Abstract
Developmental HgCl2 exposures of F1 offspring (H-2q/s) from unsociable SJL/J (H-2s) dams with high susceptibility to Hg-induced autoimmunity (SFvF1) and from highly sociable FVB/NJ (FVB; H-2q) dams with lower susceptibility to Hg-induced autoimmunity (FvSF1) were investigated. Hg exposure increased the serum IgG levels of all offspring at postnatal day 21 (pnd21) and of SJL/J dams but not of FVB dams. Serum IgG anti-brain antibody (Ab) levels of pnd21 SFvF1 offspring and SJL dams were higher than those of the FvSF1 offspring and FVB dams, but Hg only increased the titers of the FVB dams and their offspring. Hg significantly elevated the presence of IgG in all brain regions of the pnd21 SFvF1 offspring, and the SFvF1 offspring had greater amounts of IgG in the brain than the FvSF1 offspring, which had Hg-induced increases in only two brain regions. Cytokine levels were elevated in the brain regions of Hg-treated pnd21 SFvF1 but not of FvSF1 offspring, and SFvF1 females had more brain regions expressing cytokines than the males. At pnd70, the serum IgG, serum antibrain Abs, amounts of brain IgG, and brain cytokine levels of all of the Hg-treated offspring were equivalent to those of their appropriate controls, suggesting that developmental Hg exposure did not induce chronic immunological effects. However, the social behaviors of Hg-exposed SFvF1 offspring at pnd70 were significantly impaired, and SFvF1 females displayed greater decline in social behaviors than males, suggesting that the higher neuroinflammation of SFvF1 females earlier in life is associated with the altered behavior. Thus, developmental Hg exposure induces long-lasting effects on social behavior of offspring, which is dependent on sex and genetics and the induction of neuroinflammation.
Key Words: mercury, mouse social behavior, IgG antibrain antibodies, maternal influences.
Mercury (Hg) has been demonstrated to induce renal autoimmunity due to autoantibody (autoAb)–induced nephritis, which is dependent on the major histocompatibility complex (MHC) haplotype (Henry et al., 1988; Hultman and Eneström, 1988; Hultman et al., 1993). The different forms of Hg, e.g., methyl Hg (MeHg), ethyl Hg, and inorganic Hg (Hg2+), differentially affect immunity (Havarinasab and Hultman, 2005), and although MeHg is more toxic than Hg2+, Hg2+ generated more antibrain Abs in A.SW (H-2s) mice than MeHg (Zhang et al., 2011). Mice carrying H-2s genes have been shown to be the most sensitive to Hg-induced autoimmunity, whereas H-2q mice are slightly less sensitive, and mice carrying H-2a or H-2b genes are low to nonresponders (Hultman et al., 1992; Nielsen and Hultman, 2002; Zhang et al., 2011). In addition, Hg is well known to be neurotoxic (Clarkson and Magos, 2006; Farina et al., 2011; Myers and Davidson, 1998). Both MeHg and Hg2+ are able to directly interact with neurons and cause oxidative stress–induced apoptosis; however, neurons are much more sensitive to MeHg compared with Hg2+ (Clarkson and Magos, 2006; Gassó et al., 2001). Neurobehavioral changes induced by MeHg were also observed in mice carrying H-2 haplotypes resistant to Hg-induced autoimmunity (Cooper and Kusnecov, 2007; Goulet et al., 2003; Kim et al., 2000), suggesting that the neurotoxicity of MeHg is at least partially through its direct interaction with neurons and/or other cells including astrocytes and microglia (Shanker et al., 2003). At subclinical doses, neurobehavioral changes of mice exposed to Hg2+ were only observed in strains carrying H-2 haplotypes sensitive to Hg-induced autoimmunity (Zhang et al., 2011). Although high levels of exposure to Hg2+ have direct neurotoxic effects, indirect neurotoxic effects at low levels of Hg exposures are posited to occur through the induction of antibrain Abs and neuroinflammation. Because MeHg could be demethylated to form Hg2+ in vivo (Ishitobi et al., 2010), the induction of antibrain Abs by Hg2+ might also contribute to the MeHg-induced neurological alterations, dependent on the MHC haplotypes and the amount of Hg2+ released.
Because various studies have demonstrated that immature neurons are more sensitive to Hg toxicity, and Hg exposure can induce neurobehavioral alterations, a concern for the neurotoxicity of dietary exposure to MeHg in children remains (Koren and Bend, 2010; Monnet-Tschudi et al., 1996; Myers et al., 2009). The incidence of impaired social behavior has been substantially increased during the past decades of years (Center for Disease Control and Prevention [CDC], 2012; Gurney et al., 2003), questioning whether exposure to Hg may be involved (Schultz 2010). In animal models, the effects of developmental Hg exposure on the social behavior (sociability) had not been previously measured. Herein, the study was designed to investigate whether exposure to Hg during fetal development affects social behavior and whether any effects were dependent on maternal influences, including genetic susceptibilities and innate and adaptive immunity that could affect neuroinflammation. Although antibrain Abs likely may not cause impaired social behavior in all mouse strains, they have been demonstrated to be crucial in some models (Martin et al., 2008; Singer et al., 2009).
This study was designed to assess whether gestational Hg2+ exposure of pregnant dams expressing certain H-2 haplotypes are induced to develop maternal antibrain Abs, which could impair the social behavior of offspring. We hypothesize that a central mechanism for reduced social behavior is environmental toxicant induction of maternal antibrain Ab deposition in the brain of developing fetuses, which causes detrimental developmental neural functions due to inflammation.
MATERIALS AND METHODS
Animals.
SJL/J (H-2s) and FVB/NJ (H-2q) mice, 7–9 week of age from the Jackson Laboratory (Bar Harbor, ME), were bred for these experiments. Mice were housed in the AAALAC-approved Wadsworth Center Animal Facility with controlled temperature (23°C), humidity, and an artificial 12-h light-12-h dark cycle, and this study was approved by Wadsworth Center’s IACUC. The two parental SJL/J and FVB strains have different sensitivities to Hg-induced immunomodulation (Abedi-Valugerdi and Möller, 2000), whereas the reciprocal F1 offspring (SFvF1 and FvSF1) have similar genetic compositions with the exception of their sex chromosomes and mitochondria. Therefore, we selected the reciprocal F1 offspring to evaluate maternal influences on social behavior through immune modulation by Hg exposure.
Experimental groups.
Two females were mated with one male per cage with free access to water and food. At gestational day (gd) 8 (based on the appearance of vaginal plug), females were taken out and placed in individual cages. A previous study indicated that developmental exposure to the subclinical dose of 50μM HgCl2 in maternal drinking water from gd8 to postnatal day (pnd) 21 induced IgG antibrain Abs both in the sera of dams and pups of the A.SW (H-2s) strain (Zhang et al., 2011). Thus, our experiments utilized 50μM HgCl2 in maternal drinking water, which was provided ad libitum from gd8 to pnd21. Control animals were treated with normal drinking water. For the Hg-treated groups, 7 SJL/J dams and 6 FVB dams were used; for the control groups, 7 SJL/J dams and 7 FVB dams were used. SFvF1 and FvSF1 offspring were developmentally exposed to HgCl2 and were evaluated for antibrain Ab levels and altered social behavior.
Blood and tissue collection.
Serum IgG, serum IgG antibrain Abs, IgG presence in brain sections, and multiple cytokines in the brain were measured at pnd21 and pnd70. Dams and randomly selected pnd21 offspring (one male and one female per litter) at weaning were euthanized by CO2 exposure and assayed. Bloods were collected by cardiac puncture; thereafter, brains were perfused with PBS and then collected. Brains of offspring were excised and dissected to obtain substantia nigra (SN), hypothalamus (HT), frontal cortex (FCX), striatum (STR), cortex (CX), hippocampus (HC), and cerebellum (CB). Thereafter, the brain regions were homogenized immediately. The brain homogenates were sonicated for 1min and then centrifuged at 12,000 × g for 30min at 4°C. Supernatants, which were for brain IgG and cytokine assays, as described later, were collected and stored at −80°C until use. Bloods were stored at 4°C for 24h, and sera were collected after centrifugation at 12,000 × g for 10min, and then stored at −80°C until use. HgCl2 exposure was stopped at pnd21. At pnd70, all of the F1 offspring were assayed for sociability and urine protein analysis; thereafter, bloods and brains were harvested as described above. One offspring of each sex was randomly selected from each litter for brain IgG and cytokine assays as described later. In addition, within each strain, sex, and treatment at pnd1, pnd21, and pnd70, respectively, one offspring was selected randomly to make whole brain homogenate as antigens (Ags) for serum antibrain Ab detection as described later. Whole brain homogenates were also made from one pnd21 SJL/J mouse and one pnd21 FVB mouse, respectively.
