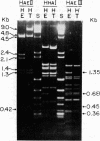
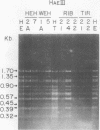
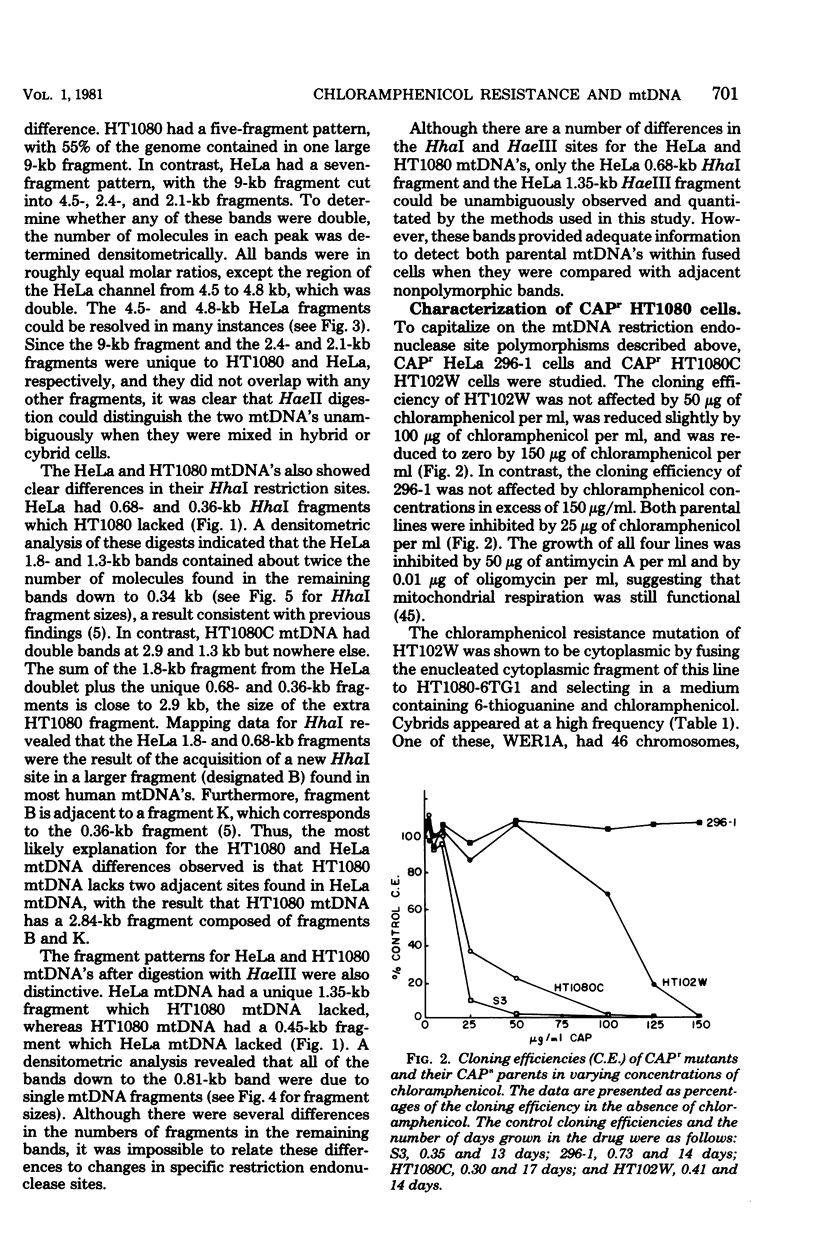
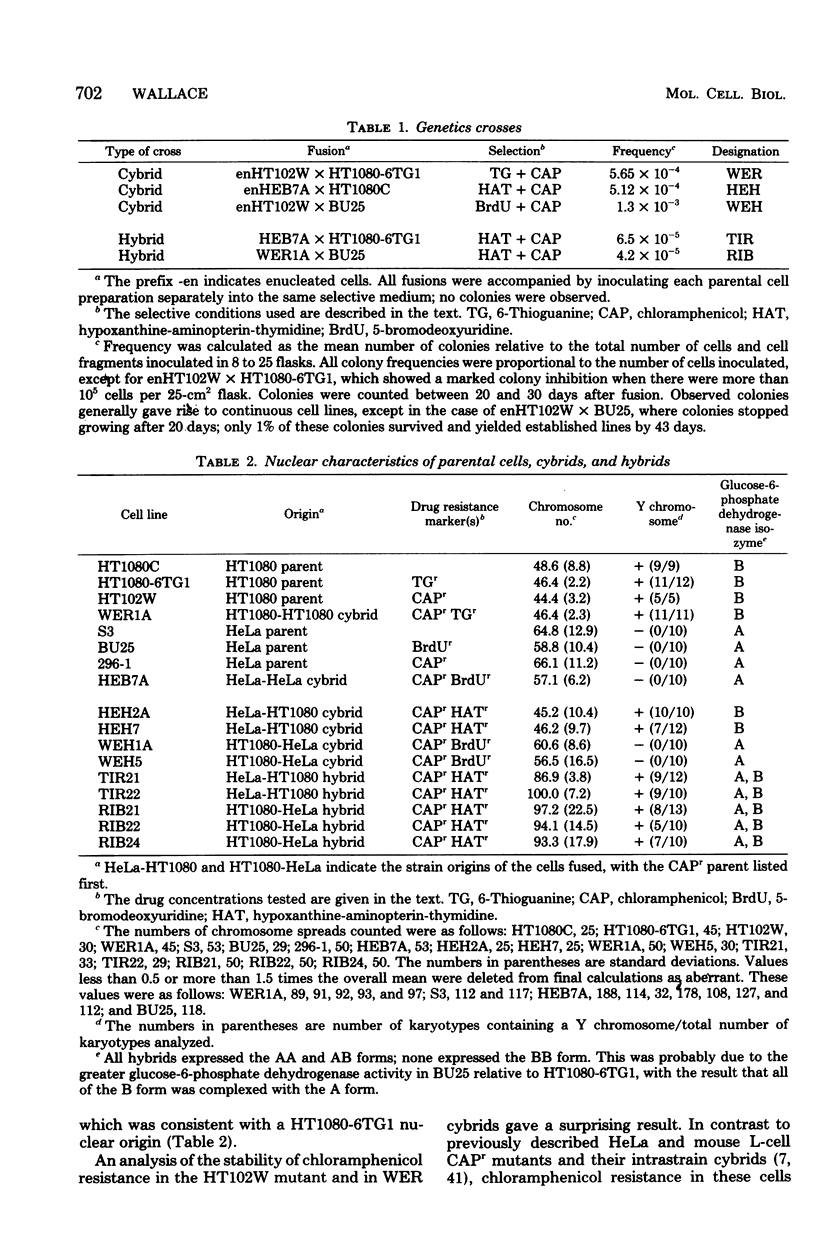
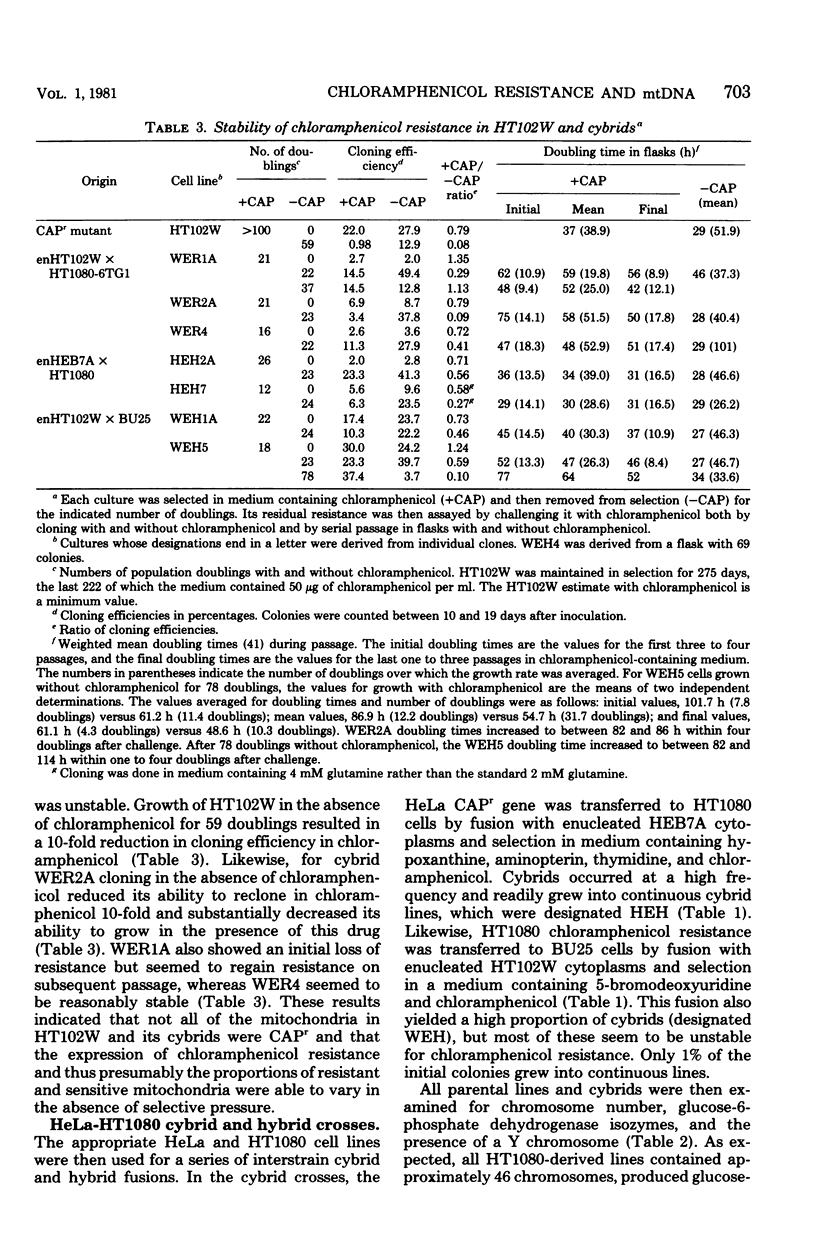
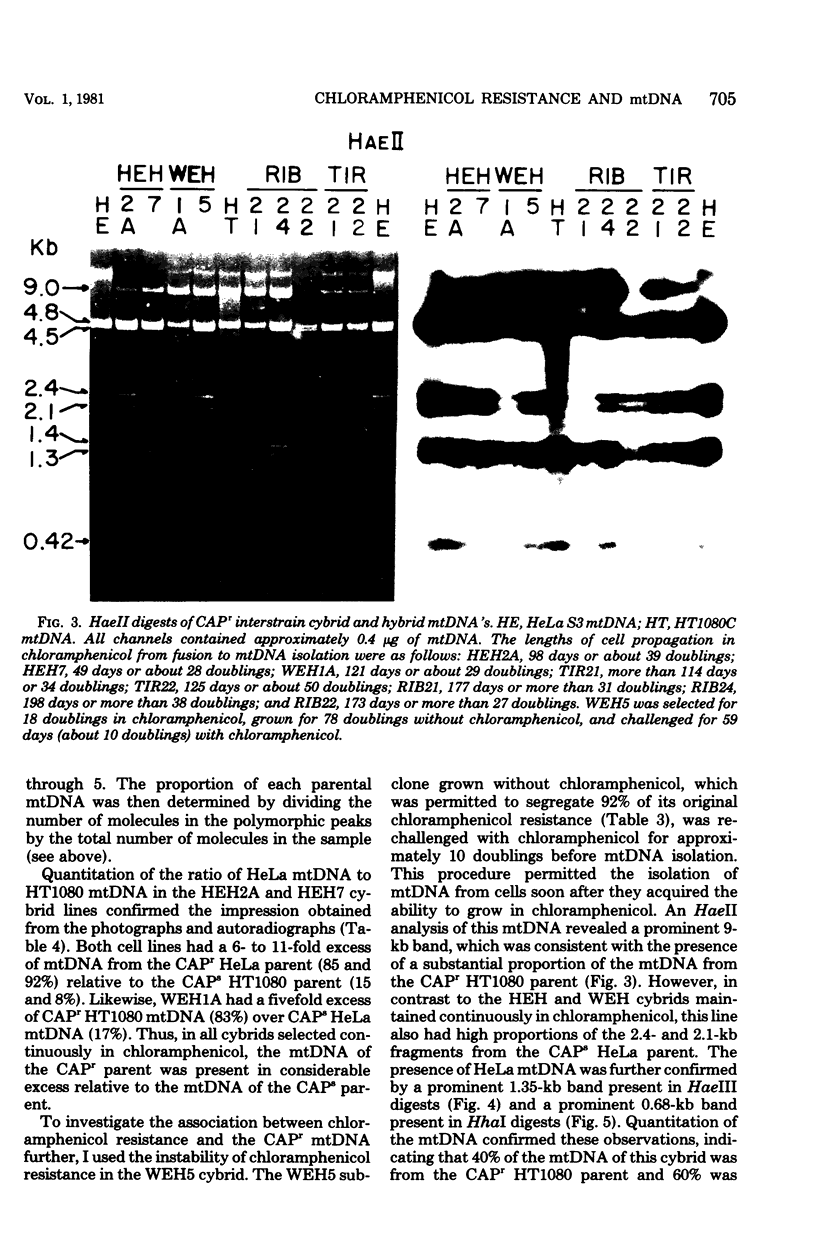
Abstract
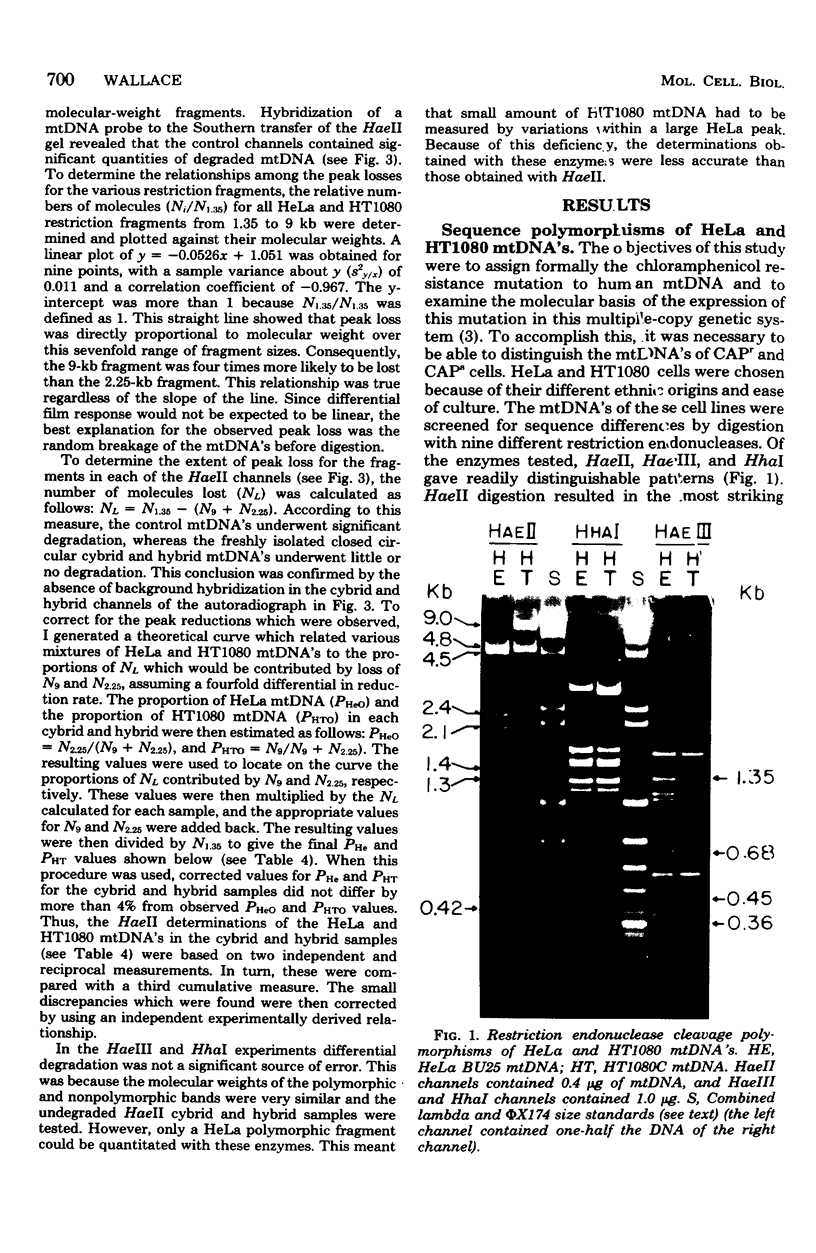
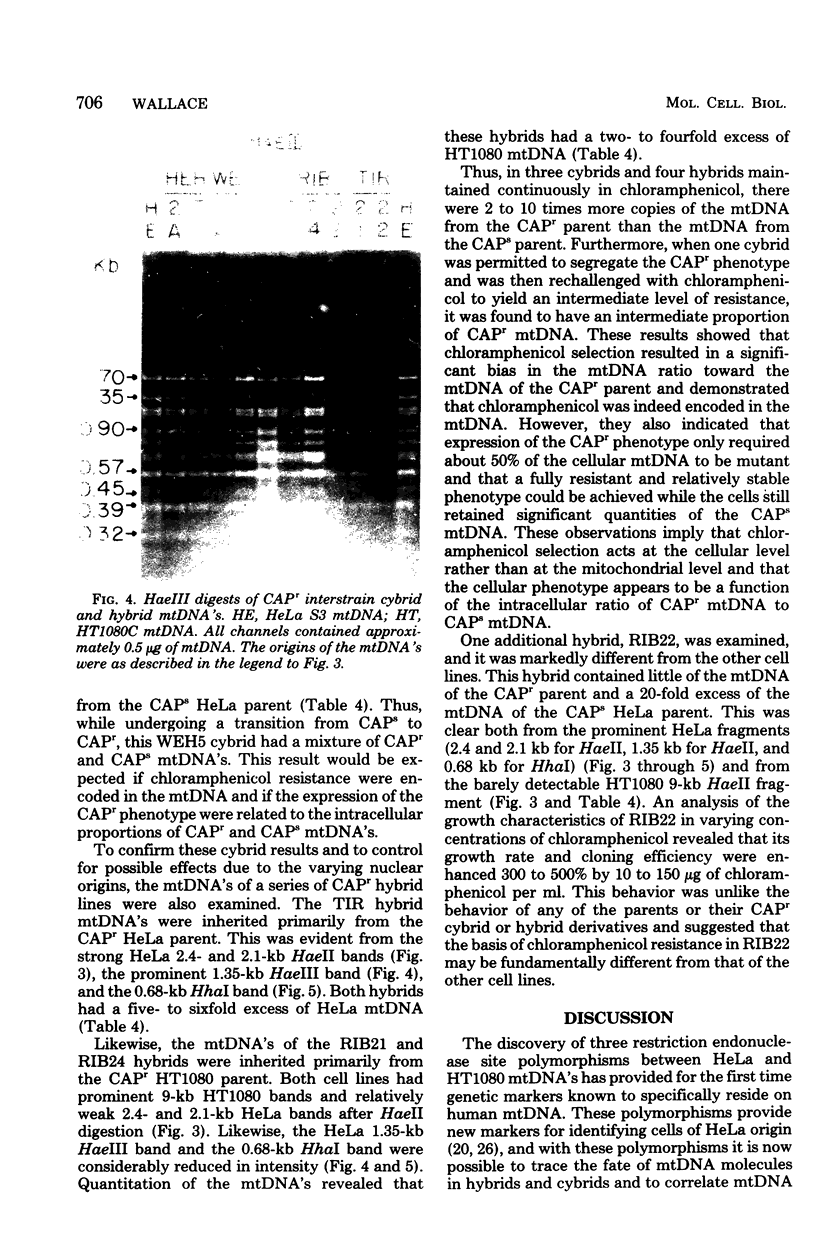
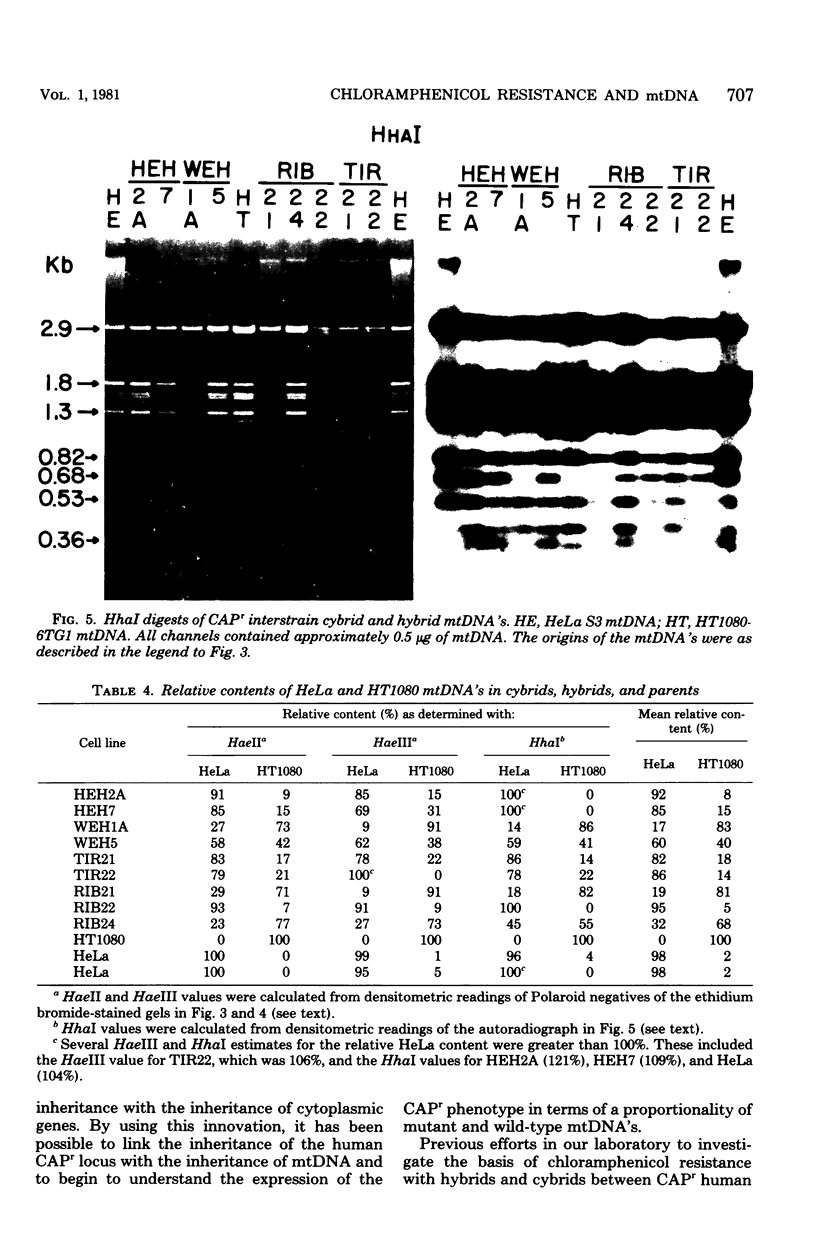
The mitochondrial deoxyribonucleic acids (mtDNA's) from human HeLa and HT1080 cells differed in their restriction endonuclease cleavage patterns for HaeII, HaeIII, and HhaI. HaeII digestion yielded a 9-kilobase fragment in HT1080, which was replaced by 4.5-, 2.4-, and 2.1-kilobase fragments in HeLa. HaeIII and HhaI yielded distinctive 1.35- and 0.68-kilobase HeLa fragments. These restriction endonuclease polymorphisms were used as mtDNA markers in HeLa-HT1080 cybrid and hybrid crosses involving the cytoplasmic chloramphenicol resistance mutation was used. mtDNA's were purified and digested with the restriction