In this international prospective cohort of 165 individuals coinfected with HIV and hepatitis B virus (HBV), 3 patterns of suboptimal response to tenofovir therapy were identified: persistent viremia, viral rebound, and viral blips. Suboptimal adherence was associated with detectable HBV DNA, even when HIV was undetectable.
Keywords: HIV, hepatitis B, antiretroviral therapy, adherence
Abstract
Background. Tenofovir (TDF) is effective for treatment of hepatitis B virus (HBV) in human immunodeficiency virus (HIV) infection; however, some individuals have ongoing HBV viremia, the reasons for which are unclear. We determined the patterns and factors associated with detectable HBV DNA in HIV-HBV–coinfected subjects on highly active antiretroviral therapy (HAART).
Methods. One hundred sixty-five HIV-HBV–coinfected individuals from the United States, Australia, and Thailand, the majority of whom were on HAART at study entry, were prospectively followed semiannually for a median of 2.8 years. Logistic regression was used to determine factors associated with detectable HBV DNA.
Results. Anti-HBV regimens were TDF/emtricitabine (57%), lamivudine or emtricitabine (19%), or TDF monotherapy (13%). During follow-up, HBV DNA was detected at 21% of study visits and was independently associated with hepatitis B e antigen (HBeAg), HAART <2 years, CD4 <200 cells/mm3, detectable HIV RNA, reporting <95% adherence, and anti-HBV regimen. TDF/emtricitabine was less likely to be associated with detectable HBV than other regimens, including TDF monotherapy (odds ratio, 2.79; P = .02). In subjects on optimal anti-HBV therapy (TDF/emtricitabine) and with undetectable HIV RNA, HBeAg, CD4 <200 mm3, and reporting <95% adherence remained associated with detectable HBV DNA. Three main patterns of HBV viremia were observed: persistent HBV viremia, viral rebound (>1 log from nadir), and viral blips. No TDF resistance was identified.
Conclusions. Tenofovir/emtricitabine was superior to other anti-HBV regimens in long-term HBV suppression. HBV viremia on therapy was identified in 1 of 3 main patterns. Suboptimal adherence was associated with detectable HBV DNA during therapy, even when HIV was undetectable.
Tenofovir disoproxil fumarate (TDF) was approved for the treatment of human immunodeficiency virus (HIV) in 2001 and is currently one of the most widely prescribed, potent, and well-tolerated antiretroviral agents. Because of its dual activity against HIV and hepatitis B virus (HBV), it has been of particular benefit for individuals with HIV-HBV coinfection. In this group, it effectively suppresses both wild-type and lamivudine-resistant HBV and reverses the parameters of advanced liver disease [1–4].
Recently, 2 European cohorts reported high rates of HBV suppression maintained between 3 and 5 years in those treated with TDF [5, 6]. In the first study, 10 participants failed to achieve a virologic response and 4 experienced virologic breakthrough without evidence of TDF resistance mutations [6]. In the second study, undetectable HBV DNA was achieved in 89% of participants [5]. Eight participants had persistent viremia, including 3 demonstrating a significant reduction from baseline without full suppression again without evidence of drug resistance.
Therefore, it is clear that although TDF is highly effective in the majority, some patients experience a suboptimal response—either in a persistently viremic pattern or as virologic breakthrough without resistance. Although obvious inadequate adherence explains some of these cases, the observation that HIV RNA remains suppressed in many such cases argues against this. In 2008, we reported our cross-sectional findings that at entry to an international cohort study of HIV-HBV–coinfected individuals, TDF combination therapy was more strongly associated with HBV DNA suppression than was TDF (or LAM) monotherapy [7]. The purpose of this study was to determine longitudinal outcomes from the same cohort with up to 3.9 years of follow-up including an in-depth analysis of the patterns and factors associated with suboptimal responses to TDF-based therapy.
