Abstract
BACKGROUND
Although higher visit-to-visit variability (VVV) of blood pressure (BP) is associated with increased cardiovascular disease risk, the physiological basis for VVV of BP is incompletely understood.
METHODS
We examined the associations of aortic distensibility (assessed by magnetic resonance imaging) and artery elasticity indices (determined by radial artery pulse contour analysis) with VVV of BP in 2,640 and 4,560 participants, respectively, from the Multi-Ethnic Study of Atherosclerosis. Arterial measures were obtained at exam 1. BP readings were taken at exam 1 and at 3 follow-up visits at 18-month intervals (exams 2, 3, and 4). VVV was defined as the SD about each participant’s mean systolic BP (SBP) across visits.
RESULTS
The mean SDs of SBP were inversely associated with aortic distensibility: 7.7, 9.9, 10.9, and 13.2mm Hg for quartiles 4, 3, 2, and 1 of aortic distensibility, respectively (P trend < 0.001). This association remained significant after adjustment for demographics, cardiovascular risk factors, mean SBP, and antihypertensive medication use (P trend < 0.01). In a fully adjusted model, lower quartiles of large artery and small artery elasticity (LAE and SAE) indices were also associated with higher mean SD of SBP (P trend = 0.02 for LAE; P trend < 0.001 for SAE).
CONCLUSIONS
In this multiethnic cohort, functional alterations of central and peripheral arteries were associated with greater long-term VVV of SBP.
Keywords: arteries, blood pressure, epidemiology, hypertension, vasculature.
There is increasing evidence to suggest that visit-to-visit variability (VVV) of blood pressure (BP) has prognostic value, independent of average BP. Increased VVV of BP is associated with a higher risk of cardiovascular events1–4 and all-cause mortality.5,6
Presently, the mechanisms underlying higher levels of VVV in BP are unknown. Because increased pulse pressure is associated with higher levels of VVV of BP, it has been hypothesized that alterations in the elastic properties of the arterial tree may contribute to higher levels of VVV of BP.1,6,7 However, few data are available on the association between functional vascular alterations and VVV of BP.
Because of its elastic properties and proximity to vital organs such as the heart, brain, and kidneys, the aorta modulates the entire vascular tree, buffering the pulsatile cardiac output to provide constant flow to the microvascular system including capillary beds. Therefore, reduced aortic distensibility may be associated with a higher level of VVV of BP. Although decreased aortic distensibility is associated with microvascular dysfunction,8 it is unclear whether reduced small arterial elasticity (SAE) is also associated with a higher level of VVV of BP. We, therefore, evaluated whether aortic distensibility and large arterial elasticity (LAE) and SAE are associated with VVV of BP in a multiethnic, population-based cohort of middle-aged and elderly men and women.
METHODS
Study population
This analysis included participants from the Multi-Ethnic Study of Atherosclerosis (MESA), a population-based study of 6,814 community-dwelling adults aged 45–84 years and free of clinically evident cardiovascular disease at baseline. Details of the MESA study design are described elsewhere.9 Participants from 4 ethnic groups (white, black, Hispanic, and Asian primarily of Chinese descent) were recruited from 6 US communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, Minnesota). The study was approved by the institutional review boards of all participating sites, and written informed consent was obtained from all participants.
Clinical and biochemical measures were obtained at exam 1 and subsequent MESA exams (exams 2–4), which were conducted at 18-month intervals. Detailed information about the measures is provided at the MESA website (http://mesa-nhlbi.org/moreinfo.aspx). The Supplementary Methods provides details on how the cardiovascular risk factors at exam 1, which were used in our analyses, were ascertained and defined.
MESA participants underwent a cardiac magnetic resonance imaging (MRI) scan at exam 1.10 Thoracic aortic distensibility determined by MRI was measured in a subset that included 3,541 participants. In addition, 6,336 participants successfully underwent measurement of LAE and SAE indices determined by radial artery pulse wave analysis at exam 1. BP data from exams 1–4 were used for the estimation of VVV of BP (see below). For these analyses of aortic distensibility, participants who did not have BP data available from all 4 exams (n = 584) or were missing covariable data (n = 317) were excluded. Similarly, for analyses of artery elasticity, participants who did not have BP data available from all 4 exams (n = 1,232) or were missing covariable data (n = 544) were excluded. After these exclusions, 2,640 participants were included in the analyses of aortic distensibility and VVV of BP, and 4,560 participants were included in the analyses of arterial elasticity and VVV of BP. The distribution of age, sex, and body mass index for MESA participants included and not included in these analyses are presented in Supplementary Table S1.
