Labrecque MP, Takhar MK, et al. Estrogen receptor expression is required for low-dose resveratrol-mediated repression of aryl hydrocarbon receptor activity. J Pharmacol Exp Ther. 2010;335(2):273–83. doi: 10.1124/jpet.110.170654. doi:jpet.110.170654 [pii] 10.1124/jpet.110.170654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tili E, Michaille JJ, Alder H, Volinia S, Delmas D, Latruffe N, et al. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem Pharmacol. 2010;80(12):2057–65. doi: 10.1016/j.bcp.2010.07.003. doi:S0006-2952(10)00514-9 [pii] 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2011;10:238. doi: 10.1186/1471-2407-10-238. doi:1471-2407-10-238 [pii] 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Um HJ, Bae JH, Park JW, Suh H, Jeong NY, Yoo YH, et al. Differential effects of resveratrol and novel resveratrol derivative, HS-1793, on endoplasmic reticulum stress-mediated apoptosis and Akt inactivation. Int J Oncol. 2010;36(4):1007–13. doi: 10.3892/ijo_00000581. [DOI] [PubMed] [Google Scholar]
- 121.Cosan D, Soyocak A, Basaran A, Degirmenci I, Gunes HV. The effects of resveratrol and tannic acid on apoptosis in colon adenocarcinoma cell line. Saudi Med J. 2009;30(2):191–5. doi:20080981’ [pii]. [PubMed] [Google Scholar]
- 122.Wu H, Liang X, Fang Y, Qin X, Zhang Y, Liu J. Resveratrol inhibits hypoxia-induced metastasis potential enhancement by restricting hypoxia-induced factor-1 alpha expression in colon carcinoma cells. Biomed Pharmacother. 2008;62(9):613–21. doi: 10.1016/j.biopha.2008.06.036. doi:S0753-3322(08)00166-2 [pii] 10.1016/j.biopha.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 123.Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52(Suppl 1):S52–61. doi: 10.1002/mnfr.200700448. doi:10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW, et al. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinog. 2008;47(10):797–805. doi: 10.1002/mc.20437. doi:10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee JS, Jung WK, Jeong MH, Yoon TR, Kim HK. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway. Int J Toxicol. 2012;31(1):70–7. doi: 10.1177/1091581811423845. doi:1091581811423845 [pii] 10.1177/1091581811423845. [DOI] [PubMed] [Google Scholar]
- 126.Kauntz H, Bousserouel S, Gosse F, Raul F. The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells. Apoptosis. 17(8):797–809. doi: 10.1007/s10495-012-0731-4. doi:10.1007/s10495-012-0731-4. [DOI] [PubMed] [Google Scholar]
- 127.Wang JY, Chang CC, Chiang CC, Chen WM, Hung SC. Silibinin suppresses the maintenance of colorectal cancer stem-like cells by inhibiting PP2A/AKT/mTOR pathways. J Cell Biochem. 2012;113(5):1733–43. doi: 10.1002/jcb.24043. doi:10.1002/jcb.24043. [DOI] [PubMed] [Google Scholar]
- 128.Velmurugan B, Gangar SC, Kaur M, Tyagi A, Deep G, Agarwal R. Silibinin exerts sustained growth suppressive effect against human colon carcinoma SW480 xenograft by targeting multiple signaling molecules. Pharm Res. 2010;27(10):2085–97. doi: 10.1007/s11095-010-0207-6. doi:10.1007/s11095-010-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143(1):58–65. doi: 10.1016/j.jss.2007.03.080. doi:S0022-4804(07)00241-7 [pii] 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 130.Yang SH, Lin JK, Huang CJ, Chen WS, Li SY, Chiu JH. Silibinin inhibits angiogenesis via Flt-1, but not KDR, receptor up-regulation. J Surg Res. 2005;128(1):140–6. doi: 10.1016/j.jss.2005.04.042. doi:S0022-4804(05)00261-1 [pii] 10.1016/j.jss.