Abstract
BACKGROUND
Blood pressure (BP) is highest during the day and lowest at night. Absence of this rhythm is a predictor of cardiovascular morbidity and mortality. Contributions of changes in posture and physical activity to the 24-hour day/night rhythm in BP are not well understood. We hypothesized that postural changes and physical activity contribute substantially to the day/night rhythm in BP.
METHODS
Fourteen healthy, sedentary, nonobese, normotensive men (aged 19–50 years) each completed an ambulatory and a bed rest condition during which BP was measured every 30–60 minutes for 24 hours. When ambulatory, subjects followed their usual routines without restrictions to capture the “normal” condition. During bed rest, subjects were constantly confined to bed in a 6-degree head-down position; therefore posture was constant, and physical activity was minimized. Two subjects were excluded from analysis because of irregular sleep timing.
RESULTS
The systolic and diastolic BP reduction during the sleep period was similar in ambulatory (−11±2mmHg/−8±1mmHg) and bed rest conditions (−8±3mmHg/−4±2mmHg; P = 0.38/P = 0.12). The morning surge in diastolic BP was attenuated during bed rest (P = 0.001), and there was a statistical trend for the same effect in systolic BP (P = 0.06).
CONCLUSIONS
A substantial proportion of the 24-hour BP rhythm remained during bed rest, indicating that typical daily changes in posture and/or physical activity do not entirely explain 24-hour BP variation under normal ambulatory conditions. However, the morning BP increase was attenuated during bed rest, suggesting that the adoption of an upright posture and/or physical activity in the morning contributes to the morning BP surge.
Keywords: ambulatory, bed rest, blood pressure, circadian, hypertension, sleep.
Ambulatory healthy adults exhibit a 24-hour rhythm in blood pressure (BP), with levels being highest during the daytime and lowest at nighttime.1 Impaired BP reduction at night (i.e., “nondipping”) is clinically relevant and is predictive of nonfatal and fatal cardiovascular events.2–4 Consequently, it is important to understand its origin. Day/night changes in behaviors such as posture, physical and mental activity, arousal (i.e., wake and sleep), and the internal circadian timing system may contribute to the 24-hour rhythm in BP.5–7 Daily changes in posture and physical activity are thought to be 2 major contributors to 24-hour BP variability.
The premise that daily changes in posture and physical activity are major contributors to 24-hour BP variability is based on the knowledge that BP is affected by dynamic exercise and transitions between postures (supine, sitting, and upright).8,9 Previous work has attempted to elucidate the effect of typical day/night changes in posture and physical activity on 24-hour BP rhythms. Athanassiadis et al.10 demonstrated a significant 24-hour BP rhythm in individuals confined to bed. However, the researchers did not compare their bed rest data to ambulatory recordings in the same individuals, thus they were unable to directly determine the influence of typical day/night changes in posture and physical activity on 24-hour BP variability. Mann et al.11 reported similar daytime BP but lower nighttime BP in ambulatory conditions compared to semi–bed rest conditions in hypertensive individuals. The semi–bed rest protocol was unable to properly determine the effect of daily changes in posture and physical activity on 24-hour BP rhythms because it did not maintain constant posture (subjects were not restricted to lying flat) and allowed subjects to leave the bed to go to the toilet and thus be physically active. Thus, no study has systematically investigated the effect of removing day/night changes in posture and physical activity on 24-hour BP levels. Therefore, our aim was to compare 24-hour BP variability in the same individuals in an ambulatory condition and in a bed rest condition that maintained constant posture and minimized physical activity. We hypothesized that daily changes in posture and physical activity contribute substantially to the day/night rhythm in BP.
METHODS
This study was part of 2 larger bed rest studies that have been published previously and described in detail.12,13 These studies examined 2 different exercise countermeasures to prevent cardiovascular deconditioning during bed rest. Only data from the control subjects who were inactive during bed rest are reported here.
