Abstract
The treatment of chronic limb ischemia involves the restoration of pulsatile blood flow to the distal extremity. Some patients cannot be treated with endovascular means or with open surgery; some may have medical comorbidities that render them unfit for surgery, while others may have persistent ischemia or pain even in the face of previous attempts at reperfusion. In spinal cord stimulation (SCS), a device with electrodes is implanted in the epidural space to stimulate sensory fibers. This activates cell-signaling molecules that in turn cause the release of vasodilatory molecules, a decrease in vascular resistance, and relaxation of smooth muscle cells. SCS also suppresses sympathetic vasoconstriction and pain transmission. When patient selection is based on microcirculatory parameters, SCS therapy can significantly improve pain relief, halt the progression of ulcers, and potentially achieve limb salvage.
Keywords: limb ischemia, rest pain, spinal cord stimulation

Spinal Cord Stimulation
While therapy with spinal cord stimulation (SCS) was initially used in patients with intractable pain, its potential benefits have been observed in various conditions. It is now clinically indicated in failed back surgery syndrome, degenerative low back or leg pain, spinal stenosis, nerve root avulsion, traumatic nerve injury, chronic regional pain syndromes, postherpetic neuralgia, neuropathic perineal pain, interstitial cystitis, urge incontinence, refractory angina, and peripheral vascular disease (PVD).1-3 Patients with chronic limb ischemia not amenable to open surgical or endovascular intervention have been identified as candidates for SCS therapy. Usually this includes patients with rest pain alone (Fontaine stage III) or those with rest pain and arterial ulcers less than 3 cm in diameter (Fontaine stage IV). In addition, patients who have undergone revascularization and are still in pain even after appropriate medical management may benefit from therapy. Patients with pain from vasospastic disorders, frostbite, or as a result of distal arterial embolization can also be considered for therapy.4
Critical limb ischemia (CLI) is defined as limb pain that occurs at rest or impending limb loss that is caused by a severe compromise of blood flow to the affected extremity. The annual incidence of CLI is approximately 0.25 to 0.45 patients per 1,000 population.5 It is often caused by atherosclerosis due to hypertension, diabetes, or smoking. Unlike individuals with claudication, patients with CLI have resting perfusion that is inadequate to sustain viability in the distal tissue bed. The term “CLI” is applied to patients with chronic ischemic rest pain, ulcers, or gangrene attributable to objectively proven arterial occlusive disease.6 At the cornerstone of treatment is the restoration of pulsatile blood flow to the distal extremity. However, even in the face of new treatment approaches, some patients cannot be treated with endovascular means or with open surgery. Some patients may have medical comorbidities that render them unfit for surgery, whereas others may have persistent ischemia or pain even in the face of previous attempts at reperfusion.7 In these patients, the aim and potential benefit of SCS is to provide resolution, improvement, or relief of rest pain; avoid or delay amputation; and improve the patient’s quality of life.3, 8
Briefly, spinal cord stimulation requires the insertion of electrodes into the epidural space, with the electrodes connected to an impulse generator.9 To treat lower-extremity pain in patients with CLI, 4 to 8 electrodes are generally placed in the epidural space between the thoracic 8 and 11 level through a paralaminar level 1 or 2 lumbar puncture.4, 10 The electrodes are usually centered in the spinal canal or directed off-center, depending on the location of the limb with the most significant symptoms.18 In general, patients may often undergo a trial use of SCS to determine the optimal location of lead placement and to ascertain if the procedure provides pain relief as expected. Patients who respond well during the trial period and achieve pain relief, improved quality of life, or increased activity become candidates for permanent lead and generator placement.4, 11
Complications from SCS include lead migration, lead connection failure, lead break, local pain, wound seroma, hematoma and infection. Hardware-related complications are the most common and can occur in 11-36% of patients, with infection being the next most common, occurring in 3-6.3% of patients.8, 9, 10, 12
Mechanism of Action in PVD
The mechanism of action of SCS is not completely clear, and several theories have been postulated. The electrodes in the epidural space stimulate sensory unmyelinated c-fibers and myelinated Aδ-fibers in the dorsal root ganglia.7, 13, 14 This leads to the activation of cell-signaling molecules such as extracellular signal-regulated kinase (ERK) and protein kinase B (AKT). Activated ERK and AKT stimulate the transient receptor potential vanilloid receptor 1 (TRPV1). Activation of TRPV1 and depolarization of the nerve terminals causes the release of vasodilators such as calcitonin gene-related peptide (CGRP), which has powerful microvascular vasodilatory effects. The release of CGRP causes endothelial nitric oxide (NO) release and stimulates smooth muscle cell relaxation. These effects lead to a decrease in vascular resistance and an increase in local blood flow (Figure 1).7, 8, 15, 16
Figure 1.