Thoracic aortic distensibility assessed by MRI (exam 1)
Aortic distensibility was assessed using 1.5-T whole-body MRI systems, Signa CV/I, or Signa LX (General Electric Medical Systems, Wisconsin), as previously described.11,12 MRI scans of the thoracic aorta were obtained using gradient-echocardiographic phase-contrast cine MRI with electrocardiographic gating. Images of the aorta were obtained in the transverse plane at the level of the right pulmonary artery perpendicular to the vessel lumen.
Distensibility of the ascending aorta was calculated using the following formula: (maximum cross-sectional area − minimum cross-sectional area)/(minimum cross-sectional area × pulse pressure). Noninvasive brachial BP was measured immediately before and after the MRI aortic measurements while the patient was in the supine position in the MRI scanner using a nonferromagnetic arm blood pressure cuff; the average systolic and diastolic values were used to calculate pulse pressure, which was defined as SBP minus diastolic BP. The maximum and minimum areas of the ascending aorta were determined using an automated contour routine using the software FLOW (Medis Medical Imaging Systems, Raleigh, NC). Aortic distensibility determined by MRI is highly reproducible with coefficient of variation for intraobserver and interobserver variability of 1% and 2%, respectively.13
LAE and SAE indices (exam 1)
LAE and SAE indices (in ml/mm Hg) were assessed using the PulseWave CR-2000 Research CardioVascular Profiling Instrument (Hypertension Diagnostics, Eagan, MN), which analyzes radial artery pulse waveforms. This methodology has been validated for the assessment of arterial elasticity14–19 and is highly reproducible.14,20,21 While the participant was supine, the pulse pressure sensor was positioned on the right wrist, supported by a wrist stabilizer, and measurements were taken for 30 seconds. An automated, oscillatory BP measurement was performed at the contralateral arm. A computer-based, third-order, 4-element Windkessel model was used to calculate an elasticity index for both large (C1) and small (C2) arteries by analyzing the diastolic pulse contour. The values estimated directly from the waveform were X and Y for LAE and SAE, respectively. LAE and SAE indices were then estimated by dividing each of X and Y by systemic vascular resistance. Systemic vascular resistance was calculated by dividing the mean arterial pressure by the cardiac output. Cardiac output was estimated from the ejection time determined from the pulse waveform, heart rate, age, height, and weight. The correlations for repeat measurements are 0.74 for LAE index and 0.84 for SAE index.22
BP measurements and antihypertensive medication use (exams 1–4)
At each exam, BP was measured 3 times at 2-minute intervals using an automated oscillometric device (Dinamap Monitor Pro 100, GE Healthcare, Milwaukee, WI) after participants had rested for 5 minutes in the seated position. Appropriate-sized cuffs were used for BP assessment. BP at each exam was defined as the average of the second and third readings. Participants were also asked to bring their medications to each exam, and antihypertensive drug use was determined using a medication inventory. The following classes were considered to be antihypertensive medications: diuretics, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, and alpha-blockers or other peripheral vasodilators.
Statistical analyses
The primary outcome of VVV of BP was defined as the SD about the participant’s mean SBP1,6 across exams 1–4. Because aortic distensibility, LAE, and SAE had skewed distributions, the study population was divided into quartiles based on the distribution of each measure. Sample characteristics and the mean level of SD of SBP were calculated by quartile of aortic distensibility. Unadjusted differences in mean SD of SBP by quartile of aortic distensibility were estimated using a linear regression model with the highest quartile (quartile 4) serving as the referent group. Additional models with multivariable adjustment for covariables that might be related to aortic distensibility and SD of SBP were also fitted. In addition to demographics (age, sex, and ethnicity) and MESA site, which were included in model 1, the following exam 1 covariables (all chosen a priori) were included in model 2: body mass index, diabetes, low-density lipoprotein and high-density lipoprotein cholesterol levels, cigarette smoking, education level (high school or above), physical activity, reduced estimated glomerular filtration rate, and C-reactive protein. Physical activity and C-reactive protein levels were natural log transformed because of skewed distributions. Subsequent models included additional adjustment for mean SBP across exams 1–4 (model 3) and antihypertensive medication use across exams 1–4 (model 4). Antihypertensive medication use across visits was modeled as never (not on antihypertensive medications at any exams), always (on antihypertensive medications at all exams), and sometimes (on antihypertensive medications at some but not all exams). Linear trends across quartiles were assessed by including the quartile-specific median aortic distensibility values as a continuous variable in the regression models. These analyses were repeated for the LAE and SAE indices.