METHODS
Study Participants and Data Collection
169 HIV-HBV–coinfected individuals were enrolled initially from sites in Australia (The Alfred Hospital, The Royal Melbourne Hospital, and Melbourne Sexual Health Clinic, Melbourne; St Vincent's Hospital and Taylor Square Clinic, Sydney); the United States (the Multicenter AIDS Cohort Study [MACS]), and, subsequently, Thailand (HIV-NAT, Thai Red Cross AIDS Research Centre, Bangkok) between October 2004 to February 2008, as previously described [7]. Subjects were included if they had HIV infection, determined by the presence of HIV antibody; hepatitis B surface antigen (HBsAg) positive on 2 occasions separated by a minimum of 6 months, with at least 1 of these occasions prior to initiation of highly active antiretroviral therapy (HAART); were on HAART at enrollment or expected to start HAART within 1 year of enrollment; and had a known date of HAART initiation. Individuals coinfected with chronic hepatitis C virus or hepatitis delta were ineligible. All subjects were followed semiannually for the study duration. Written informed consent was obtained from all participants, and the study was approved by the relevant Human Research Ethics Committees in Australia, the United States, and Thailand.
For inclusion in this analysis, all subjects had to have commenced HAART and had HBV DNA available for assessment. Consequently, 4 subjects who did not start HAART by their last study visit or had no HBV DNA data were excluded. Subjects who had previous documentation of HBsAg positivity but who had lost HBsAg during follow-up were included. Clinical and laboratory data were collected from medical records at every visit. For those who had initiated HAART prior to study enrollment, these data were abstracted from their date of HAART initiation. Adherence was assessed by standardized self-report.
Laboratory Testing
HBV DNA was extracted from serum stored at −80°C and quantified by the respective site laboratories using a real-time HBV DNA assay (RealART HBV LC PCR [Qiagen], COBAS Amplicor HBV [Roche], Abbott RealTime HBV DNA [Abbott Molecular], or Versant HBV DNA 3.0 [Bayer Diagnostics]). The lower limit of detection (LLOD) for these assays ranged from 6 IU/mL to 357 IU/mL. Although only 2.4% of tests were performed using the assay with an LLOD of 357 IU/mL, this value was used as the cutoff for undetectable HBV DNA for the primary analyses because it was the lowest common threshold across all 3 assays. Because the majority (94%) of tests were performed using assays with an LLOD of ≤20 IU/mL, sensitivity analyses were performed using only data from these more sensitive assays. HIV RNA was quantified by commercially available approved real-time polymerase chain reaction tests, performed according to the manufacturer's instructions. The LLOD for these assays ranged from 50 copies/mL to 400 copies/mL, and we considered 400 copies/mL as undetectable as the lowest common threshold. However, for the final multivariate analysis we also considered <50 copies/mL as indicative of optimal HIV control.
Statistical Methods
This longitudinal study included data from up to 11 visits for each participant. Only study visits at which participants were taking HAART were included in these analyses. For all primary analyses, detectable HBV DNA was defined as HBV DNA >357 IU/mL. An additional 123 (12.1%) HBsAg-negative person-visits were classified as undetectable HBV DNA. We conducted this person-visit analysis using multiple logistic regression models with robust variance estimation [8] to determine which characteristics were independently associated with detectable HBV DNA while accounting for within-subject correlations across repeated visits. Covariates for study visit and study site were included in all models to account for the prospective study design and the fundamental differences between the cohorts. Initially, we fit 3 preliminary models focusing separately on demographic and behavior variables, HBV-related variables, and HIV-related variables, and then constructed a comprehensive multiple regression model from the covariates forced into all models plus those that had adjusted P values <.20 in the preliminary models. The final model included study visit, site, age, and the covariates that were independently associated with detectable HBV DNA in the preliminary models. All associations were quantified using odds ratios (ORs) and 95% confidence intervals (CIs). Multiple imputation was used to account for missing data in the regression analyses [9]. All statistical analyses were performed using SAS software, version 9.22 (SAS Institute, Cary, North Carolina), and statistical significance was defined as a 2-sided P value <.05.