Mechanisms for SCS-induced increase in distal limb perfusion. ERK: extracellular signal regulated kinase; AKT: protein kinase B; TRPV1: transient receptor potential vanilloid receptor 1; CGRP: calcitonin gene related peptide; NO: nitric oxide.
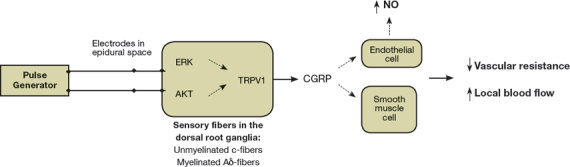
In addition, SCS suppresses sympathetic vasoconstriction through inhibition of sympathetic nicotine transmission at the ganglionar and postganglionar level.3, 4, 7 Pain relief is mediated by the suppression of pain or nociceptive transmission and the release of opioid peptides such as met-enkephalin.15
SCS and PVD
Tallis and associates reported results of 10 patients, 4 of whom had only severe claudication and 6 of whom had rest pain or ulceration. Sixty percent of patients showed clinical improvement that was maintained as long as the SCS was continued. Four patients with rest pain obtained complete or marked relief with therapy. Patient exercise tolerance improved by 61%. These changes were associated with a small increase in cutaneous and muscle blood flow measured by xenon-133 clearance.17 In a study that evaluated the effect of SCS in patients with inoperable severe leg ischemia with rest pain or ulceration, Jivegard and colleagues randomized 51 patients to either SCS with analgesic treatment or analgesic treatment alone. Patients were followed for 18 months and monitored for tissue loss and pain relief. Long-term pain relief was observed only in the SCS group. Limb salvage rates were similar in both groups, but the extent of amputations in the SCS group was smaller than those receiving only analgesic treatment. However, subgroup analysis showed that SCS was most effective in patients without arterial hypertension.18 In Reig and Abejon’s 20-year experience using SCS, 98 of their 260 patients had peripheral arterial disease, and 88% of them experienced good pain relief with therapy. The authors suggested that SCS should be considered as an important therapeutic approach in the management of patients with vascular pain or ulcers.19
Peripheral arterial disease is common in patients with end-stage renal disease who are on dialysis, and those who are not candidates for limb-preserving procedures have to undergo amputation. Brümmer and colleagues used SCS to treat 8 patients on hemodialysis and followed them for 12 months. Both intensity of pain and quality of life significantly improved during follow-up, which allowed all patients to decrease their intake of pain medications. The authors did not observe a significant improvement in ischemic skin lesions, nor did they observe the appearance of new ulcers. Limb survival was reported at 75%.8 Though a small study, it highlights the opportunity of this therapy in a patient population that has been considered at a disadvantage for revascularization and limb salvage procedures.