Subjects
Fourteen healthy, sedentary, normotensive, nonsmoking, medication- and drug-free men (mean ± SD (range): age: 33±12 years (19–50 years); body mass index: 23.6±3.2 kg/m2 (20.7–26.2 kg/m2)) completed this study. Subjects had a normal echocardiogram at rest and under exercise stress. Subjects signed an informed consent form, and the study was approved by the institutional review boards of the University of Texas Southwestern Medical Center at Dallas and Presbyterian Hospital. Nine subjects completed the study protocol described by Hastings et al., 12 and 5 subjects finished the study reported by Shibata et al.13 The study protocols differ in that bed rest measurements occurred either 7 (Shibtata et al.13) or 14 (Hastings et al.12) days after the start of bed rest. Two subjects were excluded from analysis because of irregular sleep timing. We found no effect of study protocol (Hastings et al.12 vs. Shibata et al.13) on 24-hour BP and heart rate (HR) variation (i.e., study protocol did not influence any of our 24-hour BP and HR findings; P > 0.05).
Study design
Participants completed two 24-hour BP recordings, one under ambulatory conditions and one under bed rest conditions. Ambulatory recordings always preceded the bed rest measurements, and these were interspersed by 2–4 weeks.
Ambulatory condition
Subjects were freely ambulant and were instructed to undertake their “usual” daily activities. Few other instructions were given because this period was designed to capture normal ambulatory day/night variability in BP. Subjects chose their bedtime (10:00 pm to 2:00 am) and wake time (6:30 am to 10:00 am) and recorded this in a diary. The mean ± SD sleep period duration (from bedtime to wake time) was 8.3±0.7 hours.
Bed rest condition
Subjects undertook comprehensive cardiovascular function assessments over 2 days before beginning bed rest; for more details, see Hastings et al.12 and Shibata et al.13 Bed rest consisted of a constant 6-degree head-down tilt to simulate microgravity conditions. Subjects were permitted to elevate on one elbow to consume meals, but were otherwise confined to bed at all other times, including for personal hygiene. They were allowed to read, use a computer, or watch TV but were prohibited from any exercise. Wake and sleep periods were strictly monitored and controlled. Bedtimes (lights off) occurred between 9:30 pm and 12:00 am, and wake times (lights on) occurred between 5:00 am and 8:00 am and were recorded by the investigators. The mean ± SD sleep period duration was 8.1±0.4 hours. Bed rest was monitored with a bed alarm that would alert the nurses if the subject deviated from the head-down bed rest condition, although sleep per se was not monitored. Subjects consumed a standard 3-meal isocaloric diet, but fluid intake was ad libitium. Subjects stayed at the General Clinical Research Center at the University of Texas Southwestern Medical Center/Parkland Hospital.
Measurements
For 24 hours, BP and HR were measured with a clinical ambulatory BP monitor that uses a microphone over the brachial artery to detect Korotkoff sounds gated to the echocardiograph to avoid noise (Oscar 2 or Accutracker II; Suntech Medical Instruments, Morrisville, NC). These devices have been validated according to the Association for the Advancement of Medical Instrumentation’s standards.14,15 Measurements were made every 30 minutes during the scheduled wake period and every 60 minutes during the scheduled sleep period. Ambulatory recordings occurred 2–4 weeks before the in-laboratory visits.
Physical activity counts and energy expenditure were estimated in 7 subjects by an accelerometer (Actical, Respironics, OR, USA) worn on the subject’s waist. The subjects wore the device for 1–7 days before (freely ambulatory condition) and during the bed rest trial.
Data analysis and statistics
BP and HR measurements were averaged across 24 hours and over the wake and sleep periods. Sleep and wake periods were defined as the actual time periods that the subjects were in bed attempting to sleep and when they were out of bed and awake, respectively (ambulatory trial, times obtained from subject’s diary; bed rest trial, investigator-recorded times were used). We also analyzed the data separately for the day, defined as after 6 am and before 10 pm, and for the night, defined as between 10 pm and 6 am, as is often done in clinical trials in which no information is available regarding sleep timing, to permit comparison of our results with these trials. Average daily physical activity counts and energy expenditure were estimated using the accelerometer’s associated software.