Urine protein analysis.
Urine protein analysis of SFvF1 and FvSF1 offspring was performed at pnd70. Mice were first anesthetized with CO2; thereafter, urine was harvested and tested immediately with urinalysis strips (SIEMENS, Tarrytown, NY). Protocol was according to manufacturer’s instruction.
ELISA.
ELISA was used to measure levels of serum IgG, serum IgG antibrain Abs, and IgG in brain regions. For measurement of serum IgG, goat anti-mouse (GAM) IgG γ-chain (Sigma, St Louis, MO) was used as capture Abs. Peroxidase-conjugated GAM IgG whole molecule (Sigma) was used as detection Abs; 3,3ʹ,5,5ʹ-tetramethylbenzidine (Sigma) was used as substrate. One mole of H2SO4 was used to stop the peroxidase-substrate reaction. The ELISA plates were read at OD450 on an ELISA analyzer (Bio-Tek, Winooski, VT). For the analysis of serum IgG antibrain Abs, brains of offspring were homogenized in the presence of homogenization buffer containing 1% NP-40, 50mM Tris-Cl (pH 7.6) (Sigma), 150mM NaCl, 2mM EDTA, 1mM Na-orthovanadate, 5mM NaF, and 10 μg/ml of proteinase inhibitor cocktail (Sigma). Brain homogenates from both Hg-treated and water-treated offspring (pnd1, pnd21, and pnd70) and normal FVB and SJL/J mice (pnd21) were used as Ags. Protein concentrations were assayed with a BCA Protein Assay Kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. Homogenates (200 µg of protein/ml) were coated to ELISA plates as brain Ags. Rat anti-mouse CD16/CD32 (Fc block; BD Pharmingen, San Jose, CA) was used to incubate with the coated brain Ags prior to the addition of serum samples. Peroxidase-conjugated GAM IgG γ-chain (Sigma) was used as detection Abs. Thereafter, the protocol was the same as described earlier. Because we did not observe any differences of brain Ags due to sex, age, or treatment under our setting, to compare the serum antibrain Ab levels, we used the same pnd1, pnd21, and pnd70 homogenates from the control (water) groups for analysis of all sera of each strain. For the quantification of the IgG concentration in different brain regions, the protocol was the same as serum IgG detection as described earlier. The concentration of IgG in the homogenate of each brain region was calculated as ng IgG/mg protein.
Luminex analysis.
Cytokines were measured in the brain sections of SFvF1 and FvSF1 offspring. Because autoAbs might cause elevation of multiple types of cytokines derived from multiple types of immune cells, we performed Luminex analysis to measure brain cytokines. At pnd21, classical proinflammatory cytokines (interleukin [IL]-1β, IL-6, and tumor necrosis factor [TNF]-α) and other myeloid cell or dendritic cell–related cytokines (granulocyte-macrophage colony-stimulating factor [GM-CSF], monocyte chemotactic protein [MCP]-1, macrophage inflammatory protein [MIP]-2, and IL-12p70), Th1 cytokines (interferon [IFN]-γ and IL-2), Th2 cytokines (IL-4, IL-5, IL-10, and IL-13), and Th17 cytokines (IL-17) were assayed at pnd21. Based on the data from pnd21 offspring, at pnd70, IL-1β, IL-6, MCP-1, IL-12p70, IL-4, IL-10, IL-13, and IL-17 were quantified. Fluorokine MAP multiplex mouse cytokine panel (R&D, Minneapolis, MN) was used. Protocol followed the manufacturer’s instructions. Regional brain homogenate preparation and protein assay were as described above. Plates were run on a Luminex 100 IS analyzer. The cytokine concentration was calculated as pg cytokine/mg protein.
Sociability test.
The sociability behaviors of the F1 offspring were tested using the three-chamber testing system (Dold Labs & Engineering, Seguin, TX) developed by Crawley and colleagues (Moy et al., 2007). The procedure was performed, as described (Moy et al., 2007), except that BALB/c mice (Taconic Farms, Inc., Hudson, NY) were used. The time spent in each of the three chambers and the migration among the three chambers were recorded by the affiliated social interaction software; the time sniffing the stimulus mouse and the time engaged in self-grooming behavior were recorded by hand-held timers. To avoid any left/right bias, for each test, the stimulus mouse was alternated in the left or the right chamber. Only the data collected from the second 10min were used for behavioral analysis. If more than one male or female per litter was utilized, the time in each chamber was averaged.
Statistics.
All the data were in the form of mean ± SEM. Data for the dams were first analyzed using two-way ANOVA (strain and treatment); data for the offspring were first analyzed using three-way ANOVA (strain, sex, and treatment). If there was a significant difference, post hoc Bonferroni or Holm-Sidak tests were performed as stated. A value of p < 0.05 was selected as the level of significance. All statistical analyses were performed with SigmaStat 3.1.
RESULTS
Hg Effects on Litters
The dose of HgCl2 used (50µM) was selected because it was previously shown to have no detrimental effect on A.SW offspring, a strain known to be sensitive to Hg-induced immunomodulation; there were no effects on litter size, birth weights, and growth (Zhang et al., 2011). For the SFvF1 or FvSF1 offspring, there also were no significant differences with regard to litter sizes or body weights due to the Hg treatment; the average litter sizes were 6–7 pups. Birth weights did not significantly differ (data not shown), all animals looked healthy, and no abnormal appearances were observed after Hg treatment.
Urine Protein Levels Were Not Altered at Pnd70 After Hg Treatment
Because SJL/J mice are susceptible to Hg-induced renal autoimmunity (Abedi-Valugerdi and Möller, 2000; Hultman et al., 1992), we assessed whether developmental Hg exposure could induce proteinuria in F1 offspring. The urine protein levels were measured at pnd70 when the mice were assessed for behavioral activity because poor kidney functions might affect behavior. The gestational Hg treatment did not compromise adult kidney function, in that urine protein was not changed either in SFvF1 (100±0.1 [control] vs. 98.6±33.6mg/dl) or in FvSF1 offspring (98.8±29.1 [control] vs. 107.1±19.1mg/dl).
Serum IgG Levels
Serum IgG levels of the dams were measured at weaning of the offspring, and those of the offspring were measured at pnd21 and pnd70. For dam serum IgG, two-way ANOVA of dams indicated significant treatment differences (F (1,24) = 4.686, p = 0.042) with no strain differences. No significant interactions were indicated. Post hoc Bonferroni test indicated that serum IgG levels were significantly elevated in the Hg-treated SJL/J dams (t = 3.087, p = 0.005) (Fig. 1A), but not in the FVB dams (Fig. 1B). For pnd21 offspring serum IgG, three-way ANOVA indicated significant treatment (F (1,43) = 127.188, p < 0.001) and strain (F (1,43) = 4.281, p = 0.045) differences but no sex differences. Post hoc Bonferroni test indicated that Hg treatment significantly increased serum IgG levels of both the SFvF1 (male: t = 9.208, p < 0.001; female: t = 7.746, p < 0.001) and FvSF1 offspring (male: t = 4.411, p < 0.001; female: t = 3.645, p < 0.001) (Figs. 1A and B). At pnd70, the serum IgG levels of the offspring were no longer elevated by the Hg exposure (Fig. 1).
Fig. 1.
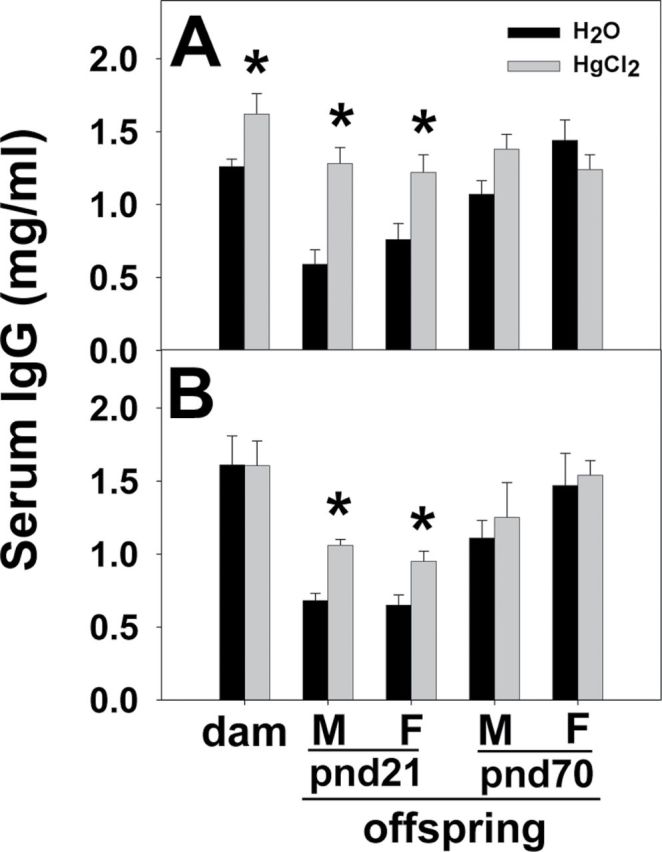
Serum IgG levels of dams and pnd21 and pnd70 offspring. GAM IgG γ-chain Abs were used as capture Abs, and peroxidase-conjugated GAM IgG whole molecule Abs were used as detection Abs. Serum IgG was quantified by ELISA for SJL/J dams and SFvF1 offspring (A) and for FVB dams and FvSF1 offspring (B). M, male; F, female. * indicates a significant difference of the Hg group compared with the counterpart water group (p < 0.05). Number of mice used (H2O; Hg): SJL/J dam (7; 7), pnd21M (5; 7) and F (5; 7) SFvF1, pnd70M (6; 7) and F (5; 7) SFvF1; FVB dam (7; 6), pnd21M (7; 6) and F (7; 6) FvSF1, pnd70M (6; 6) and F (6; 6) FvSF1.