Several sensitivity analyses were performed. First, prevalence ratios for the outcome of being in the highest quartile of SD of SBP (≥13.48mm Hg), associated with quartiles of aortic distensibility, LAE, and SAE, were calculated using log-binomial regression models with quartile 4 of each arterial measure serving as the referent group. Second, the associations of quartiles of aortic distensibility and LAE and SAE indices with other VVV measures, including coefficient of variation and variation independent of the mean,1,6 were assessed. Third, the associations between aortic distensibility, LAE, and SAE, expressed as continuous variables, and SD of SBP as a continuous variable were evaluated. Because of skewed distributions, aortic distensibility, LAE, and SAE were natural log transformed.
Pulse pressure and mean arterial pressure are both partially determined by mean SBP. Because pulse pressure is used to derive aortic distensibility, and age, height, weight, and mean arterial pressure are used to derive LAE and SAE indices, variance inflation factors were calculated to examine the possible existence of multicollinearity between aortic distensibility and mean SBP and multicollinearity among both arterial elasticity indices (LAE and SAE), age, body mass index, and mean SBP. The variance inflation factors were all <2.3, indicating multicollinearity was not present among the predictors.23
Statistical significance was defined by α = 0.05 level, 2-tailed. Statistical analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC) and SPSS 18.0 (SPSS, Chicago, IL).
RESULTS
Table 1 shows the characteristics of the sample according to aortic distensibility quartiles. Older age, female sex, black ethnicity, lower education level, diabetes, higher high-density lipoprotein levels, higher C-reactive protein levels, reduced estimated glomerular filtration rate, lower physical activity, antihypertensive medication use, and higher mean SBP and diastolic BP levels were associated with lower aortic distensibility quartiles. Chinese ethnicity was associated with higher aortic distensibility. Supplementary Tables S2 and S3 show the characteristics of the sample according to quartiles of LAE and SAE indices, respectively.
Table 1.
Characteristics of Multi-Ethnic Study of Artherosclerosis participants included in the analysis of thoracic aortic distensibility and SD of systolic blood pressure
| Quartiles of aortic distensibility | |||||
|---|---|---|---|---|---|
| Characteristics | Q4 (n = 660) | Q3 (n = 660) | Q2 (n = 660) | Q1 (n = 660) | P trend |
| Aortic distensibility, 10−3/mm Hg | >2.34 | 1.59–2.34 | 1.07–1.58 | <1.07 | — |
| Mean age (SD), years | 53.7 (7.7) | 58.2 (8.7) | 62.9 (9.1) | 66.2 (8.7) | <0.001 |
| Female, % | 52.7% | 50.2% | 55.9% | 58.8% | <0.01 |
| Race/ethnicity | |||||
| White (referent), % | 43.2% | 44.1% | 43.6% | 41.4% | — |
| Chinese American, % | 16.2% | 11.8% | 10.9% | 9.1% | <0.01 |