Paired samples t tests were used to test if averaged variables varied between the ambulatory and bed rest conditions. Moreover, linear mixed model cosine analyses were performed on raw (i.e., not averaged) BP and HR data to further test for differences between conditions.16 The cosine analyses were performed on actual clock time of measurement (i.e., not binned or averaged) and included a condition factor (ambulatory trial vs. bed rest trial) and both fundamental (24 hour) and the second harmonic (12 hour) terms of the 24-hour day. Thus, the above cosine analysis tested the effect of condition and its interactions with the fundamental and second harmonic terms. Statistical tests were performed with SPSS for Windows, version 18 (SPSS inc., IL, USA). Statistical significance was accepted at P < 0.05. Data are presented as mean ± SEM.
RESULTS
Blood pressure and heart rate
Mean 24-hour systolic BP was similar in the ambulatory (119±2mmHg) and bed rest conditions (119±3mmHg; P = 0.87). There was also no difference in mean 24-hour diastolic BP between the ambulatory (68±2mmHg) and bed rest trials (69±3mmHg; P = 0.71). Mean 24-hour HR was similar in the ambulatory (70±2 beats/min) and bed rest condition (68±1 beats/min; P = 0.16).
Mean wake period systolic BP was similar in the ambulatory (122±2mmHg) and bed rest conditions (121±3mmHg; P = 0.67) (Figure 1a). There was also no difference in mean wake period diastolic BP between the ambulatory (70±2mmHg) and bed rest trials (69±3mmHg; P = 0.92) (Figure 1b). Mean wake period HR was 4±2 beats/min higher in the ambulatory trial than in the best rest trial (P = 0.049) (Figure 1c). See Supplementary Figure S1b, d, and f for each subject’s wake period data for systolic BP, diastolic BP, and HR under ambulatory and bed rest conditions.
Figure 1.
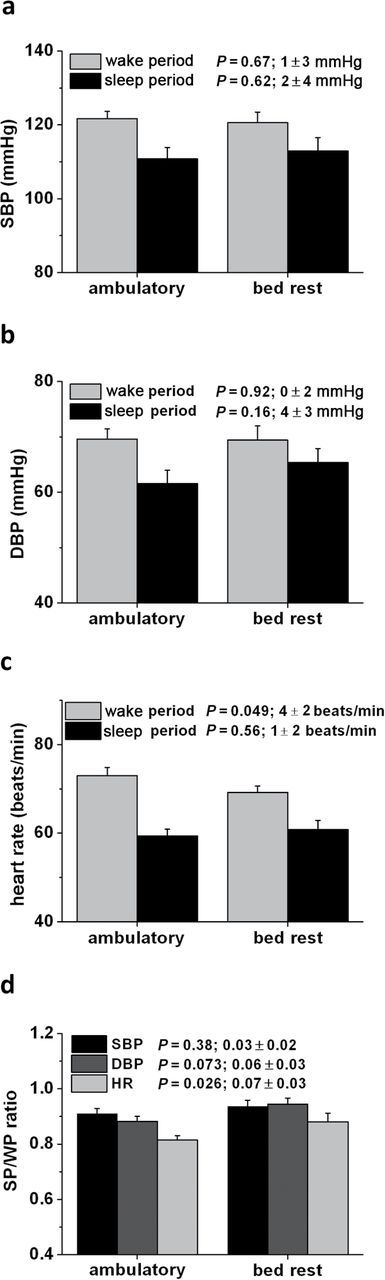
Blood pressure and heart rate measured during wake periods and sleep periods under ambulatory and bed rest conditions. (a) Systolic blood pressure (SBP) data. (b) Diastolic blood pressure (DBP) data. (c) Heart rate data. (d) Sleep period (SP)/wake period (WP)ratios. P values are given for comparison between ambulatory and bed rest trials. The additional data next to the P values indicates mean ± SEM difference between conditions.