Serum IgG Antibrain Abs
Within each strain, brain Ags from untreated pnd1 offspring were used to test the sera of the dams for IgG antibrain Abs; brains from untreated pnd21 offspring and pnd70 offspring were used as Ags for pnd21 and pnd70 offspring testing, respectively. The sera from each group of Hg-treated mice and the counterpart water (control) group were tested using the same brain homogenate. For serum antibrain Abs of dams, two-way ANOVA indicated significant strain (F (1,24) = 9.637, p = 0.005) and treatment differences (F (1,24) = 4.368, p = 0.043). Post hoc Bonferroni test indicated no significant treatment differences for the SJL/J dams; both groups of the SJL/J dams had high levels of antibrain Abs (Fig. 2A). However, the levels of antibrain Abs of Hg-treated FVB dams were significantly increased compared with those of the water group (t = 2.041, p = 0.048) (Fig. 2B). For serum antibrain Abs of pnd21 offspring, three-way ANOVA indicated significant strain (F (1,43) = 7.139, p = 0.011) and treatment differences (F (1,43) = 4.374, p = 0.043) but no sex differences. Post hoc Bonferroni test indicated no significant treatment differences between water and Hg-treated SFvF1 offspring; both groups had relatively high levels of serum antibrain Abs (Fig. 2A). The levels of antibrain Abs were significantly higher for the Hg-treated pnd21 FvSF1 offspring (male: t = 2.430, p = 0.019; female: t = 2.163, p = 0.036) compared with those of the water group (Fig. 2B). In addition, the levels of serum IgG antibrain Abs of pnd21 offspring were similar to those of their dams for both of the two strains, in that the SFvF1 dams and offspring had high levels and no differences due to Hg, whereas the FvSF1 dams and offspring had lower levels that were elevated by Hg. At pnd70, the levels of serum antibrain Abs returned to relatively low levels in the SFvF1 offspring, indicating that the pnd21 SFvF1 levels likely reflect mainly the levels of the SJL dams. There were no significant differences between the water and Hg groups of pnd70 offspring of either strain (Fig. 2). The serum antibrain Abs from the SFvF1 and FvSF1 offspring and their dams also reacted to brain Ags from pnd21 SJL/J or FVB mice, and serum IgG antibrain Abs from SJL/J dams also reacted with pnd70 SFvF1 brain Ags (data not shown). No significant differences were observed for Abs binding to brain Ags from water-treated mice and Hg-treated mice (data not shown). No significant differences were observed in brains from pnd1, pnd21, or pnd70 offspring when antibrain Abs from dam sera were tested (data not shown). No sex differences of brain Ags were observed (data not shown).
Fig. 2.
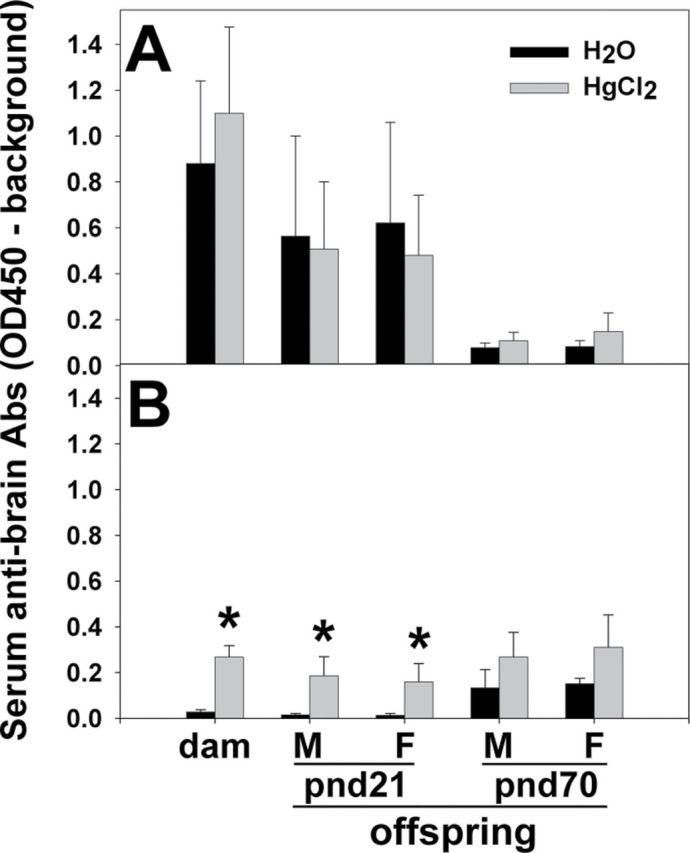
Serum antibrain IgG Ab levels of dams and pnd21 and pnd70 offspring. Brain homogenates from normal pnd21 offspring and pnd70 offspring were used as Ags to detect serum antibrain Abs in pnd21 and pnd70 offspring, respectively; normal pnd1 brain homogenate was used as brain Ags to detect dam antibrain IgG Abs. Peroxidase-conjugated GAM IgG γ-chain Abs were used as detection Abs. ELISA was used for assessment of serum antibrain IgG Abs for SJL/J dams and SFvF1 offspring (A) and FVB dams and FvSF1 offspring (B). M, male; F, female. * indicates a significant difference of the Hg group compared with the counterpart water group (p < 0.05). Number of mice used (H2O; Hg): SJL/J dam (7; 7), pnd21M (5; 7) and F (5; 7) SFvF1, pnd70M (6; 7) and F (5; 7) SFvF1; FVB dam (7; 6), pnd21M (7; 6) and F (7; 6) FvSF1, pnd70M (6; 6) and F (6; 6) FvSF1.
Deposition of IgG in the Brain
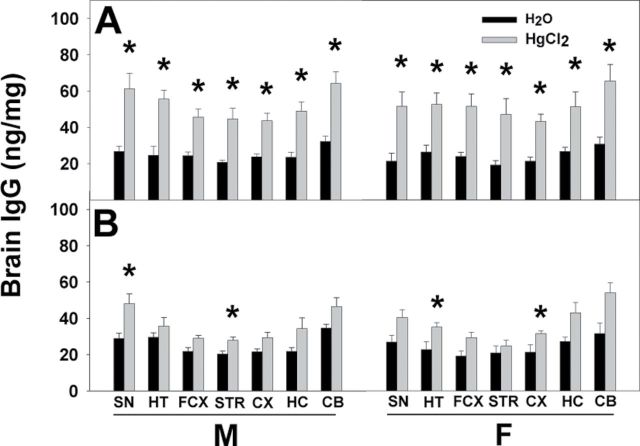
Homogenates of brain regions from the pnd21 and pnd70 offspring were quantified for the presence of IgG. At pnd21, three-way ANOVA indicated significant treatment differences for all brain regions (SN: F (1,40) = 32.096, p < 0.001; HT: F (1,40) = 37.334, p < 0.001; FCX: F (1,40) = 36.907, p < 0.001; STR: F (1,40) = 20.790, p < 0.001; CX: F (1,40) = 52.970, p < 0.001; HC: F (1,40) = 26.452, p < 0.001; CB: F (1,40) = 35.827, p < 0.001) and strain differences for many regions (HT: F (1,40) = 9.280, p = 0.004; FCX: F (1,40) = 18.332, p < 0.001; STR: F (1,40) = 7.677, p = 0.009; CX: F (1,40) = 13.212, p < 0.001), but no sex differences. Post hoc Bonferroni test indicated that the Hg-treated SFvF1 offspring had significantly elevated amounts of IgG (ng/mg protein) in all tested brain regions compared with the water group for both males and females (Fig. 3A). For the pnd21 FvSF1 offspring, the amount of IgG in some brain regions also was significantly increased by exposure to Hg (Fig. 3B). At pnd70, the significant differences between water and Hg groups of all offspring were no longer present (data not shown).
