Abstract
A 42-year-old Caucasian female with history of neurofibromatosis type 1 presented with nephrotic range proteinuria and focal segmental glomerulosclerosis (FSGS). On final dose of lisinopril 20 mg/day, protein–creatinine ratio declined to 0.33 within 10 months. We propose the hypothesis that development of FSGS in NF1 may be mediated by activation of mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) signaling pathways secondary to up-regulation of ras proteins due to deficient neurofibromin. Since mTOR signaling pathway is partially mediated through angiotensin-II activation, angiotensin-converting enzyme (ACE) inhibition may serve as an effective initial treatment beyond anti-proteinuric properties of ACE-inhibitors.
Keywords: focal segmental glomerulosclerosis, MAPK, mTOR, neurofibromatosis
Background
Neurofibromatosis type 1 (NF1) is a rare autosomal-dominant genetic disorder. Association of NF1 with focal segmental glomerulosclerosis (FSGS) is quite rare. In this report, we present clinical course of a patient with NF1 and FSGS and propose the molecular pathways explaining the association between these two entities.
Clinical report
A 42-year-old Caucasian female with a history of clinically diagnosed NF and migraine headache was referred for evaluation of nephrotic range proteinuria associated with pitting leg edema diagnosed 6 months earlier in December 2010. At that time, her protein–creatinine ratio was >11. In her first clinic visit, we found her to be asymptomatic. Her medications started with enalapril 2.5 mg/day, potassium chloride 20 meq/day and bumetanide 1 mg/day at the time of diagnosis of nephrotic syndrome. Her family history was remarkable for NF in her maternal grandmother, mother and her daughter; however, there was no known family history of proteinuria or kidney disease. She denied history of smoking, alcohol or drug abuse. On physical examination, sitting blood pressure was 136/76 mmHg, pulse rate 95–108, temperature 36.4°C and weight was 66 kg. She was alert, awake and without any response delay. Examination of her heart, lungs and abdomen was unremarkable. She had multiple neurofibromas in chest, abdomen and posterior trunk. She also had multiple café-au-lait spots. Her labs showed sodium 141 mmol/L, potassium 4.1 mmol/L, chloride 113 mmol/L, HCO3− 27 mmol/L, blood urea nitrogen (BUN) 5.0 mmol/L (14 mg/dL), creatinine 44.3 µmol/L (0.5 mg/dL), random plasma glucose 3.9 mmol/L (70 mg/dL), calcium 2.1 mmol/L (8.5 mg/dL), phosphorous 1 mmol/L (3 mg/dL), albumin 25 g/L (2.5 g/dL), ANA titer 1/80, C3 1.19 g/L [119 mg/dL (reference range 83–240)], C4 0.17 g/L [17 mg/dL (reference range 13–60)], hepatitis panel negative, white blood count 5.4 × 103/mm3, hemoglobin 134 g/L (13.4 g/dL) and platelet count 291 × 109/L. Serum protein electrophoresis showed hypoalbuminemia. In urinalysis, there was trace blood without any dysmorphic red blood cells, and protein–creatinine ratio was 7.2. Urinary protein electrophoresis showed a combined glomerular and tubular proteinuria. DNA analysis confirmed a heterozygous truncating mutation in NF1 gene on chromosome 17. All eight exones of NPHS2 gene were negative for podocin mutation. In ultrasonography, kidney size was 10.7 cm in right and 10.6 cm in left side, with normal-appearing cortex and echogenicity.