SCS and the Arterial Microcirculation
Transcutaneous oxygen (tcpO2) measurements reflect the arteriolar oxygen tension (pO2) and are almost flow-independent in healthy subjects. In severely ischemic patients, however, arteriolar pO2 becomes dependent on flow. Ubbink and colleagues have found that even though diabetes can cause changes in microcirculatory perfusion, its influence is outweighed by the effects of atherosclerosis, especially in patients with severe peripheral vascular disease. The authors conclude that techniques investigating skin microcirculation may be useful in assessing the severity of lower limb ischemia even if the arterial ankle brachial index (ABI) cannot be obtained.20
Spinal cord stimulation may improve ischemic wound healing and could benefit limb preservation through improved skin perfusion. The Dutch Multicenter Randomized Controlled Trial compared 55 patients assigned to standard medical therapy to 56 patients treated with SCS and standard medical therapy. The study investigated the effects of treatment on skin microcirculation in relation to treatment outcome in patients with nonreconstructable CLI. Skin microcirculation was assessed by means of capillary microscopy, laser Doppler perfusion, and tcpO2 measurements in the foot. A poor microcirculatory skin perfusion was associated with a higher amputation frequency in both groups. Amputation frequency was significantly lower in patients with intermediate skin perfusion who were also treated with SCS. This study suggests that patient selection on the basis of the initial microcirculatory skin perfusion can identify those patients in whom SCS can improve local skin perfusion and limb survival.21
Petrakis and Sciacca reported their experience of 150 patients with Fontaine stage III and IV disease due to atherosclerosis and diabetic vascular disease. Limb salvage was achieved in patients that experienced a significant increase in tcpO2 within the first 2 weeks of the testing period. A tcpO2 increase of more than 50% in the first 2 months after implantation was also predictive of long-term success even though the ABI did not change.22 These same authors have suggested that diabetic patients with a significant increase in tcpO2 that is associated with a clinical improvement during the test period, and not merely all patients with pain relief alone, should be considered for permanent SCS device implantation. They imply that changes in tcpO2 can be used as a predictive index of the success of therapy.23
While some have suggested that tcpO2 measurements in the supine position are of value,20 others have reported that the tcpO2 gradient between the supine and sitting position is a strong predictor of limb salvage.24 Spincemaille and associates noted an 88% limb salvage rate with SCS when the difference between the baseline supine and sitting tcpO2 was ≥15 mm Hg. In addition, a rise in tcpO2 of at least 15% after a trial of SCS resulted in a significant limb salvage of 77% at 18 months. The outcome of patients with an initial tcpO2 ≤10 mm Hg was significantly poor compared to those with higher tcpO2.24 In line with these results, Horsch and colleagues showed improved limb survival and a delay in time-to-amputation in patients with an initial baseline tcpO2 between 10-30 mm Hg compared to those with a baseline <10 mm Hg.25 As described above, in severe arterial disease, microcirculatory perfusion depends mostly on flow and not necessarily on the effects of diabetes. These results support this notion and showed that patients with diabetes did not have a worse prognosis regarding limb survival compared with nondiabetics.25 Ubbink and Vermeulen also found tcpO2 measurements to be useful in selecting the most responsive patients to SCS therapy. They recommend SCS as a treatment alternative in patients with CLI, particularly if their foot tcpO2 is between 10-30 mm Hg.26
The European Peripheral Vascular Disease Outcome Study (SCS-EPOS) was a prospective controlled multicenter study that compared patients with CLI who had baseline tcpO2 <30 mm Hg and pain relief after a 72-hour trial period of stimulation, or a baseline tcpO2 <10 mm Hg that increased to >20 mm Hg after trial stimulation, with patients who did not fit this criteria and were treated with or without SCS. Patients who showed improvement in tcpO2 based on their criteria also showed a significant improvement in arterial microcirculation, pain relief, and limb survival. The study concluded that patient selection based on tcpO2 trial screening further increases the probability of limb preservation with SCS therapy.27
In a meta-analysis of 444 patients that compared SCS to any form of conservative treatment for inoperable CLI, patients receiving the device required significantly less analgesia and improved their Fontaine stage classification. The authors observed a stronger trend towards a better rate of amputation-free salvage in the subgroup of patients selected by initial tcpO2 measurements.9 Predrini and Magnoni reviewed the efficacy of SCS in patients with untreatable CLI and found that pain relief, ulcer healing, and limb salvage were greater in nondiabetic patients, in diabetic patients without autonomic neuropathy, and in patients with rest pain or ulcer more often than in patients with gangrene.7 The Cochrane review of SCS for nonreconstructable CLI found that limb salvage after 12 months was significantly higher in patients with the device. Pain relief was also more prominent in the SCS-treated patients, and they required significantly less analgesics. The report observed no significant effect on ulcer healing between patients treated medically and those with the implant. There was no difference between diabetic and nondiabetic patients. Of interest, pain relief was substantially better in amputated patients compared to those who did not undergo amputation.28 We believe that selection of patients for SCS therapy should include those with a baseline tcpO2 between 10-30 mm Hg and who demonstrate ≥10 mm Hg increase with an initial SCS trial period (Figure 2).
Figure 2.
Algorithm for patient selection for SCS therapy.