RESULTS
Study Population and Baseline Characteristics
The study population of 165 HIV-HBV–coinfected participants were followed from their earliest eligible study visit (baseline) for a median of 2.8 years (interquartile range [IQR], 2.0–3.9 years), yielding a total of 1015 study visits. At baseline, the median duration of HAART was 3.5 years with the majority (89%) on an HBV-active regimen. Baseline demographic characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics Among HIV/Hepatitis B Virus Cohort Participants
| Characteristic | No. | % |
|---|---|---|
| All | 165 | 100 |
| Country | ||
| Australia | 67 | 40.6 |
| Thailand | 47 | 28.5 |
| United States | 51 | 30.9 |
| Age, y | ||
| <30 | 9 | 5.5 |
| 30–39 | 50 | 30.3 |
| 40–49 | 62 | 37.6 |
| ≥50 | 44 | 26.7 |
| Female | 16 | 9.7 |
| History of IDU | 16 | 9.8 |
| Homosexual contact | 120 | 72.7 |
| Heterosexual contact | 34 | 20.6 |
| Consumes >14 alcohol drinks/wk | 17 | 10.4 |
| Child-Pugh Score >5 | 13 | 9.8 |
| HBeAg positive | 74 | 48.7 |
| Anti-HBe positive | 54 | 43.9 |
| HBV genotype | ||
| A | 47 | 50.5 |
| C | 33 | 35.5 |
| All other types | 13 | 14 |
| Not tested | 57 | … |
| Currently taking HAART | 149 | 90.3 |
| Cumulative HAART use, y | ||
| <2 | 38 | 23 |
| 2–5 | 62 | 37.6 |
| >5 | 65 | 39.4 |
| Current NRTI use | 152 | 92.1 |
| Current PI use | 60 | 36.4 |
| Current NNRTI use | 100 | 60.6 |
| Less than 95% adherent to ART regimen | 24 | 15.3 |
| HBV-active components of ART regimen | ||
| No HBV-active druga | 18 | 10.9 |
| Includes LAM/FTC | 32 | 19.4 |
| Includes TDF | 21 | 12.7 |
| Includes TDF and LAM/FTC | 94 | 57 |
| Current CD4, cells/mm3 | ||
| >500 | 49 | 30.3 |
| 200–500 | 86 | 53.1 |
| <200 | 27 | 16.7 |
| Nadir CD4, cells/mm3 | ||
| >500 | 2 | 1.2 |
| 200–500 | 60 | 36.8 |
| <200 | 101 | 62 |
| HIV RNA, copies/mL | ||
| <400 | 135 | 82.8 |
| 401–9999 | 13 | 8 |
| ≥10 000 | 15 | 9.2 |
| Prior AIDS diagnosis | 58 | 35.2 |
Abbreviations: ART, antiretroviral therapy; FTC, emtricitabine; HAART, highly active antiretroviral therapy; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IDU, injection drug use; LAM, lamivudine; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir.
a Includes participants on no ART and those on ART with no HBV-active component.
The most common HBV-active component in the HAART regimen was TDF in conjunction with either lamivudine (LMV) or emtricitabine (FTC) (57% of participants). LMV or FTC monotherapy was prescribed for 19%, and 13% received TDF monotherapy. For participants on TDF (with or without FTC or LMV), the median duration of TDF by end of study follow-up was 4.2 years (IQR, 2.9–5.4 years).
At baseline, 49% of the cohort was hepatitis B e antigen (HBeAg) positive (34% Thai, 55% Australian, and 56% MACS) and HBV DNA was detectable in 29% of participants. When stratified by HBeAg status, 50% of HBeAg-positive and 9% of HBeAg-negative individuals had detectable HBV DNA. The proportion of participants with detectable HBV DNA was highest in the United States (47%), followed by Australia (31%) and Thailand (6%).
Factors Associated With Detectable HBV DNA
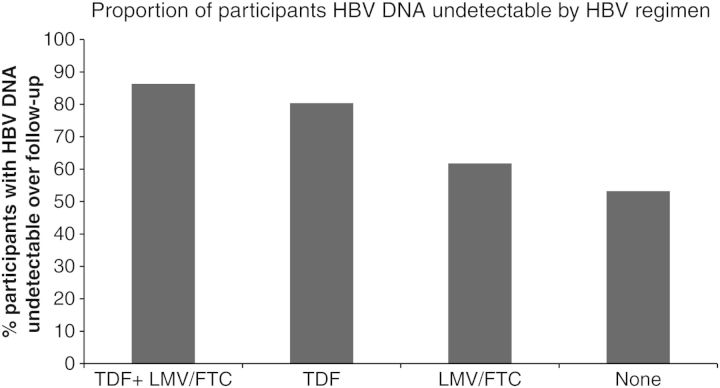
Across all follow-up, HBV DNA was detected at 20.8% of the 1015 person-visits. In univariate analyses, factors significantly associated with detectable HBV DNA included study country (USA > Australia > Thailand), homosexual versus heterosexual HIV risk, Child-Pugh score >5, positive HBeAg, negative anti-HBe, HBV genotype A, type and duration of HAART, CD4 count <200 cells/mm3, detectable HIV RNA, and <95% adherence to HAART regimen (Table 2). In particular, participants receiving TDF plus FTC or LMV had the highest proportion with undetectable HBV DNA (86.3%) across all study visits (Figure 1). In multivariate analysis, factors remaining significantly associated with detectable HBV DNA included HBeAg positivity (OR, 18.95 [95% CI, 9.00–39.88]), HAART <2 years (OR, 3.07 [95% CI, 1.51–6.24]), CD4 count <200 cells/mm3 (OR, 2.21 [95% CI, 1.30–3.77]), and detectable HIV RNA (OR, 4.51 [95% CI, 2.68–7.57]) (Table 2). Those on the TDF plus LAM/FTC combination were significantly less likely to have detectable HBV DNA than those on other regimens: FTC/LMV monotherapy (OR, 6.59 [95% CI, 3.14–13.86], P < .0001), TDF monotherapy (OR, 2.79 [95% CI, 1.17–6.64], P < .02), and no HBV-active medications (OR, 2.49 [95% CI, 1.27–4.88], P < .008). In addition, <95% adherence to HAART remained borderline significantly associated with detectable HBV DNA (OR, 1.77 [95% CI, .99–3.13], P = .05).