| Black, % | 22.1% | 27.1% | 31.8% | 34.2% | <0.001 |
| Hispanic, % | 18.5% | 17.0% | 13.6% | 15.3% | 0.19 |
| High school education or above, % | 90.5% | 87.9% | 87.3% | 84.1% | <0.01 |
| Current smoker, % | 11.4% | 12.9% | 12.4% | 10.3% | 0.52 |
| Diabetes, % | 5.6% | 7.6% | 10.0% | 12.4% | <0.001 |
| Mean BMI (SD), kg/m2 | 27.7 (4.9) | 28.1 (5.2) | 27.5 (4.9) | 27.8 (4.8) | 0.69 |
| Mean LDL-cholesterol (SD), mg/dl | 118.8 (31.2) | 117.5 (30.4) | 116.7 (30.5) | 116.7 (31.0) | 0.18 |
| Mean HDL-cholesterol (SD), mg/dl | 51.0 (14.7) | 51.3 (15.8) | 53.0 (15.7) | 52.9 (14.3) | <0.01 |
| Median CRP (25-75th percentile), mg/L | 1.6 (0.7–3.8) | 1.8 (0.8–3.8) | 1.8 (0.8–4.0) | 2.1 (0.8–4.4) | <0.01 |
| Mean eGFR (SD), mg/dl | 83.9 (15.7) | 83.1 (16.8) | 79.8 (15.9) | 78.8 (18.2) | <0.001 |
| Reduced eGFRa | 3.5% | 6.1% | 9.4% | 13.5% | <0.001 |
| Median physical activity level (25th–75th percentile), 1000 METs-min/wk | 4.6 (2.4–7.9) | 4.6 (2.3–8.0) | 4.2 (2.3–8.4) | 3.9 (2.0–7.1) | <0.01 |
| Antihypertensive medicationsb | |||||
| Never taking across exams (referent), % | 70.0% | 55.0% | 43.2% | 29.4% | — |
| Taking across all exams, % | 15.3% | 23.8% | 32.9% | 44.2% | <0.001 |
| Sometimes taking, % | 14.7% | 21.2% | 23.9% | 26.4% | <0.001 |
| Mean SBP (SD), mm Hg | 112.9 (13.8) | 120.2 (14.9) | 125.2 (17.0) | 131.0 (16.9) | <0.001 |
| Mean DBP (SD), mm Hg | 69.2 (8.4) | 71.0 (8.6) | 70.3 (8.6) | 71.2 (8.8) | 0.001 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; METs = metabolic equivalent values; SBP, systolic blood pressure.
aeGFR < 60ml/min/1.73 m2.
bParticipants were classified as: never taking across exams (on no antihypertensive medications at all exams), taking across all exams (on antihypertensive medications at all exams), and sometimes taking across exams.
Association between Aortic Distensibility and VVV of BP
For the aortic distensibility analysis (n = 2,640), the mean of the SD of SBP was 10.4mm Hg (SD = 6.5). Figure 1 (upper panel) shows mean and 95% confidence interval (CI) for SD of SBP by quartile of aortic distensibility (quartile 4: 7.7mm Hg; quartile 3: 9.9mm Hg; quartile 2: 10.9mm Hg; and quartile 1: 13.2mm Hg). Compared with participants in the highest quartile (quartile 4), those in the lower quartiles of aortic distensibility had higher mean SD of SBP (Table 2). After adjustment for age, sex, ethnicity, and MESA site (model 1, Table 2) and additional adjustment for body mass index, diabetes, low-density lipoprotein and high-density lipoprotein cholesterol levels, cigarette smoking, education level, physical activity, reduced estimated glomerular filtration rate, and C-reactive protein (model 2, Table 2), these associations remained statistically significant (P trend < 0.001 for both models 1 and 2). After further adjustment for mean SBP across exams 1–4 (model 3, Table 2) and antihypertensive medication use across exams 1–4 (model 4, Table 2), the association between aortic distensibility and SD of SBP was attenuated but remained statistically significant.