Mean sleep period systolic BP was similar in the ambulatory (111±3mmHg) and bed rest conditions (113±4mmHg; P = 0.62) (Figure 1a). Mean sleep period diastolic BP was not statistically different in the ambulatory (62±2mmHg) and bed rest trials (65±3mmHg; P = 0.16) (Figure 1b). Sleep period HR was similar in the ambulatory (59±2 beats/min) and bed rest trials (61±2 beats/min; P = 0.56) (Figure 1c). See Supplementary Figure S1a, c, and e for each subject’s sleep period data for systolic BP, diastolic BP, and HR under ambulatory and bed rest conditions.
The systolic/diastolic BP decrease from the wake period to the sleep period was not significantly different between the ambulatory trial (by 11±2mmHg/8±1mmHg) and bed rest trial (by 8±3mmHg/4±2mmHg; P = 0.38/P = 0.12). The HR decrease from the wake period to the sleep period was significantly larger during the ambulatory trial (by 14±1 beats/min) than during the bed rest trial (by 8±2 beats/min; P = 0.01). Sleep period/wake period ratios for systolic BP, diastolic BP, and HR were 0.91±0.02, 0.88±0.02, and 0.81±0.02 in the ambulatory trial and 0.94±0.02 (P = 0.38), 0.94±0.02 (P = 0.07), and 0.88±0.03 (P = 0.03) in the bed rest trial, respectively (Figure 1d). See Supplementary Figure S2 for each subject’s sleep period/wake period difference in systolic BP, diastolic BP, and HR under ambulatory and bed rest conditions.
Similar results were found when data were differentiated by day (after 6 am and before 10 pm) and night (between 10 pm and 6 am) rather than wake and sleep periods (see Supplementary Figure S3).
Twenty-four hour and hourly average SBP, DBP, and HR values for both trials are depicted in Figure 2a, c, and e.
Figure 2.
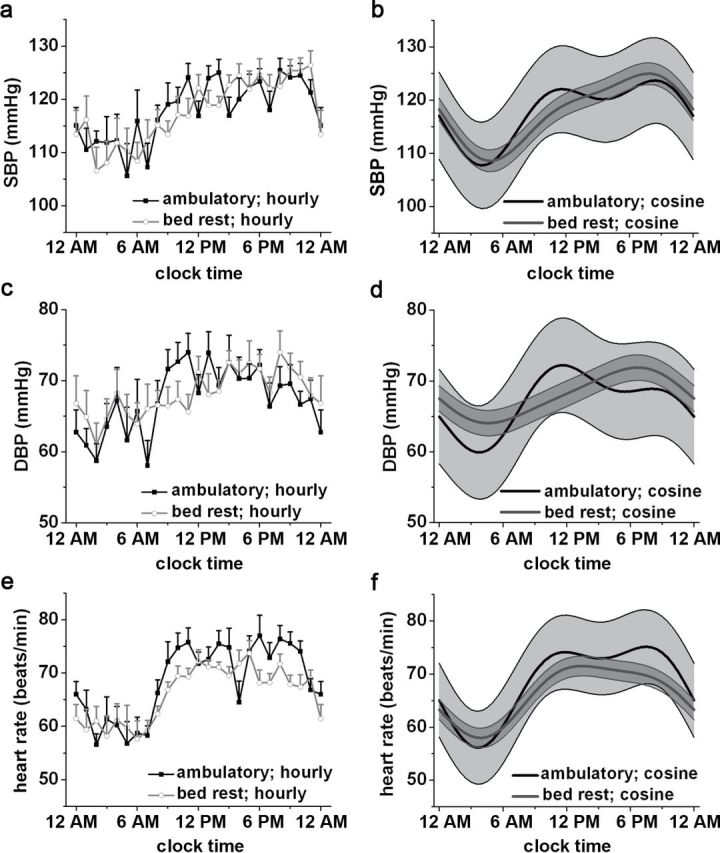
Twenty-four-hour blood pressure and heart rate profiles under ambulatory and bed rest conditions. (a and b) Systolic blood pressure (SBP) data. (c and d) Diastolic blood pressure (DBP) data. (e and f) Heart rate data. (a, c, e) Hourly plots of data (mean ± SEM). (b, d, f) Cosinor fits (mean ± 95% confidence interval). See text for P values.