Table 2.
Univariate and Multivariate Analysis of Participant Characteristics Associated With Having Detectable Hepatitis B Virus DNA
| Characteristic | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Study visit (per visit) | 0.93 | .85, 1.01 | .07 | 1.03 | .94, 1.14 | .51 |
| Country | ||||||
| Australia | 1 (ref) | 1 (ref) | ||||
| Thailand | 0.22 | .08, .62 | .004 | 0.31 | .09, 1.00 | .05 |
| United States | 2.31 | 1.17, 4.53 | .02 | 1.52 | .81, 2.84 | .19 |
| Age (per 10 y) | 0.83 | .59, 1.16 | .27 | 0.83 | .59, 1.16 | .27 |
| Female | 0.51 | .16, 1.6 | .25 | |||
| History of IDU | 0.89 | .25, 3.11 | .85 | |||
| Homosexual contact | 4.78 | 2.01, 11.38 | .0004 | |||
| Heterosexual contact | 0.19 | .06, .59 | .004 | |||
| Consumes >14 alcoholic drinks/wk | 0.99 | .59, 1.66 | .98 | |||
| Child-Pugh Score >5 | 1.63 | 1.1, 2.42 | .01 | |||
| HBeAg positive | 10.25 | 5.13, 20.48 | <.0001 | 18.96 | 9.00, 39.88 | <.0001 |
| Anti-HBe positive | 0.16 | .08, .34 | <.0001 | |||
| HBV genotype A vs all other types | 5.35 | 2.31, 12.41 | <.0001 | |||
| Currently taking HAART | 0.34 | .17, .68 | .002 | |||
| Cumulative HAART use <2 y | 2.58 | 1.72, 3.88 | <.0001 | 3.07 | 1.51, 6.24 | .002 |
| Current NRTI use | 0.36 | .18, .72 | .004 | |||
| Current PI use | 0.83 | .43, 1.61 | .59 | |||
| Current NNRTI use | 0.47 | .24, .92 | .03 | |||
| <95% adherent to ARV regimen | 1.65 | 1.16, 2.34 | .006 | 1.77 | .99, 3.13 | .05 |
| Current HBV-active ARV regimen | ||||||
| Includes TDF and LAM/FTC | 1 (ref) | 1 (ref) | ||||
| Includes LAM/FTC only | 4.47 | 2.29, 8.74 | <.0001 | 6.59 | 3.14, 13.86 | <.0001 |
| Includes TDF only | 2 | .92, 4.33 | .08 | 2.79 | 1.17, 6.64 | .02 |
| Not taking HBV-active regimen | 4.19 | 2.12, 8.28 | <.0001 | 2.49 | 1.27, 4.88 | .008 |
| Current CD4 count <200 cells/mm3 | 2.02 | 1.23, 3.31 | .006 | 2.21 | 1.30, 3.77 | .004 |
| Nadir CD4 count <200 cells/mm3 | 0.8 | .43, 1.49 | .49 | |||
| HIV RNA >400 copies/mL | 4.5 | 2.67, 7.59 | <.0001 | 4.51 | 2.68, 7.57 | <.0001 |
| Prior AIDS diagnosis | 1.06 | .58, 1.95 | .84 | |||
Abbreviations: ARV, antiretroviral; CI, confidence interval; FTC, emtricitabine; HAART, highly active antiretroviral therapy; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IDU, injection drug use; LAM, lamivudine; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor; TDF, tenofovir.
