In 2001, the Mochly-Rosen laboratory published the first evidence that inhibition of delta protein kinase C (δPKC) at the onset of reperfusion reduced tissue injury in preclinical models of acute myocardial infarction (AMI). In various models of cardiac ischemia and reperfusion (I/R)including cultured neonatal cardiac myocytes [1], ex vivo whole rat heart [2], and in vivo porcine heart [3], we demonstrated that delivering a rationally designed peptide allosteric inhibitor of δPKC (δV1-1) at reperfusion reduced infarct size by about 70% [3]. δV1-1 treatment reduced necrosis and apoptosis, accelerated ATP regeneration, preserved mitochondrial structure, and preserved organization of contractile elements [3–6]. We also found that treatment with δV1-1 protected the vascular endothelium, leading to improved microvasculature flow and rapid recovery of coronary flow reserve [5]. All the benefits of δV1-1 treatment delivered at ~500 ng/kg at reperfusion were sustained through 12 days after treatment, including a ~70% smaller infarct size than control, normal ejection fraction, and normal coronary flow reserve [5]. Finally, independent studies that contributed to the characterization of δPKC in reperfusion injury in the heart were carried out in the laboratories of Drs. Gerald Dorn and Roberto Bolli [1], and Elizabeth Murphy [5], and were also corroborated independently using other systems, e.g. when using human atrial trabeculae strips in a transient ischemia model[7]. This preclinical evidence together with δV1-1’s excellent safety profile led KAI pharmaceuticals to test whether similar benefits could be achieved in AMI patients.
The DELTA MI trial was a first-in-human, double-blinded, placebo controlled phase 1/2a trial to assess the safety of intracoronary δV1-1 (KAI-9803) in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PCI). (For patient characteristics and detailed trial design, see ref [8].) Drug administration was based on the porcine STEMI model, in which the peptide was delivered through the lumen of a balloon catheter to the area down-stream of the occlusion, just prior to balloon deflation. Based on discussions with the FDA and bridging animal studies, a second dose was given through the guide catheter after balloon deflation, to allow drug delivery to myocardium supplied by arterial side branches that might have been blocked by the inflated balloon. The trial included five arms: a concurrent placebo arm of 51 patients and 4 drug-treated arms of 0.05, 0.5, 1.25, and 5 mg, with 25–26 patients each. The lowest dose was calculated to be efficacious based on the porcine model. DELTA MI demonstrated that δV1-1 was safe at all dose levels tested. Although the trial was not designed to achieve statistical significance, a trend towards benefit was seen in biomarkers that correlate with AMI clinical outcomes when the combined treatment groups were compared to placebo. These biomarkers included resolution of ST segment elevation, as measured by ST segment area under the curve (AUC), and infarct size, as measured by creatine kinase AUC (Figure 1; summarized from data in [8]). Late ischemic events (as measured by ST segment re-elevation in the 24 hrs after treatment) also declined by 63% (unpublished data).
Figure 1. DELTA MI Biomarker Results.
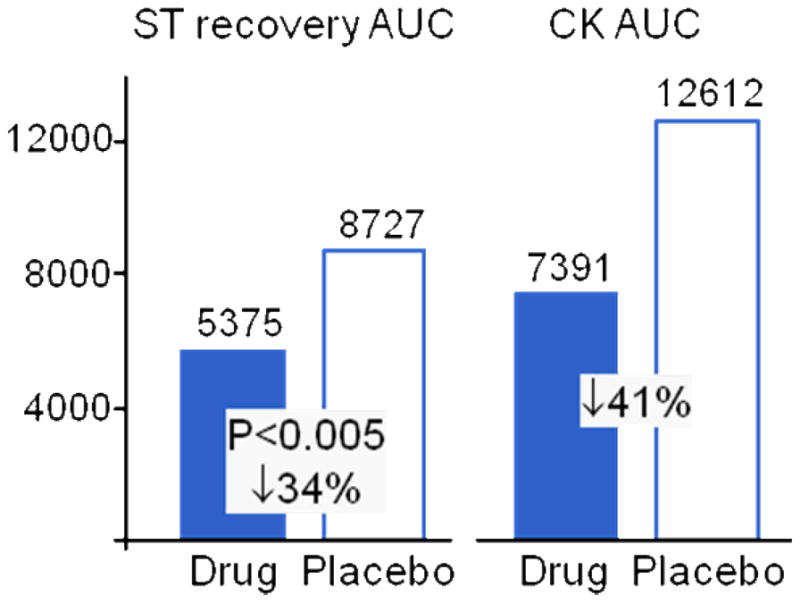
Abbreviations: ST -ST Segment elevation; AUC-area under the curve; CK -creatine kinase ST AUC: assessed through 3 hours after drug administration; median value (uV/min) of pooled drug versus placebo.