Cosinor analysis.
Overall (i.e., in both conditions), significant 24-hour and 12-hour rhythms in systolic BP (fundamental: P < 0.0001; second harmonic: P < 0.0001), diastolic BP (fundamental: P < 0.0001; second harmonic: P = 0.004), and heart rate (fundamental: P < 0.0001; second harmonic: P < 0.0001) were observed (Figure 2b, d, and f). The time of the peak/trough for systolic BP, diastolic BP, and HR was 8:30 pm/4:00 am, 11:30 am/3:30 am, 7:30 pm/3:30 am in the ambulatory trial and 8:00 pm/5:00 am, 6:30 pm/4:30 am, 1:30 pm/4:00 am in the bed rest condition, respectively. There was no main effect of condition on systolic BP (P = 0.92) or diastolic BP (P = 0.16), indicating no significant difference in average systolic BP and diastolic BP between trials. A significant main effect of condition for heart rate was observed (P = 0.001), with higher values occurring in the ambulatory trial than the bed rest trial. The morning surge in diastolic BP was attenuated in the bed rest trial (trial*fundamental component; P = 0.001), and there were statistical trends for the same effect in systolic BP (P = 0.06) and heart rate (P = 0.06). There was a significant interaction between the second harmonic and condition for heart rate (P = 0.01), statistical trend for diastolic BP (P = 0.08), and no evidence for an effect for systolic BP (P = 0.13).
Physical activity
Average daily accelerometer counts were reduced (193,514±15,588 to 61,687±5,313) during bed rest (P = 0.0003). Estimated energy expended by physical activity was also markedly and significantly reduced by approximately 67% in the bed rest condition (158±16 kcal/day) compared with the ambulatory condition (480±40 kcal/day; P = 0.0003). The above findings highlight that physical activity was significantly reduced and minimized in our bed rest condition.
DISCUSSION
The primary new finding from this study is that, contrary to our hypothesis, 24-hour BP variation was largely the same in healthy individuals regardless of whether they were under ambulatory or strict bed rest conditions. Given that the bed rest protocol maintained constant posture and minimized physical activity, these findings indicate that typical day/night changes in posture and physical activity do not entirely explain the 24-hour rhythm in BP. Conceivably, other behavioral cycles (e.g., wake/sleep and feeding/fasting rhythms) and/or the endogenous circadian timing system must be major mechanisms underlying the normal 24-hour BP variation. However, we did observe an attenuation of the rate and magnitude of the morning increase in BP (particularly diastolic) in the bed rest trial, suggesting the adoption of an upright posture and/or physical activity upon awakening during normal ambulatory conditions contributes to the morning surge in BP.