Figure 1.
Proportion of individuals coinfected with human immunodeficiency virus and hepatitis B virus (HBV) with undetectable HBV DNA by HBV active regimen. Abbreviations: FTC, emtricitabine; LMV, lamivudine; TDF, tenofovir.
Since most HBV DNA testing in clinical practice is based on assays with more sensitive LLOD, a secondary analysis was performed including only visits where the HBV DNA assay LLOD was ≤20 IU/mL (Supplementary Table 1). In this sensitivity analysis, the number of visits with detectable HBV DNA increased from 21% to 38%, indicating that very low-level viremia (20–357 IU/mL) was relatively frequent. Furthermore, only HBeAg positivity, HAART <2 years, detectable HIV RNA, and HBV-active ARV regimen (LAM/FTC monotherapy and not taking HBV-active drug) remained statistically significant.
Predictors of Detectable HBV DNA in Subjects on TDF-Based HAART
Since TDF is the recommended agent in HIV-HBV–coinfection, we determined factors associated with detectable HBV DNA among the subgroup of subjects on TDF-based HAART. We examined 3 different scenarios: (1) all subjects on TDF-based HAART, (2) only those taking TDF-based HAART for ≥6 months, and (3) only those taking TDF + LAM/FTC as part of HAART. In each scenario, HBeAg positivity, CD4 count <200 cells/mm3, and detectable HIV RNA were significantly associated with detectable HBV DNA. Furthermore, in the first 2 scenarios, combination therapy (TDF + LAM/FTC) was significantly associated with a lower odds of having detectable HBV DNA compared to TDF monotherapy (model 1: OR, 0.33 [95% CI, .13–.83], P = .02; model 2: OR, 0.29 [95% CI, .10–.80], P = .02).
To determine factors associated with detectable HBV DNA in participants with optimal HIV control, we fit additional models including only those with undetectable HIV RNA. For this analysis, we ran the model using both an HIV RNA <400 copies/mL (lowest common threshold) and an HIV RNA <50 copies/mL (excluding patients with HIV RNA values between 50 copies/mL and 400 copies/mL). No difference was seen between the models with regard to the factors associated with detectable HBV DNA. In the strictest scenario (HIV RNA <50 copies/mL) and where the model was further restricted to participants on TDF + FTC/LAM, HBeAg positivity (OR, 22.39 [95% CI, 7.81–64.15]), CD4 <200 cells/mm3 (OR, 2.32 [95% CI, 1.02–5.28]), being on HAART for <2 years (OR, 3.21 [95% CI, 1.11–9.26]), and <95% adherence to therapy (OR, 2.84 [95% CI, 1.11–7.26]) were independently associated with detectable HBV DNA (Table 3).
Table 3.
Multivariable Analysis of Factors Associated With Having Detectable Hepatitis B Virus DNA in Participants on Tenofovir Plus Lamivudine/Emtricitabine and With HIV RNA <50 Copies/mLa
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Age (per 10 y) | 0.92 | .49, 1.70 | .78 |
| HBeAg positive | 22.39 | 7.81, 64.15 | <.0001 |
| <95% adherent | 2.84 | 1.11, 7.26 | .03 |
| HAART <2 y | 3.21 | 1.11, 9.26 | .03 |
| CD4 < 200 cells/mm3 | 2.32 | 1.02, 5.28 | .05 |
Abbreviations: CI, confidence interval; HAART, highly active antiretroviral therapy; HBeAg, hepatitis B e antigen; OR, odds ratio.
a Model adjusted for study visit and country of origin.