Figure 1.
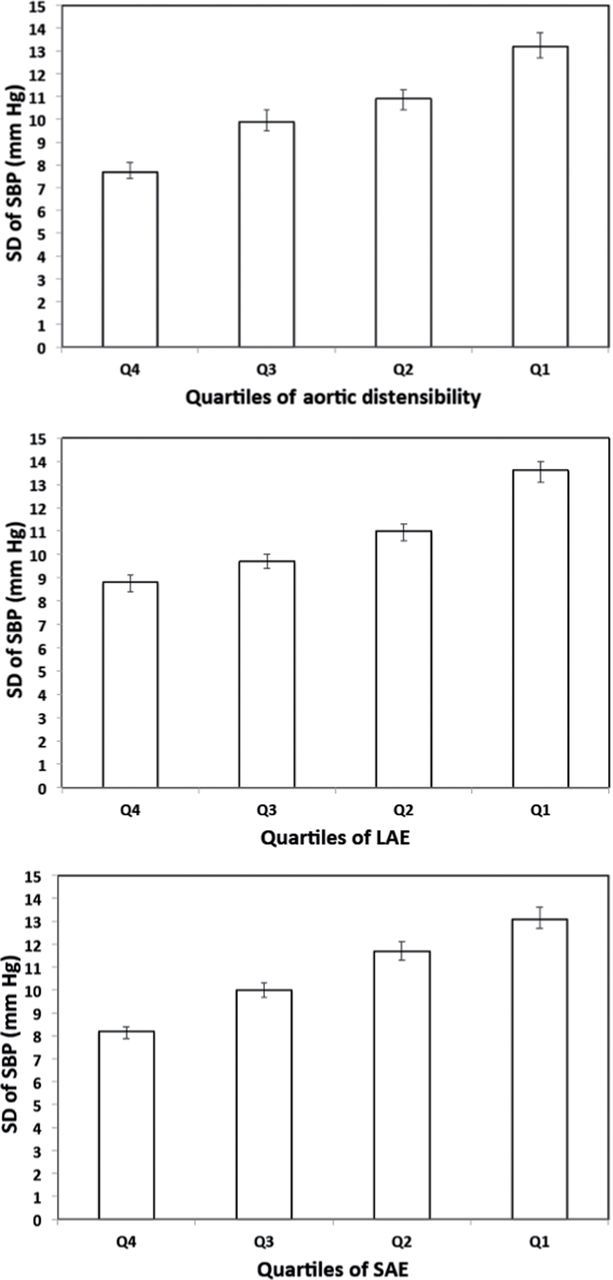
SD of systolic blood pressure (SBP) across exams 1–4 by quartile of aortic distensibility (top panel), large artery distensibility (LAE) index (middle panel), and small artery distensibility (SAE) index (bottom panel). Data are expressed as mean (bar) with 95% confidence intervals (line). For aortic distenstibility, LAE, and SAE, P trends are all < 0.001.
Table 2.
Unadjusted and adjusted differences in mean SD of systolic blood pressure by quartiles of thoracic aortic distensibility
| Quartiles of aortic distensibility | |||||
|---|---|---|---|---|---|
| Characteristics and Models | Q4 (referent) (n = 660) | Q3 (n = 660) | Q2 (n = 660) | Q1 (n = 660) | P trenda |
| Aortic distensibility, 10−3/mm Hg | >2.34 | 1.59–2.34 | 1.07–1.58 | <1.07 | — |
| Unadjusted | 0 (referent) | 2.24 (0.34) | 3.16 (0.34) | 5.54 (0.34) | <0.001 |
| Model 1b | 0 (referent) | 1.58 (0.34) | 1.82 (0.36) | 3.61 (0.38) | <0.001 |
| Model 2c | 0 (referent) | 1.48 (0.33) | 1.77 (0.35) | 3.42 (0.37) | <0.001 |
| Model 3d | 0 (referent) | 0.80 (0.32) | 0.63 (0.34) | 1.76 (0.36) | <0.001 |
| Model 4e | 0 (referent) | 0.69 (0.31) | 0.41 (0.33) | 1.42 (0.36) | <0.01 |
Numbers in table are difference in mean (SE) SD of SBP compared with quartile 4 of thoracic aortic distensibility.
aP value represents the linear trend across quartiles (with each quartile represented by the median value within the quartile).
bModel 1 includes adjustment for age, sex, race/ethnicity, and Multi-Ethnic Study of Artherosclerosis site.
cModel 2 includes adjustment for variables in model 1 and body mass index, diabetes, low-density lipoprotein and high-density lipoprotein cholesterol levels, cigarette smoking, education level (high school or above), physical activity, reduced estimated glomerular filtration rate, and C-reactive protein.
dModel 3 includes adjustment for variables in model 2 and mean systolic blood pressure levels (across exams 1–4).
eModel 4 includes adjustment for variables in model 3 and use of antihypertensive medication (never taking across exams, taking across all exams, sometimes taking across exams).
Associations between LAE and SAE indices and VVV of BP
For the artery elasticity indices analyses (n = 4,560), the mean of the SD of SBP was 10.8mm Hg (SD = 6.6). The middle and bottom panels of Figure 1 show mean and 95% CI for SD of SBP by quartile of LAE and SAE index, respectively (quartile 4: 8.8mm Hg; quartile 3: 9.7mm Hg; quartile 2: 11.0mm Hg; and quartile 1: 13.6mm Hg for LAE index; quartile 4: 8.2mm Hg; quartile 3: 10.0mm Hg; quartile 2: 11.7mm Hg; and quartile 1: 13.1mm Hg for SAE index). These associations remained statistically significant after multivariable adjustment (see models 1–4 in Supplementary Tables S4 and S5).