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 131.Zhang JS, Li DM, He N, Liu YH, Wang CH, Jiang SQ, et al. A paraptosis-like cell death induced by delta-tocotrienol in human colon carcinoma SW620 cells is associated with the suppression of the Wnt signaling pathway. Toxicology. 2011;285(1-2):8–17. doi: 10.1016/j.tox.2011.03.011. doi:S0300-483X(11)00116-8 [pii] 10.1016/j.tox.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 132.Kannappan R, Ravindran J, Prasad S, Sung B, Yadav VR, Reuter S, et al. Gamma-tocotrienol promotes TRAIL-induced apoptosis through reactive oxygen species/extracellular signal-regulated kinase/p53-mediated upregulation of death receptors. Mol Cancer Ther. 2010;9(8):2196–207. doi: 10.1158/1535-7163.MCT-10-0277. doi:1535-7163.MCT-10-0277 [pii] 10.1158/1535-7163.MCT-10-0277. [DOI] [PubMed] [Google Scholar]
- 133.Yang Z, Xiao H, Jin H, Koo PT, Tsang DJ, Yang CS. Synergistic actions of atorvastatin with gamma-tocotrienol and celecoxib against human colon cancer HT29 and HCT116 cells. Int J Cancer. 2010;126(4):852–63. doi: 10.1002/ijc.24766. doi:10.1002/ijc.24766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu WL, Liu JR, Liu HK, Qi GY, Sun XR, Sun WG, et al. Inhibition of proliferation and induction of apoptosis by gamma-tocotrienol in human colon carcinoma HT-29 cells. Nutrition. 2009;25(5):555–66. doi: 10.1016/j.nut.2008.10.019. doi:S0899-9007(08)00448-6 [pii] 10.1016/j.nut.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 135.Agarwal MK, Agarwal ML, Athar M, Gupta S. Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle. 2004;3(2):205–11. doi: 10.4161/cc.3.2.654. doi:637 [pii]. [DOI] [PubMed] [Google Scholar]
- 136.Gosslau A, En Jao DL, Huang MT, Ho CT, Evans D, Rawson NE, et al. Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vivo. Mol Nutr Food Res. 2011;55(2):198–208. doi: 10.1002/mnfr.201000165. doi:10.1002/mnfr.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Attoub S, Sperandio O, Raza H, Arafat K, Al-Salam S, Al Sultan MA, et al. Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam Clin Pharmacol. 2012 doi: 10.1111/j.1472-8206.2012.01056.x. doi:10.1111/j.1472-8206.2012.01056.x. [DOI] [PubMed] [Google Scholar]
- 138.Gali-Muhtasib H, Kuester D, Mawrin C, Bajbouj K, Diestel A, Ocker M, et al. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008;68(14):5609–18. doi: 10.1158/0008-5472.CAN-08-0884. doi:68/14/5609 [pii] 10.1158/0008-5472.CAN-08-0884. [DOI] [PubMed] [Google Scholar]
- 139.Gali-Muhtasib H, Ocker M, Kuester D, Krueger S, El-Hajj Z, Diestel A, et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J Cell Mol Med. 2008;12(1):330–42. doi: 10.1111/j.1582-4934.2007.00095.x. doi:JCMM095 [pii] 10.1111/j.1582-4934.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140••.Prasad S, Yadav VR, Sung B, Reuter S, Kannappan R, Deorukhkar A, et al. Ursolic Acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: chemosensitization with capecitabine. Clin Cancer Res. 2012;18(18):4942–53. doi: 10.1158/1078-0432.CCR-11-2805. doi:1078-0432.CCR-11-2805 [pii] 10.1158/1078-0432.CCR-11-2805. Ursolic acid is produced by a wide variety of plants and can be used both for prevention and treatment of colorectal cancer.