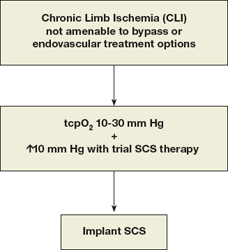
When amputation is performed, patients remain at risk for stump complications and even conversion to a higher level amputation. Elderly patients who undergo amputation are affected by depression and a continued sense of loss even after recovery.29 These patients also have to deal with the comorbidities of their postoperative recovery, rehabilitation, general deconditioning, loss of mobility and transfer power, lack of knowledge about caring for the residual limb, and oftentimes the lack of social and environmental support.30 Thus, when addressing the personal, psychological, and societal costs involved with the alternative of amputation, the expenses involved with SCS may appear to be well justified.
Conclusion
Patients who are candidates for SCS therapy are those with CLI who have exhausted both open and endovascular options, those whom are unfit for surgery, or those who continue to experience ischemic-related pain following revascularization. If patients are selected using baseline and changes in tcpO2 measurements, a trial period with an external SCS can identify those who may benefit and show pain relief, halt the progression of ulcers, and potentially achieve limb salvage.
Funding Statement
Funding/Support: The authors have no funding disclosures.
Footnotes
Conflict of Interest Disclosure: All authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Costantini A. Spinal cord stimulation. Minerva Anestesiol. 2005 Jul-Aug;71(7-8):471–4. [PubMed] [Google Scholar]
- 2.Wolff A, Vanduynhoven E, van Kleef M, Huygen F, Pope JE, Mekhail N. 21. Phantom pain. Pain Pract. 2011 Jul-Aug;11(4):403–13. doi: 10.1111/j.1533-2500.2011.00454.x. [DOI] [PubMed] [Google Scholar]
- 3.Deer TR, Raso LJ. Spinal cord stimulation for refractory angina pectoris and peripheral vascular disease. Pain Physician. 2006 Oct;9(4):347–52. [PubMed] [Google Scholar]
- 4.Reig E, Abejón D, del Pozo C, Wojcikiewicz R. Spinal cord stimulation in peripheral vascular disease: a retrospective analysis of 95 cases. Pain Pract. 2001 Dec;1(4):324–31. doi: 10.1046/j.1533-2500.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 5.Devulder J, van Suijlekom H, van Dongen R, Diwan S, Mekhail N, van Kleef M, et al. 25. Ischemic pain in the extremities and Raynaud’s phenomenon. Pain Pract. 2011 Sep-Oct;11(5):483–91. doi: 10.1111/j.1533-2500.2011.00460.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006 Mar 21;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 7.Pedrini L, Magnoni F. Spinal cord stimulation for lower limb ischemic pain treatment. Interact Cardiovasc Thorac Surg. 2007 Aug;6(4):495–500. doi: 10.1510/icvts.2006.150185. [DOI] [PubMed] [Google Scholar]
- 8.Brümmer U, Condini V, Cappelli P, Di Liberato L, Scesi M, Bonomini M, et al. Spinal cord stimulation in hemodialysis patients with critical lower-limb ischemia. Am J Kidney Dis. 2006 May;47(5):842–7. doi: 10.1053/j.ajkd.2006.02.172. [DOI] [PubMed] [Google Scholar]
- 9.Ubbink DT, Vermeulen H, Spincemaille GH, Gersbach PA, Berg P, Amann W. Systematic review and meta-analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischemia. Brit J Surg. 2004 Aug;91(8):948–55. doi: 10.1002/bjs.4629. [DOI] [PubMed] [Google Scholar]
- 10.Stojanovic MP, Abdi S. Spinal cord stimulation. Pain Physician. 2002 Apr;5(2):156–66. [PubMed] [Google Scholar]
- 11.Epstein LJ, Palmieri M. Managing chronic pain with spinal cord stimulation. Mt Sinai J Med. 2012 Jan-Feb;79(1):123–32. doi: 10.1002/msj.21289. [DOI] [PubMed] [Google Scholar]
- 12.Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011 Mar-Apr;11(2):148–53. doi: 10.1111/j.1533-2500.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Role of primary afferents in spinal cord stimulation-induced vasodilation: characterization of fiber types. Brain Res. 2003 Jan 10;959(2):191–8. doi: 10.1016/s0006-8993(02)03740-x. [DOI] [PubMed] [Google Scholar]