Sensitivity analyses
The prevalence of SD of SBP ≥ 13.48mm Hg, the highest quartile of SD of SBP, was 10.9% for quartile 4, 22.6% for quartile 3, 27.1% for quartile 2, and 39.4% for quartile 1 of aortic distensibility (Supplementary Table S6). In a fully adjusted model (model 4, Supplementary Table S6), the prevalence ratios of SD of SBP ≥ 13.48mm Hg associated with quartiles 3, 2, and 1 versus quartile 4 of aortic distensibility were 1.48 (95% CI = 1.16–1.89), 1.40 (95% CI = 1.09–1.78), and 1.58 (95% CI = 1.24–2.03), respectively. The prevalence of SD of SBP ≥ 13.48mm Hg was 17.2%, 20.4%, 26.6%, and 42.3% for quartiles 4, 3, 2, and 1 of LAE index, respectively (Supplementary Table S7), and 12.8%, 22.6%, 31.6%, and 39.5% for quartiles 4, 3, 2, and 1 of SAE index, respectively (Supplementary Table S8). In a fully adjusted model (model 4, Supplementary Table S7) and compared with quartile 4 of LAE index, the prevalence ratios of SD of SBP ≥ 13.48mm Hg were 1.03 (95% CI = 0.88–1.21), 1.10 (95% CI = 0.94–1.28), and 1.19 (95% CI = 1.02–1.40) for quartiles 3, 2, and 1, respectively (P trend = 0.03). In a fully adjusted model (model 4, Supplementary Table S8) and compared with quartile 4 of SAE index, the prevalence ratios (95% CI) were 1.28 (95% CI = 1.07–1.52), 1.44 (95% CI = 1.20–1.71), and 1.56 (95% CI = 1.30–1.87) for quartiles 3, 2 and 1, respectively (P trend < 0.001). When VVV was defined as coefficient of variation and separately as variation independent of the mean, the findings were similar in a fully adjusted model to the results obtained using SD of SBP (model 4, Supplementary Tables S9 and S10). In a fully adjusted model, lower aortic distensibility, LAE, and SAE, expressed as continuous variables, were significantly associated with higher SD of SBP (model 4, Supplementary Table S11). The associations between quartiles of aortic distensibility, LAE, and SAE and SD of SBP were similar when the slope of SBP across exams 1–4 was added to model 4 and also when SD of SBP was derived from SBP obtained from exams 2–4 rather than from exams 1–4 in model 4 (results not shown).
DISCUSSION
We found that lower aortic distensibility by MRI and lower LAE and SAE indices by pulse contour analysis were associated with higher levels of SD of SBP. These findings suggest that alterations in arterial function may contribute to greater VVV of SBP.
Scarce data are available on the association between arterial vascular function and VVV of BP.7 In an outpatient cohort of 201 elderly Japanese patients with ≥1 cardiovascular disease risk factors, Nagai et al. 7 found an association between common carotid artery stiffness and VVV of BP, independent of age, smoking, and high-density lipoprotein level. Our results extend these findings by demonstrating that reduced aortic distensibility and arterial elasticity were both associated with a higher level of VVV of BP in a large, multiethnic, population-based cohort. This association was present after adjustment for several important confounders such as age, sex, race/ethnicity, cardiovascular risk factors, mean SBP, and antihypertensive medication use.
In our study, functional alterations in the aorta and other large vessels were associated with higher levels of VVV of BP. Although the mechanisms underlying this relation are unknown, some evidence suggests that baroreceptor insensitivity may underlie the link between large artery vascular changes and VVV of BP.24,25 Increases in beat-to-beat BP variability are associated with a reduction in baroreceptor sensitivity,24 and prior evidence suggests that large artery stiffness is a major determinant of reduced baroreceptor sensitivity.25 Further, a recent report26 indicated that BP variability over a 24-hour period was associated with large artery stiffness in hypertensive patients. Whether this mechanistic pathway can be extended to the associations of aortic distensibility and LAE with VVV of BP is unclear.
In addition, our findings also suggest an association between small artery alterations and VVV of BP. Changes in SAE may be due to mechanisms that are closely linked to nitric oxide–mediated endothelial dysfunction.27 In a sample of 36 black subjects, Diaz et al. 28 showed that VVV of BP is higher in blacks with decreased endothelial function. Therefore, reduced SAE and endothelial dysfunction may be overlapping biological domains that underlie VVV of BP.
Finally, we studied long-term VVV as BP readings were taken at 18-month intervals. The optimal period between visits for the estimation of VVV is unknown. In the studies that have demonstrated associations between VVV and cardiovascular disease events or subclinical disease, the intervals between visits have ranged from several days6 to several years.3,29 Whether our results can be extended to VVV estimated from shorter or longer intervals remains unknown.