endonucleases, the fragments were separated on agarose gels, and the bands were detected by ethidium bromide staining and Southern transfer analysis. Three cybrids and four hybrids (four expressing HeLa and three expressing HT1080 chloramphenicol resistance) contained 2- to 10-fold excesses of the mtDNA of the chloramphenicol-resistant parent. One cybrid, which was permitted to segregate chloramphenicol resistance and was then rechallenged with chloramphenicol, had approximately equal proportions of the two mtDNA's. Only one hybrid was discordant. These results indicated that chloramphenicol resistance is encoded in mtDNA and that expression of chloramphenicol resistance is related to the ratio of chloramphenicol-resistant and -sensitive genomes within cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L., Davidson N., Murphy W., Lynch D., Attardi G. An electron microscope study of the relative positions of the 4S and ribosomal RNA genes in HeLa cells mitochondrial DNA. Cell. 1976 Sep;9(1):81–90. doi: 10.1016/0092-8674(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Bankier A. T., Drouin J. A different genetic code in human mitochondria. Nature. 1979 Nov 8;282(5735):189–194. doi: 10.1038/282189a0. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D., Clayton D. A. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974 Dec 25;249(24):7991–7995. [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Mitotic segregation of cytoplasmic determinants for chloramphenicol resistance in mammalian cells. I: Fusion with mouse cell lines. Somatic Cell Genet. 1977 Jan;3(1):71–92. doi: 10.1007/BF01550988. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Crews S., Attardi G. The sequences of the small ribosomal RNA gene and the phenylalanine tRNA gene are joined end to end in human mitochondrial DNA. Cell. 1980 Mar;19(3):775–784. doi: 10.1016/s0092-8674(80)80053-5. [DOI] [PubMed] [Google Scholar]