Fig. 3.
IgG levels in brain regions of pnd21 offspring. Whole brains of pnd21 SFvF1 (A) and FvSF1 (B) offspring were dissected into SN, HT, FCX, STR, CX, HC, and CB, and homogenates of each region were used to assess presence of IgG by ELISA. GAM IgG γ-chain Abs were used as capture Abs, and peroxidase-conjugated GAM IgG whole molecule Abs were used as detection Abs for quantification of brain IgG (ng/mg protein). M, male; F, female; * indicates a significant difference of the Hg group compared with the counterpart water group (p < 0.05). Number of mice used (H2O; Hg): M (5; 7) and F (5; 7) SFvF1, M (7; 6) and F (4; 6) FvSF1.
Effects of Developmental HgCl2 Exposure on Sociability
At pnd70, the social behaviors of the offspring were tested, as previously described (Moy et al., 2007). Three-way ANOVA compared the social behavior of male and female SFvF1 and FvSF1 offspring in the H2O and HgCl2 treatment groups. Both male SFvF1 and FvSF1 offspring demonstrated more interaction with the stimulus mouse than the females regardless of the Hg treatment (H2O, p = 0.002; HgCl2, p < 0.001; Fig. 4). The greater social interactions of the males also were apparent based on increased entrances to the chamber of the stimulus mouse and increased sniffing time (Table 1). SFvF1 (p < 0.001) and FvSF1 (p = 0.045) males had more sociability than the females, and female FvSF1 offspring had more sociability than the SFvF1 females (p = 0.013). The HgCl2 treatment significantly lowered interaction with the stimulus mouse for both males (p = 0.047) and females (p = 0.005), but the HgCl2 significantly lowered social behavior only for the SFvF1 offspring (p < 0.001) (Fig. 4). To address whether there was a trafficking/mobility difference, that is entrances and exits among chambers, we totaled the number for each group. Interestingly, the SFvF1 offspring migrated more than the FvSF1 offspring (F (1,41) = 9.12, p = 0.004) (Table 1), but there were no sex, treatment, or interaction differences. Thus, the lower sociability of the SFvF1 mice was not due to less trafficking. Hg treatment did not significantly change the self-grooming behavior of either strain (data not shown).
Fig. 4.
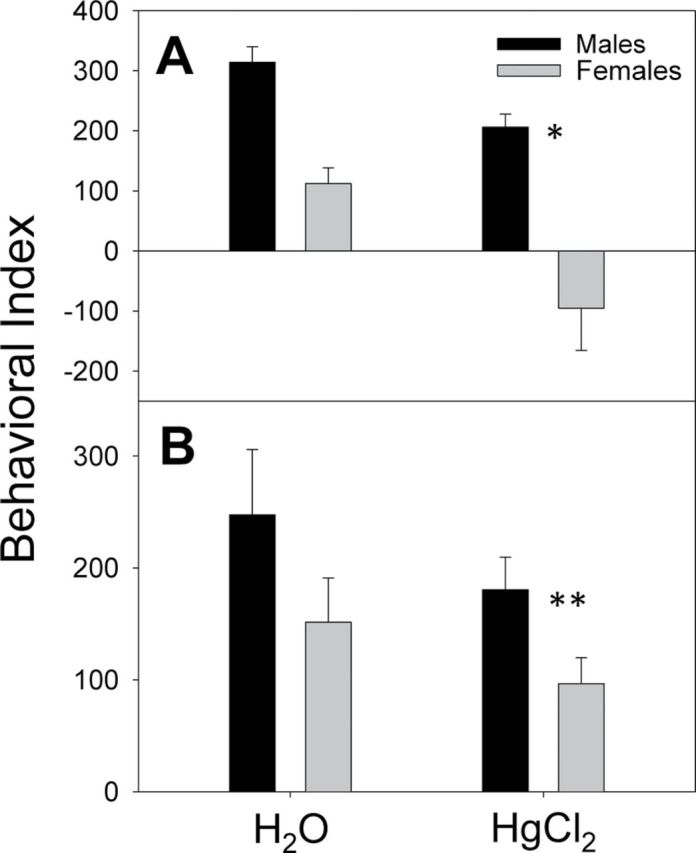
Social behavior of pnd70 offspring. Three-chamber mouse social behavior testing was performed. BALB/c mice of the same age and sex were used as stimulus mice. A 20-min test was performed in which only the behavior in the second 10min was analyzed. The behavioral index is the time (s) in the side chamber with the novel mouse of same sex minus time in the side chamber with the novel object for the SFvF1 offspring (A) and the FvSF1 offspring (B). Three-way ANOVA (sex, treatment, and strain) indicated a significant sex difference (F (1,42) = 30.68, p < 0.001), HgCl2 effect (F (1,42) = 12.57, p < 0.001) and a significant interaction (p = 0.012) between sex and strain. Pairwise multiple comparisons (Holm-Sidak method) indicated significant differences for the following: H2O versus HgCl2 for males (p = 0.047) and females (p = 0.005), male versus female for H2O (p = 0.002) and HgCl2 (p < 0.001), female SFvF1 versus female FvSF1 (p = 0.013), male versus female FvSF1 (p = 0.45), male versus female SFvF1 (p < 0.001), and H2O versus HgCl2 for SFvF1 offspring (p < 0.001). Number litters assayed for the treatment groups (H2O; Hg) were M (6; 7) and F (5; 7) SFvF1, M (7; 6) and F (6; 6) FvSF1; * indicates significant sex and treatment difference and ** indicates only sex difference.
Table 1.
Behavior Analysis of SFvF1 and FvSF1 Offspring After Developmental Exposure to HgCl2
| Strain | Chamber entrancesa | Sniffingb | ||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||
| H2O | HgCl2 | H2O | HgCl2 | H2O | HgCl2 | H2O | HgCl2 | |
| SFvF1 | 2.50±0.67 | 2.86±0.99 | 0.80±0.49 | −2.86±1.28 | 89.67±16.47 | 57.29±5.79 | 57.40±4.94 | 35.43±6.44 |
| (58.5±7.43)c | (67.7±5.85) | (72.8±10.80) | (55.5±4.36) | |||||
| FvSF1 | 4.29±2.29 | 2.83±1.33 | 2.00±2.13 | 0.83±1.11 | 76.60±13.27 | 66.33±7.89 | 55.17±10.00 | 38.5±5.97 |
| (44.1±5.12) | (52.0±5.33) | (47.0±9.97) | (53.3±4.28) | |||||
Notes. All results are reported as mean ± SEM. For chamber entrances (a), the number of times entering the chamber with the empty cage was subtracted from the entrances into the chamber with the novel mouse. All entrances and exits from chambers also were totaled (c); the SFvF1 mice differed from the FvSF1 mice (F (1,41) = 9.12, p = 0.004) by three-way ANOVA, but there were no significant sex, treatment, or interaction differences. Three-way ANOVA analysis of the chamber entrances indicated a sex difference (F (1,41) = 7.75, p = 0.008) but no treatment differences and no significant interactions. For sniffing (b), the time (s) in direct contact with the novel mouse was measured. Three-way ANOVA analysis of sniffing indicated significant sex (F (1,41) = 14.40, p < 0.001) and treatment (F (1,41) = 9.06, p = 0.004) differences. There were no strain differences or significant interactions.