- 14.Provenzano DA, Jarzabek G, Georgevich P. The utilization of transcutaneous oxygen pressures to guide decision-making for spinal cord stimulation implantation for inoperable peripheral vascular disease: a report of two cases. Pain Physician. 2008 Nov-Dec;11(6):909–16. [PubMed] [Google Scholar]
- 15.Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008 Feb 29;138 (1-2):9–23. doi: 10.1016/j.autneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka S, Komori N, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Mechanisms of sustained cutaneous vasodilation induced by spinal cord stimulation. Auton Neurosci. 2004 Jul 30;114(1-2):55–60. doi: 10.1016/j.autneu.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Tallis RC, Illis LS, Sedgwick EM, Hardwidge C, Garfield JS. Spinal cord stimulation in peripheral vascular disease. J Neurol Neurosurg Psychiatry. 1983 Jun;46(6):478–84. doi: 10.1136/jnnp.46.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jivegård LE, Augustinsson LE, Holm J, Risberg B, Ortenwall P. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischemia: a prospective randomized controlled trial. Eur J Vasc Endovasc Surg. 1995 May;9(4):421–5. doi: 10.1016/s1078-5884(05)80010-3. [DOI] [PubMed] [Google Scholar]
- 19.Reig E, Abejón D. Spinal cord stimulation: a 20-year retrospective analysis in 260 patients. Neuromodulation. 2009 Jul;12(3):232–9. doi: 10.1111/j.1525-1403.2009.00220.x. [DOI] [PubMed] [Google Scholar]
- 20.Ubbink DT, Kitslaar PJ, Tordoir JH, Reneman RS, Jacobs MJ. Skin microcirculation in diabetic and non-diabetic patients at different stages of lower limb ischemia. Eur J Vasc Surg. 1993 Nov;7(6):659–66. doi: 10.1016/s0950-821x(05)80713-3. [DOI] [PubMed] [Google Scholar]
- 21.Ubbink DT, Spincemaille GH, Prins MH, Reneman RS, Jacobs MJ. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicenter randomized controlled trial. J Vasc Surg. 1999 Aug;30(2):236–44. doi: 10.1016/s0741-5214(99)70133-3. [DOI] [PubMed] [Google Scholar]
- 22.Petrakis IE, Sciacca V. Spinal cord stimulation in critical limb ischemia of the lower extremities: our experience. J Neurosurg Sci. 1999 Dec;43(4):285–93. [PubMed] [Google Scholar]
- 23.Petrakis E, Sciacca V. Prospective study of transcutaneous oxygen tension (TcPO2) measurement in the testing period of spinal cord stimulation in diabetic patients with critical lower limb ischemia. Int Angiol. 2000 Mar;19(1):18–25. [PubMed] [Google Scholar]
- 24.Spincemaille GH, de Vet HC, Ubbink DT, Jacobs MJ. The results of spinal cord stimulation in critical limb ischemia: a review. Eur J Vasc Endovasc Surg. 2001 Feb;21(2):99–105. doi: 10.1053/ejvs.2000.1291. [DOI] [PubMed] [Google Scholar]
- 25.Horsch S, Schulte S, Hess S. Spinal cord stimulation in the treatment of peripheral vascular disease: results of a single-center study of 258 patients. Angiology. 2004 Mar-Apr;55(2):111–18. doi: 10.1177/000331970405500201. [DOI] [PubMed] [Google Scholar]
- 26.Ubbink DT, Vermeulen H. Spinal cord stimulation for critical leg ischemia: a review of effectiveness and optimal patient selection. J Pain Symptom Manage. 2006 Apr;31(4 Suppl):S30–5. doi: 10.1016/j.jpainsymman.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, Ubbink DT. European Peripheral Vascular Disease Outcome Study SCS-EPOS. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Outcome Study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003 Sep;26(3):280–6. doi: 10.1053/ejvs.2002.1876. [DOI] [PubMed] [Google Scholar]
- 28.Ubbink DT, k Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischemia (review). Cochrane Database Syst Rev. 2009;1:1–7. [Google Scholar]
- 29.Parkes CM. Psycho-social transitions: comparison between reactions to loss of a limb and loss of a spouse. Br J Psychiatry. 1975 Sep;127:204–10. doi: 10.1192/bjp.127.3.204. [DOI] [PubMed] [Google Scholar]
- 30.Frieden RA. The geriatric amputee. Phys Med Rehabil Clin N Am. 2005 Feb;16(1):179–95. doi: 10.1016/j.pmr.2004.06.004. [DOI] [PubMed] [Google Scholar]