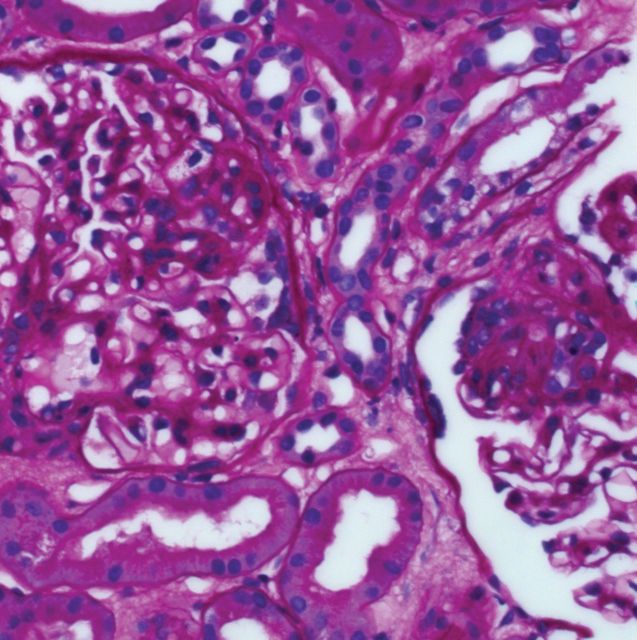
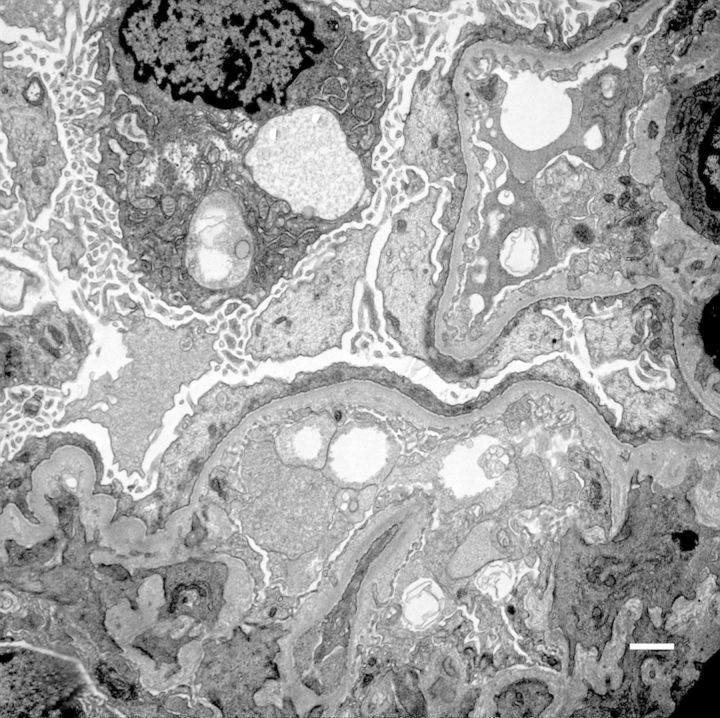
On kidney biopsy, small portions of juxtamedullary cortex were examined. Nine glomeruli without obsolescence were present. There were no crescents but there were two synechia. These glomeruli had segmental collapse associated with the adhesions to Bowman's capsule (Figure 1). There were two tip lesions. The overall glomerular size and cellularity was normal. The peripheral vascular loops were patent without fibrin, platelet or hyaline within the thrombi. The capillary loops had a normal thickness without apparent deposits. The mesangium was not expanded except in those areas adherent to Bowman's capsule. There was diffuse mild interstitial fibrosis with tubular atrophy (IFTA) without any significant tubular injury or acute interstitial infiltrate. The arterioles showed mild fibrous intimal thickening. There was a 2 to 3+ staining of granular mesangial deposition of IgM in a segmental distribution in the majority of glomeruli and a 3+ staining of granular deposition of C3 co-distributed with IgM and also present in arteriolar walls. Similarly, there was a 2+ staining of granular mesangial deposition of kappa and lambda light chains in a distribution similar to IgM. On electron microscopy, the visceral epithelial cells show confluent foot process effacement and prominent pseudovillus transformation (Figure 2). There were rare loops with duplication of lamina densa and entrapped cell detritus but no intramembranous, subendothelial or epithelial electron dense deposits are present. The mesangium is mildly expanded with mesangial matrix which entraps cell debris and scant proteinaceous material. No immune type deposits were identified. Overall these findings are consistent with primary FSGS. No other renal abnormality was identified. On her initial clinic visit, enalapril was changed to lisinopril with gradual titration to a final dose of 20 mg/day. After 10 months she had a gradual decrease of protein–creatinine ratio to 0.33.
Fig. 1.
Periodic acid Schiff staining show mesangial expansion adjacent to adhesion to Bowman's capsule, synechia and segmental collapse.
Fig. 2.
Electron microscopy shows confluent foot process effacement and prominent pseudovillus transformation.
Discussion
The first case of FSGS in association with NF1 was reported by Gersch and Taylor in 2006 [1]. They reported a 22 year-old male with diagnosis of NF1 who presented with serum creatinine of 300.6 µmol/L (3.4 mg/dL), and a urinary protein excretion of 6 g in 24 h. Kidney biopsy showed advanced FSGS and therefore no specific treatment was recommended. Tarrass reported a 58-year-old male with past medical history of NF who presented with serum creatinine of 572 µmol/L (6.5 mg/dL) [2]. Similarly, kidney biopsy showed FSGS with severe IFTA. To our knowledge, our patient is the third case of NF1 being reported in association with FSGS.