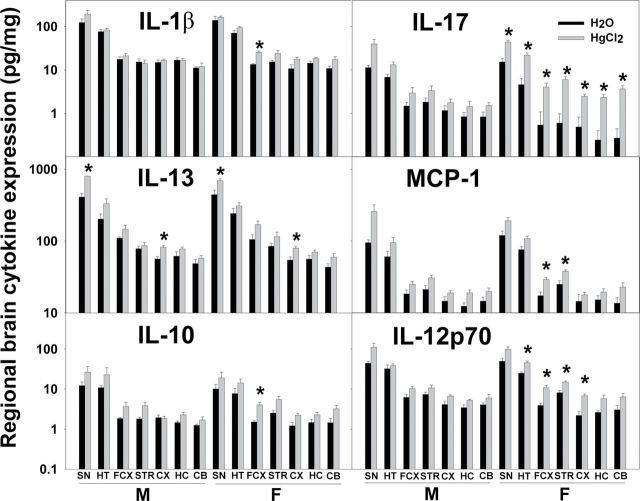
Levels of Cytokines in the Brains of the Hg-Treated Pnd21 SFvF1 Offspring Were Elevated
Because Hg enhanced the amount of IgG in multiple brain regions of male and female SFvF1 offspring but significantly lowered social behavior only of the female SFvF1 offspring, as a measure of neuroinflammation, cytokine expression in various brain regions was assayed. Hg did enhance the expression of some cytokines in different brain regions of the SFvF1 offspring, and females had more regions with cytokine levels enhanced by Hg (Fig. 5). There were no Hg effects on the cytokine levels of the FvSF1 offspring (Supplementary fig. 1). Additionally, at pnd70, brain cytokine levels of both SFvF1 and FvSF1 offspring were equivalent between the Hg-treated and the water control groups (data not shown). GM-CSF, IFN-γ, IL-2, IL-5, MIP-2, and TNF-α were not detectable (data not shown). The following cytokine results were obtained for the pnd21 brain regions. For IL-1β, three-way ANOVA indicated significant treatment differences (FCX: F (1,36) = 7.103, p = 0.012; CX: F (1,36) = 5.223, p = 0.028), strain differences (SN: F (1,36) = 18.519, p < 0.001; HT: F (1,36) = 48.896, p < 0.001; FCX: F (1,36) = 52.780, p < 0.001; STR: F (1,36) = 45.642, p < 0.001; CX: F (1,36) = 53.105, p < 0.001; HC: F (1,36) = 34.684, p < 0.001; CB: F (1,36) = 19.297, p < 0.001), and sex differences (STR: F (1,36) = 5.725, p = 0.022; CB: F (1,36) = 5.234, p = 0.028). Post hoc Bonferroni tests indicated that Hg treatment significantly increased IL-1β in the FCX for SFvF1 females. For IL-13, three-way ANOVA indicated significant treatment differences (SN: F (1,36) = 4.573, p = 0.04; CX: F (1,36) = 6.928, p = 0.012) and strain differences (SN: F (1,36) = 9.039, p = 0.005; FCX: F (1,36) = 5.040, p = 0.031; STR: F (1,36) = 64.032, p < 0.001; CX: F (1,36) = 17.083, p < 0.001; HC: F (1,36) = 20.022, p < 0.001; CB: F (1,36) = 91.057, p < 0.001) but no sex differences. Post hoc Bonferroni tests indicated that Hg treatment significantly increased IL-13 in the SN and CX for both SFvF1 males and females. For IL-10, three-way ANOVA indicated significant treatment differences (FCX: F (1,36) = 10.553, p = 0.003; STR: F (1,36) = 10.190, p = 0.003; HC: F (1,36) = 4.150, p = 0.048), strain differences (SN: F (1,36) = 15.646, p < 0.001; HT: F (1,36) = 9.413, p = 0.004; FCX: F (1,36) = 40.313, p < 0.001; STR: F (1,36) = 48.618, p < 0.001; CX: F (1,36) = 60.398, p < 0.001; HC: F (1,36) = 13.765, p < 0.001; CB: F (1,36) = 20.261, p < 0.001), and sex differences (CB: F (1,36) = 5.261, p = 0.028). Post hoc Bonferroni tests indicated that Hg treatment significantly increased IL-10 in the FCX for SFvF1 females. For IL-17, three-way ANOVA indicated significant treatment differences (SN: F (1,36) = 18.315, p < 0.001; HT: F (1,36) = 26.686, p < 0.001; FCX: F (1,36) = 8.065, p = 0.007; STR: F (1,36) = 14.127, p < 0.001; CX: F (1,36) = 6.955, p = 0.012; HC: F (1,36) = 7.510, p = 0.010; CB: F (1,36) = 14.664, p < 0.001), strain differences (SN: F (1,36) = 35.302, p < 0.001; HT: F (1,36) = 45.707, p < 0.001; FCX: F (1,36) = 13.988, p < 0.001; STR: F (1,36) = 21.288, p < 0.001; CB: F (1,36) = 5.726, p = 0.022), and sex differences (CB: F (1,36) = 5.976, p = 0.020). Post hoc Bonferroni tests indicated that Hg treatment significantly increased the inflammatory cytokine IL-17 in all of the brain regions for SFvF1 females. For MCP-1, three-way ANOVA indicated significant treatment differences (SN: F (1,36) = 8.353, p = 0.007; FCX: F (1,36) = 7.943, p = 0.008; STR: F (1,36) = 4.544, p = 0.04; CB: F (1,36) = 10.034, p = 0.003), strain differences (HT: F (1,36) = 11.463, p = 0.002; FCX: F (1,36) = 25.595, p < 0.001; CX: F (1,36) = 6.811, p = 0.013; HC: F (1,36) = 14.922, p < 0.001; CB: F (1,36) = 12.387, p = 0.001), and sex differences (STR: F (1,36) = 5.899, p = 0.02; HC: F (1,36) = 6.829, p = 0.013). Post hoc Bonferroni tests indicated that Hg treatment significantly increased MCP-1 in the FCX and STR for SFvF1 females. For IL-12p70, three-way ANOVA indicated significant treatment differences (SN: F (1,36) = 10.616, p = 0.002; HT: F (1,36) = 6.332, p = 0.017; FCX: F (1,36) = 17.436, p < 0.001; STR: F (1,36) = 9.435, p = 0.004; CX: F (1,36) = 15.590, p < 0.001; HC: F (1,36) = 5.865, p = 0.021; CB: F (1,36) = 5.608, p = 0.024), strain differences (SN: F (1,36) = 69.432, p < 0.001; HT: F (1,36) = 171.972, p < 0.001; FCX: F (1,36) = 141.127, p < 0.001; STR: F (1,36) = 123.719, p < 0.001; CX: F (1,36) = 77.515, p < 0.001; HC: F (1,36) = 59.344, p < 0.001; CB: F (1,36) = 54.475, p < 0.001), and sex differences (STR: F (1,36) = 5.812, p = 0.021). Post hoc Bonferroni tests indicated that Hg treatment significantly increased IL-12p70 in the HT, FCX, STR, and CX for SFvF1 females.
Fig. 5.
Expression of brain cytokine levels of offspring at pnd21. Brains from pnd21 SFvF1 offspring were dissected into SN, HT, FCX, STR, CX, HC, and CB, and their homogenates were assayed for cytokine expression (pg/mg protein). M, male; F, female. * indicates a significant difference of the Hg group compared with the counterpart water group (p < 0.05). Number of mice used (H2O; Hg): M (5; 6) and F (5; 6) SFvF1.
Although Hg did not affect the expression of IL-4 or IL-6 in the brain, IL-6 expression and, to a lesser extent, IL-4 expression were significantly greater in the SFvF1 offspring compared with the FvSF1 offspring, and SFvF1 females expressed higher IL-6 levels in the STR and HC than the males (Table 2).
Table 2.