Patterns of HBV Nonresponse in Participants Receiving TDF-Based HAART
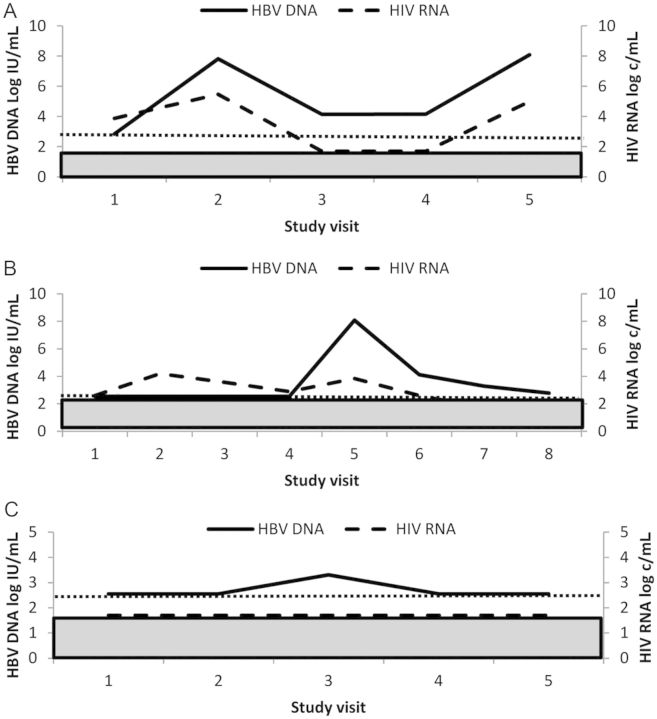
In 138 participants treated with TDF, 62 had a suboptimal response, which fell into 1 of 3 typical patterns. The first includes 25 participants (18%) who failed to achieve any undetectable HBV DNA (<357 IU/mL) despite having received at least 12 months of TDF-based HAART (persistent viremic group). Although HBV DNA was declining slowly but continuously in a few of these cases, the majority were persistently viremic with levels varying up to 3 log IU/mL and often with a similarly fluctuating HIV RNA level (Figure 2A). The second pattern includes 13 participants (9%) who experienced viral rebound of >1 log IU/mL from nadir while on TDF (viral rebound group). All but 1 of these patients were HBV undetectable (<357 IU/mL) before rebound. HIV RNA also became detectable in the majority of these cases suggesting nonadherence or therapy interruption (Figure 2B). Of note, potential TDF resistance mutations were not detected in this viral rebound group. Those with rebound who maintained an undetectable HIV RNA had HBV DNA rebounds that were generally low (1.1–3.7 log IU/mL). The third pattern of suboptimal response was achieving an undetectable HBV DNA but then experiencing intermittent low levels of HBV DNA (<1 log IU/mL increase), usually followed by a return to undetectable levels (viral blip group). This pattern of response occurred in 24 (17%) of participants and was rarely associated with loss of HIV control (Figure 2C).
Figure 2.
Patterns of suboptimal response in tenofovir-treated individuals. A, Persistent viremic pattern. B, Hepatitis B virus (HBV) rebound. C, Viral blip. Dotted line = lower limit of detection of HBV DNA. Shaded box = lower limit of detection of human immunodeficiency virus (HIV) RNA. Primary y-axis: HBV DNA log10 IU/mL; secondary y-axis: HIV RNA log10 copies/mL; x-axis: month of study visit after cohort entry. Abbreviations: c, copies; HBV, hepatitis B virus; HIV, human immunodeficiency virus.
DISCUSSION
This longitudinal analysis of a large, multinational, prospective cohort of HIV-HBV–coinfected individuals, with the majority on HBV-active HAART, confirms our prior cross-sectional findings [7] that therapy with TDF plus FTC or LMV is indeed superior to TDF monotherapy. This finding was demonstrated in the entire cohort and also when the analysis was restricted to those taking a TDF-based HAART regimen. This study also finds that CD4 cell counts <200 cells/mm3, HBeAg positivity, <2 years of HAART, and <95% compliance with HAART regimen are associated with detectable HBV DNA in those with undetectable HIV RNA while on TDF-based HAART. Last, this study defines patterns of HBV viremia for subjects on TDF-based HAART who had detectable HBV DNA.
Because this cohort was LMV-experienced at study entry, this study does not address whether the combination of TDF with FTC or LMV would be more efficacious than TDF monotherapy in treatment-naive HIV-HBV–coinfected subjects. The only study in treatment-naive subjects did not show a significant benefit for combination therapy, but only followed patients for 48 weeks [10].