- 141.Zhang P, Cheng Y, Duan RD. Ursolic Acid Inhibits Acid Sphingomyelinase in Intestinal Cells. Phytother Res. 2012 doi: 10.1002/ptr.4709. doi:10.1002/ptr.4709. [DOI] [PubMed] [Google Scholar]
- 142.Shan JZ, Xuan YY, Ruan SQ, Sun M. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin J Integr Med. 2011;17(8):607–11. doi: 10.1007/s11655-011-0815-y. doi:10.1007/s11655-011-0815-y. [DOI] [PubMed] [Google Scholar]
- 143.Shan JZ, Xuan YY, Zheng S, Dong Q, Zhang SZ. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J Zhejiang Univ Sci B. 2009;10(9):668–74. doi: 10.1631/jzus.B0920149. doi:10.1631/jzus.B0920149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prasad S, Yadav VR, Kannappan R, Aggarwal BB. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem. 2011;286(7):5546–57. doi: 10.1074/jbc.M110.183699. doi:M110.183699 [pii] 10.1074/jbc.M110.183699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Mulabagal V, Subbaraju GV, Rao CV, Sivaramakrishna C, Dewitt DL, Holmes D, et al. Withanolide sulfoxide from Aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother Res. 2009;23(7):987–92. doi: 10.1002/ptr.2736. doi:10.1002/ptr.2736. [DOI] [PubMed] [Google Scholar]
- 146.Wang Y, Chen Y, Wang J, Chen J, Aggarwal BB, Pang X, et al. Xanthohumol, a prenylated chalcone derived from hops, suppresses cancer cell invasion through inhibiting the expression of CXCR4 chemokine receptor. Curr Mol Med. 2012;12(2):153–62. doi: 10.2174/156652412798889072. doi:BSP/CMM/E-Pub/00022 [pii]. [DOI] [PubMed] [Google Scholar]
- 147.Lee SH, Kim HJ, Lee JS, Lee IS, Kang BY. Inhibition of topoisomerase I activity and efflux drug transporters’ expression by xanthohumol. from hops. Arch Pharm Res. 2007;30(11):1435–9. doi: 10.1007/BF02977368. [DOI] [PubMed] [Google Scholar]
- 148.Pan L, Becker H, Gerhauser C. Xanthohumol induces apoptosis in cultured 40-16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol Nutr Food Res. 2005;49(9):837–43. doi: 10.1002/mnfr.200500065. doi:10.1002/mnfr.200500065. [DOI] [PubMed] [Google Scholar]
- 149.Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009;69(16):6581–9. doi: 10.1158/0008-5472.CAN-09-1161. doi:0008-5472.CAN-09-1161 [pii] 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Murakami A, Miyamoto M, Ohigashi H. Zerumbone, an anti-inflammatory phytochemical, induces expression of proinflammatory cytokine genes in human colon adenocarcinoma cell lines. Biofactors. 2004;21(1-4):95–101. doi: 10.1002/biof.552210118. [DOI] [PubMed] [Google Scholar]
- 151.Tanaka T, Kawabata K, Kakumoto M, Makita H, Matsunaga K, Mori H, et al. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by a xanthine oxidase inhibitor, 1′-acetoxychavicol acetate. Jpn J Cancer Res. 1997;88(9):821–30. doi: 10.1111/j.1349-7006.1997.tb00457.x. doi:S0910505097855901 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu K, Yang Q, Mu Y, Zhou L, Liu Y, Zhou Q, et al. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/beta-catenin signaling. Int J Oncol. 2012;41(1):292–8. doi: 10.3892/ijo.2012.1423. doi:10.3892/ijo.2012.1423. [DOI] [PubMed] [Google Scholar]
- 153.Thirupurasundari CJ, Padmini R, Devaraj SN. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem Biol Interact. 2009;177(3):190–5. doi: 10.1016/j.cbi.2008.09.027. doi:S0009-2797(08)00526-7 [pii] 10.1016/j.cbi.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 154.Fukutake M, Yokota S, Kawamura H, Iizuka A, Amagaya S, Fukuda K, et al. Inhibitory effect of Coptidis Rhizoma and Scutellariae Radix on azoxymethane-induced aberrant crypt foci formation in rat colon. Biol Pharm Bull. 1998;21(8):814–7. doi: 10.1248/bpb.21.814. [DOI] [PubMed] [Google Scholar]
- 155.Yoshitani SI, Tanaka T, Kohno H, Takashima S. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by dietary capsaicin and rotenone. Int J Oncol. 2001;19(5):929–39. doi: 10.3892/ijo.19.5.929. [DOI] [PubMed] [Google Scholar]