IL-6 and IL-4 Expression in Different Brain Regions of SFvF1 and FvSF1 Offspring at Pnd21 (pg/mg protein)
| Brain sections | Treatment | IL-6 | IL-4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SFvF1 | FvSF1 | SFvF1 | FvSF1 | ||||||
| Males | Females | Males | Females | Males | Females | Males | Females | ||
| SN | H2O | 48.6±7.6 | 57.4±8.6 | 7.3±1.2 | 6.5±1.5 | 1.6±1.3 | 1.9±1.8 | 4.8±1.0 | 4.8±0.9 |
| HgCl2 | 98.7±30.3 | 79.1±11.4 | 8.3±1.5 | 6.8±0.8 | 0.8±0.8 | 6.0±6.0 | 4.6±0.7 | 5.5±0.5 | |
| HT | H2O | 33.5±8.8 | 36.0±8.6 | 3.9±0.7 | 3.8±0.6 | 4.5±0.5 | 3.6±2.3 | 4.2±0.6 | 3.6±0.4 |
| HgCl2 | 21.8±3.6 | 74.4±23.1 | 4.1±0.3 | 2.8±0.4 | 5.6±4.8 | 4.8±2.8 | 4.2±0.8 | 4.3±0.4 | |
| FCX | H2O | 12.0±5.2 | 6.2±4.9 | 0.6±0.2 | 0.6±0.4 | 1.1±0.4 | 0.8±0.1 | 2.1±0.3 | 2.4±0.3 |
| HgCl2 | 6.2±0.4 | 8.2±3.1 | 0.5±0.1 | 0.5±0.1 | 0.7±0.5 | 2.5±0.9 | 2.7±0.6 | 2.9±0.5 | |
| STR | H2O | 3.0±1.2 | 5.1±0.5a | 2.7±1.3 | 1.2±0.5 | 1.2±0.4 | 0.7±0.1 | 1.8±0.7 | 1.7±0.2 |
| HgCl2 | 3.2±0.1 | 7.6±0.9a | 1.8±0.7 | 1.0±0.3 | 0.5±0.3 | 1.2±0.9 | 2.5±0.4 | 2.4±0.4 | |
| CX | H2O | 2.0±0.7 | 7.9±4.2 | 1.0±0.3 | 0.8±0.3 | 1.2±0.3 | 1.0±0.3 | 1.7±0.6 | 1.6±0.3 |
| HgCl2 | 3.0±0.2 | 7.9±3.0 | 0.7±0.3 | 0.7±0.5 | 1.5±0.4 | 2.3±0.7 | 2.0±0.3 | 2.3±0.3 | |
| HC | H2O | 2.2±0.9b | 4.0±0.9a,b | 0.7±0.4b | 0.8±0.3b | 1.2±0.3 | 0.9±0.2 | 2.0±0.8 | 2.3±0.3 |
| HgCl2 | 1.6±0.1b | 4.6±0.3a,b | 1.4±0.7b | 0.9±0.7b | 0.5±0.3 | 2.4±0.7 | 2.1±0.3 | 2.3±0.2 | |
| CB | H2O | 3.6±1.2 | 4.0±1.4 | 0.8±0.4 | 1.1±0.3 | 1.3±0.2c | 0.9±0.2 | 2.1±0.3 | 2.0±0.3 |
| HgCl2 | 10.9±5.0 | 5.3±0.9 | 2.3±1.5 | 1.6±0.3 | 0.8±0.2c | 2.7±0.9 | 2.1±0.3 | 2.3±0.2 | |
Notes. All results are reported as mean ± SEM. For IL-6, three-way ANOVA indicated significant strain differences for all regions with p < 0.001–0.006; aindicates sex difference of SFvF1 (STR: t = 2.75, p = 0.012; HC: t = 3.16, p = 0.004) and females of each strain (STR: t = 4.73, p = 0.001; HC: t = 5.12, p = 0.001); bindicates strain difference (HC for H2O, t = 3.53, p = 0.002; HgCl2, t = 2.78, p = 0.01). For IL-4, three-way ANOVA indicated significant strain differences for FCX, STR, HC, and CB (p = 0.003–0.024); cindicates that males of each strain differ (t = 2.48, p = 0.018).
DISCUSSION
The parental strains utilized herein were selected based on their H-2 haplotypes (H-2s and H-2q) and their known social behaviors. Mice carrying H-2s genes (SJL/J mice) are known to be susceptible to Hg-induced autoimmune disease, whereas mice of H-2q haplotype (FVB mice) are less susceptible (Abedi-Valugerdi and Möller, 2000; Hansson and Abedi-Valugerdi, 2003; Hultman et al., 1992; Warfvinge et al., 1995). The parental strains were posited to have different sensitivities to Hg-induced autoimmunity, in that although Hg induces antinucleolar autoAbs in SJL and FVB mice, the Ab levels of FVB mice were lower compared with those of SJL/J mice; additionally, Hg treatment did not induce renal IgG deposition in FVB mice (Abedi-Valugerdi and Möller, 2000), which is suggested to be critical for Hg-induced nephritis (Hultman and Eneström, 1988). With regard to social behavior, we anticipated that the highly social FVB strain would dominate or at least lessen the unsocial nature of SJL mice without any treatment but would be sensitive to environmentally induced impairment of social behavior. As expected, the untreated SFvF1 and FvSF1 offspring were more sociable compared with SJL/J mice. Thus, the SFvF1 and FvSF1 strains are a good model to test HgCl2 effects on social behavior because both parental strains are sensitive to HgCl2-induced autoimmunity, and the F1 offspring have similar genetics and normal social behavior.
Maternal autoantibody induction by Hg was considered to have potential detrimental consequences to fetal brains because more IgG can enter fetal brains, in that the blood-brain barrier (BBB) is absent or less intact in the developing central nervous system (Lossinsky et al., 1986; Vorbrodt et al., 1986). However, there is evidence supporting the concept of an existing BBB during the fetal period (Ek et al., 2012). The higher amounts of IgG in the brains of the Hg-treated SFvF1 and FvSF1 offspring do not seem to just reflect higher serum antibrain Ab levels because the SFvF1 mice had equivalent serum antibrain Ab levels in the water and Hg groups. The higher amounts of brain IgG in Hg-treated SFvF1 offspring relative to those in control SFvF1 or Hg-exposed FvSF1 mice suggest that the BBB may have been compromised or delayed in formation of tight junctions by the Hg, thus allowing more maternal IgG to enter. Additionally, brain vascular integrity may be different between the two strains. However, the male and female SFvF1 mice had equivalent levels of IgG in the brain, but the females had more cytokine expression and lower sociability. Thus, the differential effects of Hg on sociability likely are related to parameters in addition to BBB integrity.
The increased amounts of IgG in the Hg-exposed mice are related to the ability of Hg to enhance lymphocyte activation (Badou et al., 1997; Jiang and Möller, 1995). The elevated levels of maternal SJL/J IgG could account for the elevated serum levels of the pnd21 SFvF1 offspring. However, the FvSF1 offspring also had elevated levels of serum IgG at pnd21 even though their dams did not have increased IgG due to the Hg exposure. This suggests that the higher levels at pnd21 could relate to increased neonatal expansion of B cells or increased early activation of the B cells or T cells (Johansson et al., 1997) and/or the differences in genetics. The normal serum IgG levels of the offspring at pnd70 might be due to the absence of any Hg exposure after pnd21 and/or an induction of CD8+ suppressive T cells (Vas and Monestier, 2008). It is not surprising that the levels of IgG in the brain are elevated when there is early production of antibrain Abs due to delay in BBB development. The pnd70 SFvF1 and FvSF1 offspring had higher levels of serum IgG compared with the counterpart pnd21 offspring, which is due to the immune system not being fully developed at pnd21, and over time the Hg-enhanced Ab levels can accumulate in tissues. The pnd70 FvSF1 offspring also had similar antibrain Ab levels compared with Hg-treated pnd21 FvSF1, which may relate to the normal baseline levels for adult animals with H-2s/q because pnd70 SFvF1 offspring also have comparable adult levels of serum antibrain Abs. Many mouse strains and humans develop Abs that can react with self-Ags, but these Abs do not necessarily induce any pathology. The Hg-treated pnd21 FvSF1 offspring had significantly increased serum antibrain Abs, whereas their brain cytokines and social behavior were not altered by Hg exposure, indicating this level of serum antibrain Abs or their specificities have less or no pathophysiological significance. In addition, the pnd70 FvSF1 offspring had similar or higher levels of IgG in the brain compared with the pnd21 offspring. Higher serum IgG levels lead to higher brain IgG levels within each strain even in control mice, but more IgG in the brain does not necessarily mean a detrimental change in behaviors.
IgG antibrain Abs have been detected in the sera of humans and mice with impaired social behaviors (Heo et al., 2011), and aberrant behaviors have been induced in animals after fetal exposure to IgG from mothers who had children with impaired social behavior (Braunschweig et al., 2008; Cabanlit et al., 2007; Singer et al., 2009; Zimmerman et al., 2007). Because the SFvF1 and FvSF1 offspring have the same H-2 haplotype, the commonality of the immune response genes suggests that the specificities of the Abs could be similar. This also suggests the majority of antibrain Abs detected in pnd21 SFvF1 offspring might be from SJL/J dams, and SJL/J Abs likely could be more detrimental to behavior than FVB Abs because SJL/J mice have low sociability. Thus, it is likely that the higher levels of serum antibrain Abs detected in SFvF1 offspring or the dam’s Ab specificities have a greater influence than the Ab specificities of the F1 offspring or the FVB dams. In addition, it is interesting to note that the control SJL/J dams also had high levels of serum antibrain Abs, which are posited to relate to the impaired social behaviors of SJL/J mice (Moy et al., 2008). Because Hg treatment induced serum antibrain Abs in A.SW mice (H-2s) (Zhang et al., 2011) and FVB mice, it is likely that antibrain Abs were also induced by Hg in SJL/J dams, but the pre-existing high levels of serum antibrain Abs in SJL/J dams may cause the undetectable change between Hg-treated SJL/J dams and control dams. The greater elevation of brain cytokine levels of the SFvF1 mice than those of the FvSF1 mice may be due to the slightly higher and more brain-reactive IgG in the brains of the Hg-exposed SFvF1 mice or due to Hg causing greater activation of cytokines unrelated to IgG levels. Ganor et al. (2005) reported that in healthy and epileptic monozygotic twins, both twins had serum autoAbs to some brain Ags, indicating that the presence of autoAbs in the sera may not be directly responsible for their neurobehavioral differences. Thus, we suggest that the presence of antibrain Abs in the brain and/or increased sensitivity to the Abs due to Hg modifications influence the behaviors. Because there were no differences in the Ab levels or cytokine levels in the brains of the SFvF1 mice at pnd70, the developmental Hg exposure and levels of IgG antibrain Abs and brain cytokines during development until pnd21 seem to be the critical parameters affecting the social behavior impairment.