There are several potential limitations to our study. Pulse wave velocity, considered by some investigators to be the gold standard methodology to assess arterial stiffness,30 was not measured in MESA. However, MRI for the assessment of distensibility has been shown to have better quality of validation and less operator bias compared with other methods of aortic stiffness assessment.31 Another possible limitation is that peripheral BP rather than central BP was used to calculate aortic distensibility. Further, although some studies have questioned the assumptions about the arterial tree when using the modified Windkessel model of circulation,31 the method of arterial elasticity used in our study has a high degree of correlation with the same measurements obtained invasively19 and is associated with important clinical outcomes. In MESA, estimates of LAE and SAE are associated with incident hypertension, cardiovascular outcomes, and kidney function decline.14,17,32 In addition, despite our use of different noninvasive techniques to measure the functional properties of large and small arteries, the findings were similar, providing stronger evidence in support of our hypotheses linking these changes with VVV of BP. Whether similar associations exist between VVV of BP and pulse wave velocity or other vascular measures such as 24-hour ambulatory arterial stiffness index,33 is unknown. Further, this study cannot ascertain whether functional arterial alterations precede or are a result of higher levels of VVV of BP. However, the results were similar when VVV of BP was derived from BP obtained from exams 2–4, suggesting that alterations in the arterial tree preceded VVV of BP in our study. Finally, because we only had 4 visits to assess VVV of BP, it is possible that measurement error was present. However, the presence of measurement error would have biased our results toward the null: the association between VVV of BP and the vascular measures would have been even stronger if measurement error was eliminated.
Major strengths of this study include the large sample size, the inclusion of a multiethnic, population-based sample that was drawn from several communities in the United States, and the careful and standardized assessment of vascular measures and cardiovascular risk factors including BP readings across time. Further, this is one of the first studies to examine the independent associations of aortic distensibility, LAE, and SAE with VVV of BP.
In summary, we found that aortic distensibility, LAE, and SAE were associated with higher long-term VVV of SBP in a large, multiethnic, population-based study. These findings are consistent with the hypothesis that functional alterations in central and peripheral arteries contribute to the long-term variability in SBP obtained over time in the clinic setting. Future studies should confirm these findings by examining the longitudinal associations of aortic distensibility and artery elasticity indices with VVV of BP. Also the biological mechanisms underlying the association between these arterial parameters and greater VVV of BP should be examined.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. MESA was supported by contracts N01-HC-95159 through N01-HC-95166, N01-HC-95167, and R01-HL077612 from the National Heart, Lung, and Blood Institute; and UL1 RR024156 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health. Dr Newman is supported by 5T32HL007854-16 from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1. Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375: 895–905 [DOI] [PubMed] [Google Scholar]
- 2. Shimbo D, Newman JD, Aragaki AK, Lamonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller S. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension 2012; 60: 625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grove JS, Reed DM, Yano K, Hwang LJ. Variability in systolic blood pressure—a risk factor for coronary heart disease? Am J Epidemiol 1997; 145: 771–776 [DOI] [PubMed] [Google Scholar]
- 4. Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens 2012; 25: 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsieh Y-T, Tu S-T, Cho T-J, Chang S-J, Chen J-F, Hsieh M-C. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5·5-year prospective analysis. Eur J Clin Invest 2012; 42: 245–253 [DOI] [PubMed] [Google Scholar]
- 6. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension 2011; 57: 160–166 [DOI] [PubMed] [Google Scholar]
- 7. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens 2011; 5: 184–192 [DOI] [PubMed] [Google Scholar]
- 8. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 2008; 105: 1652–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871–881 [DOI] [PubMed] [Google Scholar]