Neurofibromatosis is most commonly associated with renal artery stenosis and seen in a few case reports of membranous and IgA nephropathy [3–8]. The NF1 gene is mapped on pericentromeric region of chromosome 17q11.2 [9]. The product of NF1 gene is neurofibromin, a 2818 amino acid protein with an estimated weight of 327 kDa [10]. Neurobibromin is widely expressed in many tissues including the kidney [11, 12]. It is homologous to various members of GTPase-activating protein (GAP) superfamily, including mammalian GAP [13, 14]. All GAP-like proteins are capable of down-regulating the ras protein activities, including the activity of p21-ras proto-oncogene. The p21-ras protein binds to guanine nucleotides, possesses intrinsic GTPase activity and functions as an important regulator of cell growth [15, 16]. Under normal physiologic conditions, neurofibromin functions as a downregulator of p21-ras protein. In neurofibromatosis type 1, mutation in NF1 leads to deficiency or diminished activity of neurofibromin resulting in overactivity of p21-ras protein [17]. Other than oncogenic properties, the upregulation of ras also induces activation of stem cell factor (SCF)/c-kit, mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) signaling pathways [18, 19].
mTOR complex-1 (mTORC1) is a regulator of cell growth by maintaining balance between anabolic and catabolic cell processes. Ito et al. showed that activation of mTORC1 induced unfolded protein response activation and altered localization of nephrin in podocyte leading to proteinuria in a minimal change disease rat model possibly through a series of energy-consuming mechanisms which include stimulation of initiation and elongation phase of mRNA translation, increased cell-cycle progression, selective enhancement in gene transcription and increased ribosome [20]. In another study, Godel et al. have shown that loss of mTORC1 results in progressive glomerulosclerosis which can further be aggregated by additional deletion of mTORC2, while significant activation of mTORC1 subsequent to induction of diabetes was associated with subsequent hypertrophy of podocyte, foot process effacement and eventual detachment from glomerular basement membrane, suggesting a major role for mTOR signaling in podocyte homeostasis [21]. In models of puromycin aminonucleoside (PAN) and mouse adriamycin (ADR) nephropathy, Koshikawa et al. showed enhanced phosphorylation of p38 MAPK in glomeruli in the first week of insult preceding proteinuria, and marked suppression of podocyte injury and proteinuria upon inhibition of p38 MAPK [22]. They also showed enhanced phosphorylation of p38 MAPK in clinical nephrotic syndrome, all together suggestive of an upstream role for p38 MAPK activation for development of proteinuria.
To understand the role of NF1 gene in normal development, Brannan et al. have created mice models that carry a null mutation at Nf1 locus induced via homologous recombination in embryonic stem cells [23]. Examination of visceral organs showed that the mutant embryos had an 18- to 24-h developmental delay in renal, hepatic and musculoskeletal system. Renal developmental delay was also associated with retardation in cephalad repositioning of the metanephros, and reduced number of glomeruli.
We have not measured activity of MAPK or mTOR pathways in the glomeruli or in podocytes in our patient, as determination of the relative activity of the pathways requires comparison with standard controls. The occurrence of FSGS with NF in our reported patient may also be a coincidence as we have not performed any linkage investigation. However, to explain the association beyond a mere coincidence, we propose the hypothesis that mechanisms for development of FSGS and nephrotic range proteinuria in NF1 may include activation of MAPK, and mTOR signaling pathways secondary to upregulation of ras proteins due to deficient neurofibromin, in association with possible reduced nephron numbers and developmental delay of the kidney. In our reported patient, among the entire panel of familial causes of FSGS, we tested only NPHS2 gene and ruled out podocin mutation and, therefore, the possibility of the presence of other familial causes of FSGS is not entirely ruled out. It is unclear why not all patients with NF1 develop proteinuria and FSGS, but few explanations may be variation in point of mutation in NF gene, variation in nephron numbers, requirement for a second hit and interaction with environmental risk factors. mTOR signaling pathway is partially mediated through angiotensin-II activation [24] and therefore angiotensin-converting enzyme inhibition may serve as an effective initial treatment beyond class associated anti-proteinuric and anti-fibrotic properties of ACE-inhibitors.
Acknowledgements
The authors would also like to thank Dr Matthis Kretzler for his remarks. F.A. is supported by the grant 5T32DK7378-32.
Conflict of interest statement. None declared.