Brain inflammation has been suggested to be involved in many neurodevelopmental diseases (Gao et al., 2011; Lee et al., 2010; Mondal et al., 2008). Elevated antibrain Ab deposition during early brain development has been posited to initiate neuroinflammation, which subsequently affects behavior (Heo et al., 2011). The absence of brain IgG and cytokine differences of the H2O and Hg-exposed pnd70 SFvF1 offspring, but presence of aberrant behavior for those females developmentally exposed to Hg, indicates that the elevated brain cytokines observed at pnd21 may be responsible for the altered behaviors. Interestingly, SFvF1 mice may be more susceptible to neuroinflammation modulating behavior, in that their brain levels of IL-6, a cytokine associated with stress (Atzori et al., 2012), are high without any treatment. In pnd21 female SFvF1 offspring, Th1, Th2, and Th17 cytokines were increased in many brain regions. IFN-γ, a key cytokine of Th1 cells, was not detectable in the brain, suggesting that inflammatory glial, Th2, and Th17 cells seem to be more involved in the behavioral changes observed in SFvF1 offspring. Further experiments are required to identify the source of these cytokines in the brain. The self-grooming assessment, a mouse behavior which might resemble the repetitive behaviors observed in humans with impaired social behaviors, was not changed in Hg-treated pnd70 SFvF1 offspring, suggesting that the balance of direct pathway and indirect pathway of STR was not altered (Lewis and Kim, 2009; Tanimura et al., 2011).
There have been studies indicating that Hg effects on the dam should be considered with regard to strain differences of Hg toxicity (Ekstrand et al., 2010; Hultman and Nielsen, 1998). We did not observe any significant differences in water consumption between SJL/J and FVB dams; the mice drank 4–5ml per day. The parental SJL/J and FVB dams do have different social behaviors. Hg treatment might alter the behavior of the dams, which might affect the behavior of the offspring, but no obvious differences in pup rearing were noticed. Yang et al. (2007) reported that cross-fostering of BTBR and B6 offspring with B6 and BTBR dams did not affect their social behavior development. As a result, we suggest that the reduced social behavior of Hg-treated SFvF1 offspring observed in our study was less connected to the pre-existing SJL/J dams’ impaired social behavior or their possible change in behaviors due to Hg exposure. Although yet inconclusive, we speculate that the Hg-induced impairment of the social behavior of SFvF1 offspring was due to the neural damage from heightened brain cytokines. We suggest that certain autoAbs in the brain enhance activation of microglia, as evidenced by the increase in IL-1β and IL-12. Additionally, we suggest that Hg enhances the cellular sensitivity to the increased brain cytokines. A similar IgG-related response has been described for loss of dopaminergic neurons of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTPT)-treated mice; the IgG activation of microglia via their Fc receptors has been implicated in the pathology (Lira et al., 2011). The Hg-induced decline of sociability was most evident in the SFvF1 female offspring, which did not have more brain IgG, but they did have more brain regions with elevated cytokines and more cytokine expressions in some brain regions, especially IL-17. IL-6 and IL-17 have been implicated in maternal immune activation causing austim-like behaviors (Hsiao et al., 2012). Multiple brain regions may contribute to the decreased mouse sociability as elevated cytokine levels were not restricted to any one region. However, the sex differences observed for the elevation of IL-17 in CB of Hg-treated pnd21 SFvF1 offspring are interesting because there have been studies indicating that CB is altered in humans with impaired social behaviors (Bolduc et al., 2012; Webb et al., 2009). It appears that only the early Hg-induced elevated levels of cytokines in the brain affect the sociability.
Because Hg is well known to affect renal function, kidney failure could have caused brain damage through a high level of nitrogen compounds retained in the blood or a build-up of other metabolites that could alter neuronal functions (Buchman et al., 2009; Khatri et al., 2007; Yakushiji et al., 2010). However, unchanged urine protein levels in Hg-treated SFvF1 and FvSF1 mice at pnd70 indicate that any change in social behavior after Hg treatment was less likely to be linked with any kidney failure.
In conclusion, developmental exposure to HgCl2 significantly decreased the sociability of SFvF1, but not of FvSF1, offspring. Moreover, the Hg exposure affected the female SFvF1 offspring to a greater extent than their male littermates. The decreased sociability appears to be best correlated with the genetics of the dam and the increased presence of certain cytokines in the brains of the SFvF1 offspring, which may partially have been elevated by the antibrain Abs. Although performed with mice, our study provides evidence that Hg exposure might contribute to increased prevalence of low social behavior through maternal influences in a genetic- and sex-dependent manner.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Environmental Health Sciences (R01 ES011135, R21 ES013857).
ACKNOWLEDGMENTS
The authors thank Donghong Gao for her technical support and Nancy Andersen for her aid with the Luminex analysis. The authors declare no conflict of interest.
REFERENCES
- Abedi-Valugerdi M., Möller G. (2000). Contribution of H-2 and non-H-2 genes in the control of mercury-induced autoimmunity. Int. Immunol. 12, 1425–1430 [DOI] [PubMed] [Google Scholar]
- Atzori M., Garcia-Oscos F., Mendez J. A. (2012). Role of IL-6 in the etiology of hyperexcitable neuropsychiatric conditions: Experimental evidence and therapeutic implications. Future Med. Chem. 4, 2177–2192 [DOI] [PubMed] [Google Scholar]
- Badou A., Savignac M., Moreau M., Leclerc C., Pasquier R., Druet P., Pelletier L. (1997). HgCl2-induced interleukin-4 gene expression in T cells involves a protein kinase C-dependent calcium influx through L-type calcium channels. J. Biol. Chem. 272, 32411–32418 [DOI] [PubMed] [Google Scholar]
- Bolduc M. E., du Plessis A. J., Sullivan N., Guizard N., Zhang X., Robertson R. L., Limperopoulos C. (2012). Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum 11, 531–542 [DOI] [PubMed] [Google Scholar]
- Braunschweig D., Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Croen L. A., Pessah I. N., Van de Water J. (2008). Autism: Maternally derived antibodies specific for fetal brain proteins. Neurotoxicology 29, 226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. S., Tanne D., Boyle P. A., Shah R. C., Leurgans S. E., Bennett D. A. (2009). Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 73, 920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanlit M., Wills S., Goines P., Ashwood P., Van de Water J. (2007). Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann. N. Y. Acad. Sci. 1107, 92–103 [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC) (2012). Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill. Summ. 61, 1–19 [PubMed] [Google Scholar]
- Clarkson T. W., Magos L. (2006). The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 36, 609–662 [DOI] [PubMed] [Google Scholar]
- Cooper J. F., Kusnecov A. W. (2007). Methylmercuric chloride induces activation of neuronal stress circuitry and alters exploratory behavior in the mouse. Neuroscience 148, 1048–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek C. J., Dziegielewska K. M., Habgood M. D., Saunders N. R. (2012). Barriers in the developing brain and Neurotoxicology. Neurotoxicology 33, 586–604 [DOI] [PubMed] [Google Scholar]
- Ekstrand J., Nielsen J. B., Havarinasab S., Zalups R. K., Söderkvist P., Hultman P. (2010). Mercury toxicokinetics–dependency on strain and gender. Toxicol. Appl. Pharmacol. 243, 283–291 [DOI] [PubMed] [Google Scholar]
- Farina M., Rocha J. B., Aschner M. (2011). Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 89, 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y., Freilinger M., Dulac O., Levite M. (2005). Monozygotic twins discordant for epilepsy differ in the levels of potentially pathogenic autoantibodies and cytokines. Autoimmunity 38, 139–150 [DOI] [PubMed] [Google Scholar]
- Gao H. M., Zhang F., Zhou H., Kam W., Wilson B., Hong J. S. (2011). Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ. Health Perspect. 119, 807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassó S., Cristòfol R. M., Selema G., Rosa R., Rodríguez-Farré E., Sanfeliu C. (2001). Antioxidant compounds and Ca(2+) pathway blockers differentially protect against methylmercury and mercuric chloride neurotoxicity. J. Neurosci. Res. 66, 135–145 [DOI] [PubMed] [Google Scholar]