- 156.Nalini N, Sabitha K, Chitra S, Viswanathan P, Menon VP. Histopathological and lipid changes in experimental colon cancer: effect of coconut kernal (Cocos nucifera Linn.) and (Capsicum annum Linn.) red chilli powder. Indian J Exp Biol. 1997;35(9):964–71. [PubMed] [Google Scholar]
- 157.Karthik Kumar V, Vennila S, Nalini N. Inhibitory effect of morin on DMH-induced biochemical changes and aberrant crypt foci formation in experimental colon carcinogenesis. Environ Toxicol Pharmacol. 2010;29(1):50–7. doi: 10.1016/j.etap.2009.09.006. doi:S1382-6689(09)00165-3 [pii] 10.1016/j.etap.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 158.Sreedharan V, Venkatachalam KK, Namasivayam N. Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Invest New Drugs. 2009;27(1):21–30. doi: 10.1007/s10637-008-9136-1. doi:10.1007/s10637-008-9136-1. [DOI] [PubMed] [Google Scholar]
- 159.Kwon Y, Magnuson BA. Age-related differential responses to curcumin-induced apoptosis during the initiation of colon cancer in rats. Food Chem Toxicol. 2009;47(2):377–85. doi: 10.1016/j.fct.2008.11.035. doi:S0278-6915(08)00660-1 [pii] 10.1016/j.fct.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 160.Sengottuvelan M, Deeptha K, Nalini N. Influence of dietary resveratrol on early and late molecular markers of 1,2-dimethylhydrazine-induced colon carcinogenesis. Nutrition. 2009;25(11-12):1169–76. doi: 10.1016/j.nut.2009.03.009. doi:S0899-9007(09)00173-7 [pii] 10.1016/j.nut.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 161.Kauntz H, Bousserouel S, Gosse F, Marescaux J, Raul F. Silibinin, a natural flavonoid, modulates the early expression of chemoprevention biomarkers in a preclinical model of colon carcinogenesis. Int J Oncol. 2012;41(3):849–54. doi: 10.3892/ijo.2012.1526. doi:10.3892/ijo.2012.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sangeetha N, Viswanathan P, Balasubramanian T, Nalini N. Colon cancer chemopreventive efficacy of silibinin through perturbation of xenobiotic metabolizing enzymes in experimental rats. Eur J Pharmacol. 2012;674(2-3):430–8. doi: 10.1016/j.ejphar.2011.11.008. doi:S0014-2999(11)01441-5 [pii] 10.1016/j.ejphar.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 163.Sengupta A, Ghosh S, Das RK, Bhattacharjee S, Bhattacharya S. Chemopreventive potential of diallylsulfide, lycopene and theaflavin during chemically induced colon carcinogenesis in rat colon through modulation of cyclooxygenase-2 and inducible nitric oxide synthase pathways. Eur J Cancer Prev. 2006;15(4):301–5. doi: 10.1097/00008469-200608000-00005. doi:00008469-200608000-00005 [pii]. [DOI] [PubMed] [Google Scholar]
- 164.Harzallah HJ, Grayaa R, Kharoubi W, Maaloul A, Hammami M, Mahjoub T. Thymoquinone, the Nigella sativa bioactive compound, prevents circulatory oxidative stress caused by 1,2-dimethylhydrazine in erythrocyte during colon postinitiation carcinogenesis. Oxid Med Cell Longev. 2012;2012:854065. doi: 10.1155/2012/854065. doi:10.1155/2012/854065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tanaka T, Shimizu M, Kohno H, Yoshitani S, Tsukio Y, Murakami A, et al. Chemoprevention of azoxymethane-induced rat aberrant crypt foci by dietary zerumbone isolated from Zingiber zerumbet. Life Sci. 2001;69(16):1935–45. doi: 10.1016/s0024-3205(01)01277-2. [DOI] [PubMed] [Google Scholar]
- 166.Kubota M, Shimizu M, Sakai H, Yasuda Y, Terakura D, Baba A, et al. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer. 2012;64(1):72–9. doi: 10.1080/01635581.2012.630554. doi:10.1080/01635581.2012.630554. [DOI] [PubMed] [Google Scholar]
- 167.Lai CS, Wu JC, Yu SF, Badmaev V, Nagabhushanam K, Ho CT, et al. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res. 2011;55(12):1819–28. doi: 10.1002/mnfr.201100290. doi:10.1002/mnfr.201100290. [DOI] [PubMed] [Google Scholar]