Although TDF is a highly potent and successful agent for both HIV and HBV, a proportion of individuals do not achieve HBV DNA suppression. In studies to date, failure to achieve an undetectable HBV DNA (<10–20 IU/mL) has been reported in 11%–12% of individuals [5, 6]. The reasons for nonsuppression are ill defined and not clearly related to HBV resistance, which has rarely been reported in the context of TDF [11, 12], and did not occur in our participants. In our prospectively followed TDF-treated participants, a much higher proportion (44%) were shown to have a suboptimal (ie, were not persistently undetectable) response to TDF characterized by 1 of 3 responses—persistent viremia, viral rebound, or viral blip. Two likely reasons for our higher rate of suboptimal responses are the inclusion of the latter 2 definitions, especially of those with viral blip, and the assessment of prospective viral profiles rather than in a cross-sectional manner at last follow-up. The viral blip category is interesting because those participants had an undetectable HIV RNA and low levels of HBV DNA on rebound. Whether these blips represent ongoing replication or release of virions from the hepatic reservoir requires further study. In HIV-infected individuals on treatment, it has been shown that blips in HIV RNA likely represent release from reservoirs and not replication [13, 14].
In the multivariable analysis restricted to those on combination therapy and with undetectable HIV RNA, it is not surprising that HBeAg positivity is associated with detectable HBV DNA, because HBV DNA is higher in untreated participants who are HBeAg positive. The association of lower CD4 counts with detectable HBV DNA is intriguing because it suggests that the degree of immunosuppression affects the ability to respond to anti-HBV therapy. Although the mechanism for this association is not clear, one explanation is that a contribution from the immune system is important in achieving an undetectable HBV DNA during therapy. These data support recommendations for early initiation of HAART in HIV-HBV–coinfected participants. Further studies are needed to determine whether there is a difference in response to anti-HBV therapy at higher CD4 counts of 350 or 500 cells/mm3. One of the most fascinating findings is that among subjects with excellent adherence in terms of having undetectable HIV RNA, those with <95% adherence were more likely to have detectable HBV DNA, which has not been previously documented. Because replication rates of HBV are greater than HIV, it is reasonable that a higher level of compliance is needed to have a persistently undetectable HBV DNA. The consequences for these blips and rebounds that may be due to <95% adherence are unknown; thus, further study of such individuals is warranted. However, it is important to note that the majority of subjects who had been taking a TDF-based regimen for a median of 4 years had maintained an undetectable HBV DNA level during this period. Together with 2 other cohorts that reported high HBV DNA suppression rates over a similar follow-up period [5, 6], our findings demonstrate that TDF is an effective long-term anti-HBV agent in HIV-HBV coinfection.
This study has several strengths including the large number of HIV-HBV coinfected subjects on TDF for an extended period, the inclusion of subjects who received TDF monotherapy as well as TDF with FTC/LMV, and the prospective follow-up allowing determination of patterns of detectable HBV DNA. The study is limited by the fact that we did not follow all subjects since TDF initiation and, thus, do not have HBV DNA levels pretherapy which could aid in our understanding of the HBV DNA patterns we observed. Second, few patients in the cohort underwent liver biopsy and none had Fibroscan recorded; thus, any association between level of hepatic fibrosis and HBV replication could not be explored. Third, we are not aware of why a particular HBV-active HAART regimen was chosen for each subject, so we could not account for this in our analysis. Last, a range of different HBV DNA assays were performed over the duration of the cohort; however, the sensitivity analysis with 20 IU/mL as the LLOD demonstrated that most of the associations remained, with the major exception being the <95% adherence covariate. This suggests that, unlike with higher levels of HBV DNA, suboptimal adherence is not associated with episodes of low-level viremia between 20 IU/mL and 357 IU/mL, which would support the theory (as in HIV) that very low-level HBV viremia is possibly driven by other mechanisms, such as release from viral reservoirs, and may not be clinically significant. Further work is needed to confirm this hypothesis.
In summary, this study demonstrates that in HIV-HBV–coinfected subjects with prior LMV experience, combination therapy with TDF and FTC/LMV increases the likelihood for sustained HBV DNA suppression. In addition, we identified several patterns of HBV DNA in individuals who are not suppressed and demonstrated that more advanced immunodeficiency and suboptimal compliance with the anti-HBV regimen decreases the likelihood of a sustained response in HIV-HBV–coinfected subjects on TDF with FTC or LMV. Emphasizing the importance of full adherence to maintain both HIV and HBV control is critical in HIV-HBV–coinfected individuals.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS; http://www.statepi.jhsph.edu/macs/macs.html) with centers (principal investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick, Lisa P. Jacobson), Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles R. Rinaldo).