References
- 1.Gersch MS, Talor Z. Focal segmental glomerular sclerosis in a patient with neurofibromatosis type I. Am J Kidney Dis. 2006;47:e17–e19. doi: 10.1053/j.ajkd.2005.09.017. doi:10.1053/j.ajkd.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Tarrass F. Focal and segmental glomerulosclerosis and von Recklinghausen's neurofibromatosis: coincidental or associated? Saudi J Kidney Dis Transpl. 2008;19:453–454. [PubMed] [Google Scholar]
- 3.Kokubo T, Hiki Y, Horii A, et al. Recklinghausen's neurofibromatosis associated with membranous nephropathy. Nephron. 1993;65:486. doi: 10.1159/000187543. doi:10.1159/000187543. [DOI] [PubMed] [Google Scholar]
- 4.Malav IC, Kothari SS. Renal artery stenosis due to neurofibromatosis. Ann Pediatr Cardiol. 2009;2:167–169. doi: 10.4103/0974-2069.58323. doi:10.4103/0974-2069.58323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saif I, Seriki D, Moore R, et al. Midaortic syndrome in neurofibromatosis type 1 resulting in bilateral renal artery stenosis. Am J Kidney Dis. 2010;56:1197–1201. doi: 10.1053/j.ajkd.2010.04.023. doi:10.1053/j.ajkd.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi Y, Yorioka N, Kanbe M, et al. Parent and child cases of IgA nephropathy associated with von Recklinghausen's disease. Nephron. 1997;75:113–114. doi: 10.1159/000189515. doi:10.1159/000189515. [DOI] [PubMed] [Google Scholar]
- 7.Toth T, Trinn C, Simon L, et al. A case of membranous glomerulonephritis associated with Recklinghausen's neurofibromatosis. Clin Nephrol. 1996;45:271–272. [PubMed] [Google Scholar]
- 8.Wani MM, Reshi AR, Banday KA, et al. von Recklinghausen's neurofibromatosis associated with membranous glomerulonephritis. Saudi Med J. 2006;27:534–535. [PubMed] [Google Scholar]
- 9.Ledbetter DH, Rich DC, O'Connell P, et al. Precise localization of NF1 to 17q11.2 by balanced translocation. Am J Hum Genet. 1989;44:20–24. [PMC free article] [PubMed] [Google Scholar]
- 10.Marchuk DA, Saulino AM, Tavakkol R, et al. cDNA cloning of the type 1 neurofibromatosis gene: complete sequence of the NF1 gene product. Genomics. 1991;11:931–940. doi: 10.1016/0888-7543(91)90017-9. doi:10.1016/0888-7543(91)90017-9. [DOI] [PubMed] [Google Scholar]
- 11.Wallace MR, Marchuk DA, Andersen LB, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. doi:10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 12.Stocker KM, Baizer L, Coston T, et al. Regulated expression of neurofibromin in migrating neural crest cells of avian embryos. J Neurobiol. 1995;27:535–552. doi: 10.1002/neu.480270408. doi:10.1002/neu.480270408. [DOI] [PubMed] [Google Scholar]
- 13.Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis type 1 (NF1) J Med Genet. 1996;33:2–17. doi: 10.1136/jmg.33.1.2. doi:10.1136/jmg.33.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormick F. ras GTPase activating protein: signal transmitter and signal terminator. Cell. 1989;56:5–8. doi: 10.1016/0092-8674(89)90976-8. doi:10.1016/0092-8674(89)90976-8. [DOI] [PubMed] [Google Scholar]
- 15.Martin GA, Viskochil D, Bollag G, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–849. doi: 10.1016/0092-8674(90)90150-d. doi:10.1016/0092-8674(90)90150-D. [DOI] [PubMed] [Google Scholar]
- 16.Xu GF, O'Connell P, Viskochil D, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. doi:10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 17.Bollag G, McCormick F. Ras regulation. NF is enough of GAP. Nature. 1992;356:663–664. doi: 10.1038/356663a0. doi:10.1038/356663a0. [DOI] [PubMed] [Google Scholar]
- 18.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. doi:10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 19.Fehrenbacher N, Bar-Sagi D, Philips M. Ras/MAPK signaling from endomembranes. Mol Oncol. 2009;3:297–307. doi: 10.1016/j.molonc.2009.06.004. doi:10.1016/j.molonc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito N, Nishibori Y, Ito Y, et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab Invest. 2011;91:1584–1595. doi: 10.1038/labinvest.2011.135. doi:10.1038/labinvest.2011.135. [DOI] [PubMed] [Google Scholar]
- 21.Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. doi:10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koshikawa M, Mukoyama M, Mori K, et al. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol. 2005;16:2690–2701. doi: 10.1681/ASN.2004121084. doi:10.1681/ASN.2004121084. [DOI] [PubMed] [Google Scholar]
- 23.Brannan CI, Perkins AS, Vogel KS, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. doi:10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 24.Whaley-Connell A, Habibi J, Panfili Z, et al. Angiotensin II activation of mTOR results in tubulointerstitial fibrosis through loss of N-cadherin. Am J Nephrol. 2011;34:115–125. doi: 10.1159/000329327. doi:10.1159/000329327. [DOI] [PMC free article] [PubMed] [Google Scholar]