This is the first study comparing 24-hour BP recordings within the same individuals in both freely ambulatory and strict bed rest conditions. Mann et al.11 reported that daytime BP was the same in ambulatory and partial bed rest conditions, but BP was higher at night in the semi–bed rest trial. The higher nighttime BP may have been because of the disturbed sleep that occurs during the first night of sleeping in a hospital/laboratory.17 This adverse first-night effect on sleep diminishes over a few consecutive sleep periods. Our sleep period BP recordings were obtained either on the 7th or 14th in-laboratory night; thus our findings are significantly less likely to be effected by poor in-laboratory sleep. Fritsch-Yelle et al.18 compared 24-hour BP variability in humans under normal ground conditions and while in space, where BP effects of posture are negated and the level of physical activity is reduced. The reduction in BP during sleep was smaller in space, which contrasts our findings, where no significant difference between ambulatory and bed rest conditions was found in regard to sleep period BP reduction. It is difficult to pinpoint why our findings differ, but the attenuated reduction in sleep BP in space is likely associated with disturbed sleep during space missions.19 Athanassiadis et al.10 also reported a day/night rhythm in BP in bed rest conditions, although ambulatory measurements were not made. These findings, like our own, suggest that 24-hour BP variation is not majorly influenced by day/night changes in posture and physical activity (i.e., routine daily activity that does not include structured exercise). However, we did find evidence for the morning increase in systolic (trend) and diastolic BP being attenuated in the bed rest trial, indicating that the transition to an upright posture and/or physical activity upon awakening is important for the morning increase in BP under ambulatory conditions.
Posture is traditionally thought as a behavior that significantly contributes to the 24-hour rhythm in BP. This concept stems from studies demonstrating that BP changes when transitioning between supine, sitting, and standing postures.8 Dynamic exercise, even of low intensity (e.g., walking), raises BP.9 Thus, day/night changes in posture and physical activity are often thought to explain the 24-hour rhythm in BP. Our subjects were sedentary (i.e., not engaged in regular exercise) and thus probably mainly performed light activities during the ambulatory trial. Despite this, it was expected that constant posture and minimization of physical activity during complete bed rest would abolish or severely blunt the 24-hour rhythm in BP, but surprisingly we only found evidence for attenuation in the morning systolic (trend) and diastolic BP surge, and an overall 24-hour rhythm in BP was still present
Under ambulatory conditions, BP decreases during sleep.1 It is not clear if sleep decreases BP irrespective of concurrent changes in behaviors such as posture and physical activity and independent of internal circadian rhythms. Nevertheless, BP does vary with changes in sleep architecture.20 Relative to wake, BP decreases as sleep becomes deeper (stage 1 to slow-wave sleep). During sleep, BP is highest during rapid eye movement sleep, but it is still lower than during wakefulness. In the abovementioned study, the mean decrease in systolic BP and diastolic BP during slow wave sleep was approximately 15mmHg and approximately 10mmHg, respectively. Although slow-wave sleep only makes up a small percentage of the whole sleep episode, such large reductions during slow-wave sleep and smaller reductions during lighter stages of sleep could help explain the decrease in BP during sleep in both the ambulatory and bed rest conditions of this study.
The internal circadian timing system, independent of exogenous factors (e.g., sleep, activity, and feeding), has been reported to influence BP by some researchers,5–7,21 but not all.22,23 The 2 latter studies22,23 may not have detected an endogenous circadian rhythm in BP because of methodological limitations (for details, see Shea et al.6). The endogenous circadian timing system causes a peak in the biological evening (corresponding to approximately 9 pm) and a decrease across the biological night (habitual rest period in humans). Peak-to-trough amplitudes are relatively small, between 2 and 6mmHg. Thus, this rhythm, to some degree, may contribute to the fall in BP observed during the sleep period and the evening peak in BP during the bed rest study. Endogenous circadian rhythms are present in many physiological variables (e.g., cortisol, catecholamines, cardiac vagal modulation, and HR) that normally regulate BP.6,24 However, the timing of these rhythms does not align with the peak in BP, suggesting other unknown control mechanisms are involved.