Financial support. This work was supported by the National Institutes of Health (grant number R56AI60449). K. R. has been awarded Senior Researcher Scholar, Thai Research Fund; the National Research University Project of CHE (HR1161A), Ministry of Education; and the Professional Research Team Strengthening Fund, from the National Science and Technology Development Agency, BIOTEC, Ministry of Science and Technology, Thailand. The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute. UO1-AI-35042, UL1-RR025005, UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041.
Potential conflicts of interest. G. V. M. has received grant funding from Gilead and Merck Sharp & Dohme (MSD); has served on the speakers’ bureaus for Gilead, Roche, MSD, Bristol-Myers Squibb (BMS), and Janssen; and has received travel support from Janssen and MSD. S. R. L. has received grant funding from Gilead and Merck; has served as a paid consultant and speaker for Gilead and Viiv Healthcare; and has served on the advisory boards of Gilead and Merck. J. S. has received research funding from Gilead, Merck, BMS, and Roche; has received meeting sponsorships from Gilead and Merck; and has been a paid speaker and served on the advisory board for Roche. S. B. has been a speaker for and received honoraria from Gilead, BMS, Roche, and Merck. R. F. has served as a board member of Abbott, BMS, Boehringer Ingelheim, Gilead, Janssen, Merck, and ViiV. P. A. R. has received grant funding from Gilead Sciences. J. F. H.'s institution has received research funding from MSD and Gilead Sciences, and she has served on the advisory boards for MSD, Gilead Sciences, ViiV Healthcare, and Janssen (Tibotec), and has received conference sponsorship from MSD. S. L. has received royalties from Melbourne Health; holds intellectual property rights and patents from Melbourne Health; has been paid as a consultant for Evivar Pty Ltd, Gilead, and BMS; has received payment for non-CME services received directly from a commercial interest or their agent from BMS and MSD. K. R. has served as a consultant for Merck and Tibotec and has been a paid speaker for BMS, Merck, Roche, Janssen-Cilag, GlaxoSmithKline, and GPO. G. J. D. has served on the board of directors of Roche, Merck, Janssen, Gilead, and BMS; has received honoraria from Roche, Merck, Janssen, Gilead, and BMS; has received research grants from Roche, Merck, Janssen, Gilead, and BMS; and has received scholarships from Roche, Merck, and Janssen. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–9. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 2.Matthews G, Cooper DA, Dore G. Improvements in parameters of end stage liver disease in patients with HIV/HBV-related cirrhosis treated with tenofovir. Antiviral Therapy. 2007;12:119–22. [PubMed] [Google Scholar]
- 3.Benhamou Y, Fleury H, Trimoulet P, et al. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–55. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 4.Nelson M, Portsmouth S, Stebbing J, et al. An open-label study of tenofovir in HIV-1 and hepatitis B virus coinfected individuals. AIDS. 2003;17:F7–10. doi: 10.1097/00002030-200301030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Carbonero L, Teixeira T, Poveda E, et al. Clinical and virological outcomes in HIV-infected patients with chronic hepatitis B on long-term nucleos(t)ide analogues. AIDS. 2011;25:73–9. doi: 10.1097/QAD.0b013e328340fde2. [DOI] [PubMed] [Google Scholar]
- 6.de Vries-Sluijs TE, Reijnders JG, Hansen BE, et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology. 2010;139:1934–41. doi: 10.1053/j.gastro.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 7.Matthews GV, Seaberg E, Dore GJ, et al. Combination HBV therapy is linked to greater HBV DNA suppression in a cohort of lamivudine-experienced HIV/HBV coinfected individuals. AIDS. 2009;23:1707–15. doi: 10.1097/QAD.0b013e32832b43f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 9.Schafer J. Analysis of incomplete multivariate data. New York: Chapman and Hall/CRC Press; 1997. [Google Scholar]
- 10.Matthews GV, Avihingsanon A, Lewin SR, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naive individuals in Thailand. Hepatology. 2008;48:1062–9. doi: 10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 11.Sheldon J, Camino N, Rodes B, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–34. [PubMed] [Google Scholar]
- 12.Delaney WEt, Ray AS, Yang H, et al. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471–7. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293:817–29. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 14.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]