- Goulet S., Doré F. Y., Mirault M. E. (2003). Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol. Teratol. 25, 335–347 [DOI] [PubMed] [Google Scholar]
- Gurney J. G., Fritz M. S., Ness K. K., Sievers P., Newschaffer C. J., Shapiro E. G. (2003). Analysis of prevalence trends of autism spectrum disorder in Minnesota. Arch. Pediatr. Adolesc. Med. 157, 622–627 [DOI] [PubMed] [Google Scholar]
- Hansson M., Abedi-Valugerdi M. (2003). Xenobiotic metal-induced autoimmunity: Mercury and silver differentially induce antinucleolar autoantibody production in susceptible H-2s, H-2q and H-2f mice. Clin. Exp. Immunol. 131, 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havarinasab S., Hultman P. (2005). Organic mercury compounds and autoimmunity. Autoimmun. Rev. 4, 270–275 [DOI] [PubMed] [Google Scholar]
- Henry G. A., Jarnot B. M., Steinhoff M. M., Bigazzi P. E. (1988). Mercury-induced renal autoimmunity in the MAXX rat. Clin. Immunol. Immunopathol. 49, 187–203 [DOI] [PubMed] [Google Scholar]
- Heo Y., Zhang Y., Gao D., Miller V. M., Lawrence D. A. (2011). Aberrant immune responses in a mouse with behavioral disorders. PLoS ONE. 6, e20912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E. Y., McBride S. W., Chow J., Mazmanian S. K., Patterson P. H. (2012). Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. U.S.A. 109, 12776–12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman P., Bell L. J., Eneström S., Pollard K. M. (1992). Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin. Immunol. Immunopathol. 65, 98–109 [DOI] [PubMed] [Google Scholar]
- Hultman P., Bell L. J., Eneström S., Pollard K. M. (1993). Murine susceptibility to mercury. II. autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin. Immunol. Immunopathol. 68, 9–20 [DOI] [PubMed] [Google Scholar]
- Hultman P., Eneström S. (1988). Mercury induced antinuclear antibodies in mice: Characterization and correlation with renal immune complex deposits. Clin. Exp. Immunol. 71, 269–274 [PMC free article] [PubMed] [Google Scholar]
- Hultman P., Nielsen J. B. (1998). The effect of toxicokinetics on murine mercury-induced autoimmunity. Environ. Res. 77, 141–148 [DOI] [PubMed] [Google Scholar]
- Ishitobi H., Stern S., Thurston S. W., Zareba G., Langdon M., Gelein R., Weiss B. (2010). Organic and inorganic mercury in neonatal rat brain after prenatal exposure to methylmercury and mercury vapor. Environ. Health Perspect. 118, 242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Möller G. (1995). In vitro effects of HgCl2 on murine lymphocytes. I. Preferable activation of CD4+ T cells in a responder strain. J. Immunol. 154, 3138–3146 [PubMed] [Google Scholar]
- Johansson U., Sander B., Hultman P. (1997). Effects of the murine genotype on T cell activation and cytokine production in murine mercury-induced autoimmunity. J. Autoimmun. 10, 347–355 [DOI] [PubMed] [Google Scholar]
- Khatri M., Wright C. B., Nickolas T. L., Yoshita M., Paik M. C., Kranwinkel G., Sacco R. L., DeCarli C. (2007). Chronic kidney disease is associated with white matter hyperintensity volume: The Northern Manhattan Study (NOMAS). Stroke 38, 3121–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. Y., Nakai K., Kasanuma Y., Satoh H. (2000). Comparison of neurobehavioral changes in three inbred strains of mice prenatally exposed to methylmercury. Neurotoxicol. Teratol. 22, 397–403 [DOI] [PubMed] [Google Scholar]
- Koren G., Bend J. R. (2010). Fish consumption in pregnancy and fetal risks of methylmercury toxicity. Can. Fam. Physician. 56, 1001–1002 [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., Han S. B., Nam S. Y., Oh K. W., Hong J. T. (2010). Inflammation and Alzheimer’s disease. Arch. Pharm. Res. 33, 1539–1556 [DOI] [PubMed] [Google Scholar]
- Lewis M., Kim S. J. (2009). The pathophysiology of restricted repetitive behavior. J. Neurodev. Disord. 1, 114–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira A., Kulczycki J., Slack R., Anisman H., Park D. S. (2011). Involvement of the Fc gamma receptor in a chronic N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of dopaminergic loss. J. Biol. Chem. 286, 28783–28793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossinsky A. S., Vorbrodt A. W., Wisniewski H. M. (1986). Characterization of endothelial cell transport in the developing mouse blood-brain barrier. Dev. Neurosci. 8, 61–75 [DOI] [PubMed] [Google Scholar]
- Martin L. A., Ashwood P., Braunschweig D., Cabanlit M., Van de Water J., Amaral D. G. (2008). Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav. Immun. 22, 806–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal T. K., Saha S. K., Miller V. M., Seegal R. F., Lawrence D. A. (2008). Autoantibody-mediated neuroinflammation: Pathogenesis of neuropsychiatric systemic lupus erythematosus in the NZM88 murine model. Brain Behav. Immun. 22, 949–959 [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F., Zurich M. G., Honegger P. (1996). Comparison of the developmental effects of two mercury compounds on glial cells and neurons in aggregate cultures of rat telencephalon. Brain Res. 741, 52–59 [DOI] [PubMed] [Google Scholar]
- Moy S. S., Nadler J. J., Young N. B., Nonneman R. J., Segall S. K., Andrade G. M., Crawley J. N., Magnuson T. R. (2008). Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 191, 118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy S. S., Nadler J. J., Young N. B., Perez A., Holloway L. P., Barbaro R. P., Barbaro J. R., Wilson L. M., Threadgill D. W., Lauder J. M., et al. (2007). Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav. Brain Res. 176, 4–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers G. J., Davidson P. W. (1998). Prenatal methylmercury exposure and children: Neurologic, developmental, and behavioral research. Environ. Health Perspect. 106(Suppl. 3), 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers G. J., Thurston S. W., Pearson A. T., Davidson P. W., Cox C., Shamlaye C. F., Cernichiari E., Clarkson T. W. (2009). Postnatal exposure to methyl mercury from fish consumption: A review and new data from the Seychelles Child Development Study. Neurotoxicology 30, 338–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Hultman P. (2002). Mercury-induced autoimmunity in mice. Environ. Health Perspect. 110(Suppl. 5), 877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. T. (2010). Does thimerosal or other mercury exposure increase the risk for autism? A review of current literature. Acta Neurobiol. Exp. (Wars). 70, 187–195 [DOI] [PubMed] [Google Scholar]
- Shanker G., Syversen T., Aschner M. (2003). Astrocyte-mediated methylmercury neurotoxicity. Biol. Trace Elem. Res. 95, 1–10 [DOI] [PubMed] [Google Scholar]
- Singer H. S., Morris C., Gause C., Pollard M., Zimmerman A. W., Pletnikov M. (2009). Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J. Neuroimmunol. 211, 39–48 [DOI] [PubMed] [Google Scholar]
- Tanimura Y., King M. A., Williams D. K., Lewis M. H. (2011). Development of repetitive behavior in a mouse model: Roles of indirect and striosomal basal ganglia pathways. Int. J. Dev. Neurosci. 29, 461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas J., Monestier M. (2008). Immunology of mercury. Ann. N. Y. Acad. Sci. 1143, 240–267 [DOI] [PubMed] [Google Scholar]
- Vorbrodt A. W., Lossinsky A. S., Dobrogowska D. H., Wisniewski H. M. (1986). Distribution of anionic sites and glycoconjugates on the endothelial surfaces of the developing blood-brain barrier. Brain Res. 394, 69–79 [DOI] [PubMed] [Google Scholar]
- Warfvinge K., Hansson H., Hultman P. (1995). Systemic autoimmunity due to mercury vapor exposure in genetically susceptible mice: Dose-response studies. Toxicol. Appl. Pharmacol. 132, 299–309 [DOI] [PubMed] [Google Scholar]
- Webb S. J., Sparks B. F., Friedman S. D., Shaw D. W., Giedd J., Dawson G., Dager S. R. (2009). Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 172, 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushiji Y., Nanri Y., Hirotsu T., Nishihara M., Hara M., Nakajima J., Eriguchi M., Nishiyama M., Hara H., Node K. (2010). Marked cerebral atrophy is correlated with kidney dysfunction in nondisabled adults. Hypertens. Res. 33, 1232–1237 [DOI] [PubMed] [Google Scholar]
- Yang M., Zhodzishsky V., Crawley J. N. (2007). Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int. J. Dev. Neurosci. 25, 515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gao D., Bolivar V. J., Lawrence D. A. (2011). Induction of autoimmunity to brain antigens by developmental mercury exposure. Toxicol. Sci. 119, 270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. W., Connors S. L., Matteson K. J., Lee L. C., Singer H. S., Castaneda J. A., Pearce D. A. (2007). Maternal antibrain antibodies in autism. Brain Behav. Immun. 21, 351–357 [DOI] [PubMed] [Google Scholar]