- Crews S., Ojala D., Posakony J., Nishiguchi J., Attardi G. Nucleotide sequence of a region of human mitochondrial DNA containing the precisely identified origin of replication. Nature. 1979 Jan 18;277(5693):192–198. doi: 10.1038/277192a0. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Loss of mouse chromosomes in somatic cell hybrids between HT-1080 human fibrosarcoma cells and mouse peritioneal macrophages. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3248–3252. doi: 10.1073/pnas.73.9.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- Ditta G., Soderberg K., Scheffler I. E. Chinese hamster cell mutant with defective mitochondrial protein synthesis. Nature. 1977 Jul 7;268(5615):64–67. doi: 10.1038/268064a0. [DOI] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Stroynowski I., Wallace D. C. Characterization of mitochondrial DNA in chloramphenicol-resistant interspecific hybrids and a cybrid. Somatic Cell Genet. 1980 Jul;6(4):543–554. doi: 10.1007/BF01539155. [DOI] [PubMed] [Google Scholar]
- Horak I., Coon H. G., Dawid I. B. Interspecific recombination of mitochondrial DNA molecules in hybrid somatic cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1828–1832. doi: 10.1073/pnas.71.5.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. H., Schacter B. Z., Delaney N. L., Miller T. B., McKusick V. A., Kennett R. H., Bodmer J. G., Young D., Bodmer W. F. Genetic characteristics of the HeLa cell. Science. 1976 Jan 30;191(4225):392–394. doi: 10.1126/science.1246620. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. HeLa cells resistant to bromodeoxyuridine and deficient in thymidine kinase activity. Int J Cancer. 1966 Jan;1(1):19–30. doi: 10.1002/ijc.2910010105. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais R., Giguère L. On the adaptation of cultured chick embryo cells to growth in the presence of chloramphenicol. J Cell Physiol. 1979 Oct;101(1):77–88. doi: 10.1002/jcp.1041010110. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Flandermeyer R. R. HeLa cultures defined. Science. 1976 Jan 9;191(4222):96–98. doi: 10.1126/science.1246601. [DOI] [PubMed] [Google Scholar]