- 168.Villegas I, Sanchez-Fidalgo S, de la Lastra CA. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol Nutr Food Res. 2011;55(2):259–67. doi: 10.1002/mnfr.201000225. doi:10.1002/mnfr.201000225. [DOI] [PubMed] [Google Scholar]
- 169.Chiou YS, Tsai ML, Nagabhushanam K, Wang YJ, Wu CH, Ho CT, et al. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem. 2011;59(6):2725–33. doi: 10.1021/jf2000103. doi:10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- 170.Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer. 2009;61(6):847–54. doi: 10.1080/01635580903285072. doi:916753645 [pii] 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- 171.Ravichandran K, Velmurugan B, Gu M, Singh RP, Agarwal R. Inhibitory effect of silibinin against azoxymethane-induced colon tumorigenesis in A/J mice. Clin Cancer Res. 2010;16(18):4595–606. doi: 10.1158/1078-0432.CCR-10-1213. doi:1078-0432.CCR-10-1213 [pii] 10.1158/1078-0432.CCR-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Miyoshi N, Nagasawa T, Mabuchi R, Yasui Y, Wakabayashi K, Tanaka T, et al. Chemoprevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by freeze-dried yam sanyaku and its constituent diosgenin. Cancer Prev Res (Phila) 2011;4(6):924–34. doi: 10.1158/1940-6207.CAPR-10-0279. doi:1940-6207.CAPR-10-0279 [pii] 10.1158/1940-6207.CAPR-10-0279. [DOI] [PubMed] [Google Scholar]
- 173.Li S, Ghaleb AM, He J, Bughani U, Bialkowska AB, Yang VW, et al. Chemoprevention of familial adenomatous polyposis by bromo-noscapine (EM011) in the Apc(Min/+) mouse model. Int J Cancer. 2012;131(6):1435–44. doi: 10.1002/ijc.27344. doi:10.1002/ijc.27344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen C, Liu Y, Chen Y, Xu J. C086, a novel analog of curcumin, induces growth inhibition and down-regulation of NFkappaB in colon cancer cells and xenograft tumors. Cancer Biol Ther. 2011;12(9):797–807. doi: 10.4161/cbt.12.9.17671. doi:17671 [pii] 10.4161/cbt.12.9.17671. [DOI] [PubMed] [Google Scholar]
- 175.Lai L, Liu J, Zhai D, Lin Q, He L, Dong Y, et al. Plumbagin inhibits tumour angiogenesis and tumour growth through the Ras signalling pathway following activation of the VEGF receptor-2. Br J Pharmacol. 2012;165(4b):1084–96. doi: 10.1111/j.1476-5381.2011.01532.x. doi:10.1111/j.1476-5381.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Paul S, Mizuno CS, Lee HJ, Zheng X, Chajkowisk S, Rimoldi JM, et al. In vitro and in vivo studies on stilbene analogs as potential treatment agents for colon cancer. Eur J Med Chem. 2010;45(9):3702–8. doi: 10.1016/j.ejmech.2010.05.019. doi:S0223-5234(10)00359-4 [pii] 10.1016/j.ejmech.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4(8):1035–8. doi: 10.1016/j.cgh.2006.03.020. doi:S1542-3565(06)00278-3 [pii] 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 178.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 179.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 180.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270(42):24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 181.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69(1):195–206. doi: 10.1124/mol.105.017400. doi:mol.105.017400 [pii] 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 182.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171(7):3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 183.Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15(6):1557–62. [PubMed] [Google Scholar]
- 184.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G20–30. doi: 10.1152/ajpgi.00474.2002. doi:10.1152/ajpgi.00474.2002 00474.2002 [pii]. [DOI] [PubMed] [Google Scholar]
- 185.Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213–26. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 186.Yan C, Jamaluddin MS, Aggarwal B, Myers J, Boyd DD. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol Cancer Ther. 2005;4(2):233–41. [PubMed] [Google Scholar]
- 187.Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer. II. Curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol Urol. 2000;4(1):1–6. [PubMed] [Google Scholar]