Mean wake period HR was lower (4 beats/min) in the bed rest trial than in the ambulatory condition. Cosine analysis also revealed HR to be higher in the ambulatory trial than in the bed rest trial, particularly during the daytime. This finding is not unexpected, as HR is well-known to be sensitive to changes in posture and physical activity,25 both of which were permissible in the ambulatory trial but strictly minimized in the bed rest trial. Despite the difference in HR between conditions, average BP was similar in the ambulatory and best rest trials. This suggests that normal daily changes in BP are not dramatically influenced by small changes in HR. The daily rhythm in HR in the bed rest condition is somewhat similar to what is caused by the endogenous circadian system per se (i.e., a peak in the biological afternoon/early evening and a trough in the biological night).5–7,26
Our study has limitations that should be considered when interpreting the findings. First, our study only included normotensive individuals and had a small sample size. Thus, our findings may not be representative for diseased populations, and extrapolation to patients with hypertension should be done with caution. For this purpose, this study should be replicated with a larger sample that includes hypertensives. Second, ambulatory systolic BP and diastolic BP decreased by 9% ± 2% and 12% ± 2% during sleep periods, respectively, which is somewhat lower than typically expected in normal populations. Therefore, our subjects appeared to have somewhat attenuated 24-hour BP profiles. This attenuation could decrease the chance of detecting a blunting effect of bed rest on BP variation and increase the chance of a type 2 error. Third, we had no measure of posture during the ambulatory recordings. Thus, we are unable to objectively compare posture between our ambulatory and bed rest conditions. Fourth, sleep was not assessed; therefore it is not clear if total sleep time and structure were similar in both trials. Despite this uncertainty, we observed no BP differences between both trials. Fifth, our findings may have been confounded by adverse effects of prolonged 6-degree head-down bed rest on cardiovascular function. Moreover, subjects were studied either after 7 or 14 days of bed rest, but this difference had no effect on our results.
Despite a marked reduction in physical activity and elimination of changes in posture, a large 24-hour BP rhythm was still present in our bed rest trial, indicating that typical daily changes in posture and/or physical activity do not entirely explain 24-hour BP variation under normal ambulatory conditions. Our findings suggest that day/night changes in other factors than posture and physical activity (e.g., sleep and the internal circadian timing system) substantially contribute to the 24-hour BP rhythm in normal individuals. There was, however, a significant attenuation of the morning surge in BP during bed rest, suggesting that the transition from a supine position to an upright position and/or physical activity in the morning contributes to the morning BP surge.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
B.D. Levine and F.A.J.L. Scheer were co–senior authors on this paper and provided substantial direction of the analysis and interpretation of the data. This study was supported by National Aeronautics and Space Administration (NASA) grant NAS 96-OLMSA-01B, National Space Biomedical Research Institute (NSBRI) grant NNH047ZUU003N to B.D.L., and the University of Texas Southwestern Clinical and Translational Research Center (formerly General Clinical Research Center) RR-00804. C.J.M. was supported by the NSBRI through NASA NCC 9–58. J.A.H. was supported by American Heart Association postdoctoral fellowship grant 0525077Y. F.A.J.L.S. was supported by National Institutes of Health grants P30-HL101299 and R01-HL094806. We are grateful for the assistance of Julie H. Zuckerman, RN, and Daniel J. Creson, RN, for the analysis of the ambulatory BP recordings and all the nurses of the Clinical and Translational Research Center for their outstanding care during the bed rest studies. J.A. Hastings is currently in the Division of Cardiology at the Veterans Administration Medical Center, Dallas, TX, and M.A. Perhonen is currently in the Department of Preventive Medicine, Jyvaskyla, Finland.