- Prunell A. A photographic method to quantitate DNA in gel electrophoresis. Methods Enzymol. 1980;65(1):353–358. doi: 10.1016/s0076-6879(80)65045-9. [DOI] [PubMed] [Google Scholar]
- Prunell A., Goutorbe F., Strauss F., Bernardi G. Yield of restriction fragments from yeast mitochondrial DNA. J Mol Biol. 1977 Feb 15;110(1):47–52. doi: 10.1016/s0022-2836(77)80097-1. [DOI] [PubMed] [Google Scholar]
- Prunell A., Strauss F., Leblanc B. Photographic quantitation of DNA in gel electrophoresis. Anal Biochem. 1977 Mar;78(1):57–65. doi: 10.1016/0003-2697(77)90008-2. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Baluda M. C., Townsend D. E. Cellulose acetate electrophoresis of human glucose-6-phosphate dehydrogenase. J Lab Clin Med. 1969 Mar;73(3):531–534. [PubMed] [Google Scholar]
- Spolsky C. M., Eisenstadt J. M. Chloramphenicol-resistant mutants of human HeLa cells. FEBS Lett. 1972 Sep 15;25(2):319–324. doi: 10.1016/0014-5793(72)80514-3. [DOI] [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Stable alterations at the cell membrane of Chinese hamster ovary cells resistant to the cytotoxicity of phytohemagglutinin. Somatic Cell Genet. 1975 Jan;1(1):3–26. doi: 10.1007/BF01538729. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975 Oct;67(1):174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Mitotic segregation of cytoplasmic determinants for chloramphenicol resistance in mammalian cells II: Fusions with human cell lines. Somatic Cell Genet. 1977 Jan;3(1):93–119. doi: 10.1007/BF01550989. [DOI] [PubMed] [Google Scholar]
- Wigler M. H., Weinstein I. B. A preparative method for obtaining enucleated mammalian cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):669–674. doi: 10.1016/s0006-291x(75)80436-0. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Hirsch J. Induction of cytoplasmic respiratory deficient mutants in yeast by the folic acid analogue, methotrexate. I. Studies on the mechanism of petite induction. Mol Gen Genet. 1973 Oct 16;126(1):61–70. doi: 10.1007/BF00333482. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Hirsch J. Induction of cytoplasmic respiratory deficient mutants on yeast by the folic acid analogue, methotrexate. II. Genetic analysis of the methotrexate-induced petites. Mol Gen Genet. 1973 Oct 16;126(1):71–74. doi: 10.1007/BF00333483. [DOI] [PubMed] [Google Scholar]
- Wiseman A., Attardi G. Cytoplasmically inherited mutations of a human cell line resulting in deficient mitochondrial protein synthesis. Somatic Cell Genet. 1979 Mar;5(2):241–262. doi: 10.1007/BF01539164. [DOI] [PubMed] [Google Scholar]
- Wu M., Davidson N., Attardi G., Aloni Y. Expression of the mitochondrial genome in HeLa cells. XIV. The relative positions of the 4 S RNA genes and of the ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Oct 28;71(1):81–93. doi: 10.1016/0022-2836(72)90402-0. [DOI] [PubMed] [Google Scholar]