- 10. Shimbo D, Muntner P, Mann D, Barr RG, Tang W, Post W, Lima J, Burke G, Bluemke D, Shea S. Association of left ventricular hypertrophy with incident hypertension: the multi-ethnic study of atherosclerosis. Am J Epidemiol 2011; 173: 898–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stacey RB, Bertoni AG, Eng J, Bluemke DA, Hundley WG, Herrington D. Modification of the effect of glycemic status on aortic distensibility by age in the multi-ethnic study of atherosclerosis. Hypertension 2010; 55: 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, Cotch MF, Klein BE, Wong TY. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension 2007; 50: 617–622 [DOI] [PubMed] [Google Scholar]
- 13. Nelson AJ, Worthley SG, Cameron JD, Willoughby SR, Piantadosi C, Carbone A, Dundon BK, Leung MC, Hope SA, Meredith IT, Worthley MI. Cardiovascular magnetic resonance-derived aortic distensibility: validation and observed regional differences in the elderly. J Hypertens 2009; 27: 535–542 [DOI] [PubMed] [Google Scholar]
- 14. Duprez DA, Jacobs DR, Jr., Lutsey PL, Bluemke DA, Brumback LC, Polak JF, Peralta CA, Greenland P, Kronmal RA. Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. Am J Epidemiol 2011; 174: 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finkelstein SM, Collins VR, Cohn JN. Arterial vascular compliance response to vasodilators by Fourier and pulse contour analysis. Hypertension 1988; 12: 380–387 [DOI] [PubMed] [Google Scholar]
- 16. Cohn JN, Wilson DJ, Neutel J, Houston M, Weinberger MH, Grimm R, Jr, Smith DH, Sun W. Coadministered amlodipine and atorvastatin produces early improvements in arterial wall compliance in hypertensive patients with dyslipidemia. Am J Hypertens 2009; 22: 137–144 [DOI] [PubMed] [Google Scholar]
- 17. Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Jr., Duprez D, Bluemke D, Polak J, Psaty B, Kestenbaum BR. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2010; 171: 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, Cohn JN. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension 1999; 33: 1392–1398 [DOI] [PubMed] [Google Scholar]
- 19. Cohn JN, Finkelstein S, McVeigh G, Morgan D, LeMay L, Robinson J, Mock J. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension 1995; 26: 503–508 [DOI] [PubMed] [Google Scholar]
- 20. Acree LS, Montgomery PS, Gardner AW. The influence of obesity on arterial compliance in adult men and women. Vasc Med 2007; 12: 183–188 [DOI] [PubMed] [Google Scholar]
- 21. Zimlichman R, Shargorodsky M, Boaz M, Duprez D, Rahn KH, Rizzoni D, Payeras AC, Hamm C, McVeigh G. Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: reliability, repeatability, and establishment of normal values for healthy European population—the seven European sites study (SESS). Am J Hypertens 2005; 18: 65–71 [DOI] [PubMed] [Google Scholar]
- 22. Brumback LC, Jacobs DR, Jr., Dermond N, Chen H, Duprez DA. Reproducibility of arterial elasticity parameters derived from radial artery diastolic pulse contour analysis: the multi-ethnic study of atherosclerosis. Blood Press Monit 2010; 15: 312–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsieh CL, Sheu CF, Hsueh IP, Wang CH. Trunk control as an early predictor of comprehensive activities of daily living function in stroke patients. Stroke 2002; 33: 2626–2630 [DOI] [PubMed] [Google Scholar]
- 24. Omboni S, Parati G, Di Rienzo M, Wieling W, Mancia G. Blood pressure and heart rate variability in autonomic disorders: a critical review. Clin Auton Res 1996; 6: 171–182 [DOI] [PubMed] [Google Scholar]
- 25. Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension 2012; 59: 98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, Battista F, Settimi L, Desamericq G, Dolbeau G, Faini A, Salvi P, Mannarino E, Parati G. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension 2012; 60: 369–377 [DOI] [PubMed] [Google Scholar]
- 27. McVeigh GE, Allen PB, Morgan DR, Hanratty CG, Silke B. Nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci (Lond) 2001; 100: 387–393 [PubMed] [Google Scholar]
- 28. Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Brown MD. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res 2012; 35: 55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol 2010; 67: 564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 2011; 57: 1511–1522 [DOI] [PubMed] [Google Scholar]
- 31. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol 2003; 23: 554–566 [DOI] [PubMed] [Google Scholar]
- 32. Peralta CA, Jacobs DR, Jr., Katz R, Ix JH, Madero M, Duprez DA, Sarnak MJ, Criqui MH, Kramer HJ, Palmas W, Herrington D, Shlipak MG. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60mL/min/1.73 m(2): the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2012; 59: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aznaouridis K, Vlachopoulos C, Protogerou A, Stefanadis C. Ambulatory systolic-diastolic pressure regression index as a predictor of clinical events: a meta-analysis of longitudinal studies. Stroke 2012; 43: 733–739 [DOI] [PubMed] [Google Scholar]