- 188.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. doi:1472-6882-6-10 [pii] 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18. doi: 10.1021/mp700113r. doi:10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 190.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 191.Deshpande SS, Ingle AD, Maru GB. Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice. Cancer Lett. 1997;118(1):79–85. doi: 10.1016/s0304-3835(97)00238-3. doi:S0304-3835(97)00238-3 [pii]. [DOI] [PubMed] [Google Scholar]
- 192.Deshpande SS, Ingle AD, Maru GB. Chemopreventive efficacy of curcumin-free aqueous turmeric extract in 7,12-dimethylbenz[a]anthracene-induced rat mammary tumorigenesis. Cancer Lett. 1998;123(1):35–40. doi: 10.1016/s0304-3835(97)00400-x. doi:S0304-3835(97)00400-X [pii]. [DOI] [PubMed] [Google Scholar]
- 193.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95®CG (biocurcumaxTM), a novel bioenhanced preparation of curcumin. Indian J Pharmaceutical Sci. 2008;70(4):445–50. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abdel-Aziz H, Nahrstedt A, Petereit F, Windeck T, Ploch M, Verspohl EJ. 5-HT3 receptor blocking activity of arylalkanes isolated from the rhizome of Zingiber officinale. Planta Med. 2005;71(7):609–16. doi: 10.1055/s-2005-871265. [DOI] [PubMed] [Google Scholar]
- 195.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428(1-2):305–27. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 196.Surh YJ, Park KK, Chun KS, Lee LJ, Lee E, Lee SS. Anti-tumor-promoting activities of selected pungent phenolic substances present in ginger. J Environ Pathol Toxicol Oncol. 1999;18(2):131–9. [PubMed] [Google Scholar]
- 197.Bode AM, Ma WY, Surh YJ, Dong Z. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61(3):850–3. [PubMed] [Google Scholar]
- 198.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, et al. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Commun. 2005;335(2):300–8. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 199.Kim JK, Kim Y, Na KM, Surh YJ, Kim TY. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic Res. 2007;41(5):603–14. doi: 10.1080/10715760701209896. [DOI] [PubMed] [Google Scholar]
- 200.Kim SO, Kundu JK, Shin YK, Park JH, Cho MH, Kim TY, et al. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse skin. Oncogene. 2005;24(15):2558–67. doi: 10.1038/sj.onc.1208446. [DOI] [PubMed] [Google Scholar]
- 201.Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 2008;47(3):197–208. doi: 10.1002/mc.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009;382(1):134–9. doi: 10.1016/j.bbrc.2009.02.160. [DOI] [PubMed] [Google Scholar]
- 203.Ray A. Cancer preventive role of selected dietary factors. Indian J Cancer. 2005;42(1):15–24. doi: 10.4103/0019-509x.15095. [DOI] [PubMed] [Google Scholar]
- 204.Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett. 1998;134(2):163–8. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- 205.Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998;129(2):139–44. doi: 10.1016/s0304-3835(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 206.Yoshimi N, Wang A, Morishita Y, Tanaka T, Sugie S, Kawai K, et al. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res. 1992;83(12):1273–8. doi: 10.1111/j.1349-7006.1992.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brown AC, Shah C, Liu J, Pham JT, Zhang JG, Jadus MR. Ginger’s (Zingiber officinale Roscoe) inhibition of rat colonic adenocarcinoma cells proliferation and angiogenesis in vitro. Phytother Res. 2008;23(5):640–5. doi: 10.1002/ptr.2677. [DOI] [PubMed] [Google Scholar]
- 208.Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, et al. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol Nutr Food Res. 2008;52(5):527–37. doi: 10.1002/mnfr.200700157. [DOI] [PubMed] [Google Scholar]