REFERENCES
- 1. Bevan AT, Honour AJ, Stott FH. Direct arterial pressure recording in unrestricted man. Clin Sci 1969; 36: 329–344 [PubMed] [Google Scholar]
- 2. Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring. Hypertension 2007; 49: 1235–1241 [DOI] [PubMed] [Google Scholar]
- 3. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population-based study. Am J Hypertens 2006; 19: 243–250 [DOI] [PubMed] [Google Scholar]
- 4. Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens 2009; 23: 645–653 [DOI] [PubMed] [Google Scholar]
- 5. Hu K, Scheer FAJL, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 2011; 123: 961–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shea SA, Hilton MF, Hu K, Scheer FAJL. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circulation Res 2011; 108: 980–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA 2010; 107: 20541–20546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gellman M, Spitzer S, Ironson G, Llabre M, Saab P, DeCarlo Pasin R, Weidler DJ, Schneiderman N. Posture, place, and mood effects on ambulatory blood pressure. Psychophysiology 1990; 27: 544–551 [DOI] [PubMed] [Google Scholar]
- 9. Wolthuis RA, Froelicher VF, Jr, Fischer J, Triebwasser JH. The response of healthy men to treadmill exercise. Circulation 1977; 55: 153–157 [DOI] [PubMed] [Google Scholar]
- 10. Athanassiadis D, Draper GJ, Honour AJ, Cranston WI. Variability of automatic blood pressure measurements over 24 hour periods. Clin Sci 1969; 36: 147–156 [PubMed] [Google Scholar]
- 11. Mann S, Craig MW, Melville DI, Balasubramanian V, Raftery EB. Physical activity and the circadian rhythm of blood pressure. Clin Sci (Lond) 1979; 57: 291S–294S [DOI] [PubMed] [Google Scholar]
- 12. Hastings JL, Krainski F, Snell PG, Pacini E, Jain M, Bhella PS, Shibata S, Fu Q, Palmer MD, Levine BD. The effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol 2012; 112:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol 2010; 108: 1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor R, Chidley K, Goodwin J, Broeders M, Kirby B. Accutracker II (version 30/23) ambulatory blood pressure monitor: clinical validation using the British Hypertension Society and Association for the Advancement of Medical Instrumentation standards. J Hypertens 1993; 11: 1275–1282 [PubMed] [Google Scholar]
- 15. Goodwin J, Finn P, Bilous M, Winship S, Jones S. Validation of the OSCAR 2 ambulatory blood pressure monitor according to the the association for the advancement of medical instrumentation (AAMI) protocol. Am J Hypertens 2005; 18(S4):31A [Google Scholar]
- 16. Nelson W, Tong YL, Lee J, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia 1979; 6: 305–323 [PubMed] [Google Scholar]
- 17. Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiol 1966; 2: 263–266 [DOI] [PubMed] [Google Scholar]
- 18. Fritsch-Yelle JM, Charles JB, Jones MM, Wood ML. Microgravity decreases heart rate and arterial pressure in humans. J Appl Physiol 1996; 80: 910–914 [DOI] [PubMed] [Google Scholar]
- 19. Dijk DJ, Neri DF, Wyatt JK, Ronda JM, Riel E, Ritz-De Cecco A, Hughes RJ, Elliott AR, Prisk GK, West JB, Czeisler CA. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am J Physiol 2001; 281: R1647–R1664 [DOI] [PubMed] [Google Scholar]
- 20. Coccagna G, Mantovani M, Brignani F, Manzini A, Lugaresi E. Arterial pressure changes during spontaneous sleep in man. Electroencephalogr Clin Neurophysiol 1971; 31: 277–281 [DOI] [PubMed] [Google Scholar]
- 21. Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res 2012; 199: 337–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kerkhof GA, Van Dongen HPA, Bobbert AC. Absence of endogenous circadian rhythmicity in blood pressure? Am J Hypertens 1998; 11: 373–377 [DOI] [PubMed] [Google Scholar]
- 23. Van Dongen HPA, Maislin G, Kerkhof GA. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiol Int 2001; 18: 85–98 [DOI] [PubMed] [Google Scholar]
- 24. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol 2012; 349: 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rowell LB. Human Cardiovascular Control Oxford University Press: New York: 1993. [Google Scholar]
- 26. Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol Heart Circ Physiol 1997; 273: H1761–H1768 [DOI] [PubMed] [Google Scholar]