- 209.Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28(6):1188–96. doi: 10.1093/carcin/bgl241. [DOI] [PubMed] [Google Scholar]
- 210.Platel K, Srinivasan K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung. 2000;44(1):42–6. doi: 10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 211.Srinivasan K. Molecular targets and therapeutic uses of black pepper (Piper nigrum) and its bioactive compound piperine. In: Aggarwal BBaK A, editor. Molecular targets and therapeutic uses of spices: Modern uses for ancient medicine. vol 2. World Scientific Publishing; Singapore: 2009. (in press) [Google Scholar]
- 212.Durgaprasad S, Pai CG, Vasanthkumar, Alvres JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian J Med Res. 2005;122(4):315–8. [PubMed] [Google Scholar]
- 213.Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol Biochem Behav. 2009;92(1):39–43. doi: 10.1016/j.pbb.2008.10.007. doi:S0091-3057(08)00350-X [pii] 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 214.Prakash UN, Srinivasan K. Beneficial influence of dietary spices on the ultrastructure and fluidity of the intestinal brush border in rats. Br J Nutr. 2010;104(1):31–9. doi: 10.1017/S0007114510000334. doi:S0007114510000334 [pii] 10.1017/S0007114510000334. [DOI] [PubMed] [Google Scholar]
- 215.Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9(3):224–31. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- 216.Manoharan S, Balakrishnan S, Menon VP, Alias LM, Reena AR. Chemopreventive efficacy of curcumin and piperine during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Singapore Med J. 2009;50(2):139–46. [PubMed] [Google Scholar]
- 217.Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, et al. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128(10):539–46. doi: 10.1007/s00432-002-0373-y. doi:10.1007/s00432-002-0373-y. [DOI] [PubMed] [Google Scholar]
- 218.Lin JG, Chung JG, Wu LT, Chen GW, Chang HL, Wang TF. Effects of berberine on arylamine N-acetyltransferase activity in human colon tumor cells. Am J Chin Med. 1999;27(2):265–75. doi: 10.1142/S0192415X99000306. doi:S0192415X99000306 [pii]. [DOI] [PubMed] [Google Scholar]
- 219.Apisariyakul A, Vanittanakom N, Buddhasukh D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae) J Ethnopharmacol. 1995;49(3):163–9. doi: 10.1016/0378-8741(95)01320-2. doi:0378874195013202 [pii]. [DOI] [PubMed] [Google Scholar]
- 220.Jayaprakasha GK, Negi PS, Anandharamakrishnan C, Sakariah KK. Chemical composition of turmeric oil--a byproduct from turmeric oleoresin industry and its inhibitory activity against different fungi. Z Naturforsch C. 2001;56(1-2):40–4. doi: 10.1515/znc-2001-1-207. [DOI] [PubMed] [Google Scholar]
- 221.Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J Agric Food Chem. 1999;47(10):4297–300. doi: 10.1021/jf990308d. doi:jf990308d [pii]. [DOI] [PubMed] [Google Scholar]
- 222.Tripathi AK, Prajapati V, Verma N, Bahl JR, Bansal RP, Khanuja SP, et al. Bioactivities of the leaf essential oil of Curcuma longa (var. ch-66) on three species of stored-product beetles (Coleoptera) J Econ Entomol. 2002;95(1):183–9. doi: 10.1603/0022-0493-95.1.183. [DOI] [PubMed] [Google Scholar]
- 223.Roth GN, Chandra A, Nair MG. Novel bioactivities of Curcuma longa constituents. J Nat Prod. 1998;61(4):542–5. doi: 10.1021/np970459f. doi:10.1021/np970459f np970459f [pii]. [DOI] [PubMed] [Google Scholar]
- 224.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch C. 2002;57(9-10):828–35. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 225.Joshi J, Ghaisas S, Vaidya A, Vaidya R, Kamat DV, Bhagwat AN, et al. Early human safety study of turmeric oil (Curcuma longa oil) administered orally in healthy volunteers. J Assoc Physicians India. 2003;51:1055–60. [PubMed] [Google Scholar]
- 226.Benny M, Antony B. Bioavailability of Biocurcumax (BCM – 095) Spice India. 2006:11–5. [Google Scholar]
- 227.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28(8):1765–73. doi: 10.1093/carcin/bgm123. doi:bgm123 [pii] 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 228.Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 1993;41(9):1640–3. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- 229.Naganuma M, Saruwatari A, Okamura S, Tamura H. Turmeric and curcumin modulate the conjugation of 1-naphthol in Caco-2 cells. Biol Pharm Bull. 2006;29(7):1476–9. doi: 10.1248/bpb.29.1476. [DOI] [PubMed] [Google Scholar]
- 230.Kapadia GJ, Azuine MA, Tokuda H, Hang E, Mukainaka T, Nishino H, et al. Inhibitory effect of herbal remedies on 12-O-tetradecanoylphorbol-13-acetate-promoted Epstein-Barr virus early antigen activation. Pharmacol Res. 2002;45(3):213–20. doi: 10.1006/phrs.2001.0936. [DOI] [PubMed] [Google Scholar]
- 231.Thapliyal R, Deshpande SS, Maru GB. Mechanism(s) of turmeric-mediated protective effects against benzo(a)pyrene-derived DNA adducts. Cancer Lett. 2002;175(1):79–88. doi: 10.1016/s0304-3835(01)00675-9. doi:S0304383501006759 [pii]. [DOI] [PubMed] [Google Scholar]
- 232.Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22(6C):4179–81. [PubMed] [Google Scholar]
- 233.Garg R, Ingle A, Maru G. Dietary turmeric modulates DMBA-induced p21ras, MAP kinases and AP-1/NF-kappaB pathway to alter cellular responses during hamster buccal pouch carcinogenesis. Toxicol Appl Pharmacol. 2008;232(3):428–39. doi: 10.1016/j.taap.2008.07.007. doi:S0041-008X(08)00307-4 [pii] 10.1016/j.taap.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 234.Villasenor IM, Simon MK, Villanueva AM. Comparative potencies of nutraceuticals in chemically induced skin tumor prevention. Nutr Cancer. 2002;44(1):66–70. doi: 10.1207/S15327914NC441_9. doi:10.1207/S15327914NC441_9. [DOI] [PubMed] [Google Scholar]
- 235.Thapliyal R, Naresh KN, Rao KV, Maru GB. Inhibition of nitrosodiethylamine-induced hepatocarcinogenesis by dietary turmeric in rats. Toxicol Lett. 2003;139(1):45–54. doi: 10.1016/s0378-4274(02)00440-x. doi:S037842740200440X [pii]. [DOI] [PubMed] [Google Scholar]
- 236.Azuine MA, Bhide SV. Adjuvant chemoprevention of experimental cancer: catechin and dietary turmeric in forestomach and oral cancer models. J Ethnopharmacol. 1994;44(3):211–7. doi: 10.1016/0378-8741(94)01188-5. [DOI] [PubMed] [Google Scholar]
- 237.Bhide SV, Azuine MA, Lahiri M, Telang NT. Chemoprevention of mammary tumor virus-induced and chemical carcinogen-induced rodent mammary tumors by natural plant products. Breast Cancer Res Treat. 1994;30(3):233–42. doi: 10.1007/BF00665965. [DOI] [PubMed] [Google Scholar]
- 238.Prasad NS, Raghavendra R, Lokesh BR, Naidu KA. Spice phenolics inhibit human PMNL 5-lipoxygenase. Prostaglandins Leukot Essent Fatty Acids. 2004;70(6):521–8. doi: 10.1016/j.plefa.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 239.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50(11):2191–3. doi: 10.1007/s10620-005-3032-8. doi:10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 240.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4(12):1502–6. doi: 10.1016/j.cgh.2006.08.008. doi:S1542-3565(06)00800-7 [pii] 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 241.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4(3):354–64. doi: 10.1158/1940-6207.CAPR-10-0098. doi:4/3/354 [pii] 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(7):1894–900. [PubMed] [Google Scholar]
- 243.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–54. doi: 10.1158/1078-0432.CCR-04-0744. doi:10/20/6847 [pii] 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 244.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14(1):120–5. doi:14/1/120 [pii]. [PubMed] [Google Scholar]
- 245••.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29(3):208–13. doi: 10.3109/07357907.2010.550592. doi:10.3109/07357907.2010.550592. This is very important clinical trial with curcumin showing that very low dose is effective in suppression of colorectal cancer.