CK AUC: determined through curve-fitting on samples drawn at 3–6 hrs, 6–12 hrs, 18–24 hrs, and 36 hrs; median value (ng/ml x hr) of pooled drug versus placebo
These encouraging data on the safety and potential efficacy of δPKC inhibition led to a phase 2b trial testing intravenous δV1-1 in STEMI patients undergoing primary angioplasty (sponsored by KAI Pharmaceuticals with Bristol-Myers Squibb). The rationale for switching from intracoronary to intravenous administration was three-fold: 1) to allow earlier and more timely drug delivery to the increasing percentage of patients (up to 40%) who were achieving partial reperfusion prior to PCI as evidenced by TIMI 2/3 flow on initial angiography; 2) given δV1-1’s exceptionally short serum half-life (<2 minutes), continuous intravenous infusion allowed longer exposure of ischemic myocardium to the drug with the potential to increase efficacy, and 3) ease of use for the treating cardiologists. The trial sponsors had performed further preclinical [9]and human phase 1 studies to predict efficacious dose range and duration of therapy, establish safety, and characterize pharmacokinetics of intravenous infusion of δV1-1. The PROTECTION AMI trial enrolled 908 patients across four groups (~227 patients/group). Subjects received a 2.5 hour infusion of placebo or one of three doses (125, 375 or 1125 mg)of δV1-1 (called delcasertib in the trial).
Preliminary results of PROTECTION AMI were presented at the American College of Cardiology meeting in March, 2011. The data showed that the inhibitor was safe at all doses tested, but failed to show a statistically significant benefit in the primary endpoint of CK-MB area under the curve with δV1-1 administration. Subgroup analysis revealed a trend towards reduced infarct size (~14%) in the two highest doses in patients with anterior MI and TIMI 0/1 flow on initial angiography, which represented 60–65% of the patients in each treatment arm [10]. Although the full analysis of PROTECTION MI has yet to be completed and published, several issues may inform the design of future myocardial salvage studies.
1. The steady improvement in STEMI clinical outcomes over the past decade must be considered in future study design
STEMI patient outcomes have shown continued improvement in successive trials over the past decade (Table 1). Mortality at three months decreased by nearly two thirds, from 9.4% in COMPLY (2000–2002) to 3.2% in PROTECTION MI (2008–2010) [11]. This was accompanied by a 46% drop in the occurrence of CHF. The composite incidence of death and heart failure at 3 months decreased by 57%, (from 17.5% to 7.6%)[12].
Table 1.
STEMI Trial Clinical Outcomes (Placebo Group)
| Trial (Intervention) | Year Enrolled | PCI vs. Lysis | MI Location Sx Duration | Study Size/# Placebo | Mortality (%) | CHF/Shock (%) | Reference |
|---|---|---|---|---|---|---|---|
| Protection AMI (delcasertib) | 2008–10 | PCI | Ant/large inf < 6 hours | 748/249 | 3m 3.2 | 3m 4.4/3.6 | [10] |
| CICERO (IC vs. IV abciximab) | 2008–10 | PCI | All < 12 hrs | 534/263 | 1m 2.7 | NR | [21] |
| REVEAL (erythropoietin) | 2006–09 | PCI | All (TIMI 0/1) < 8 hrs | 222/97 | 0 | 2.1/NR | [22] |
| HORIZONS-AMI (bivalrudin) | 2005–07 | PCI | All STEMI < 12 hrs | 3602/1800 | 1m 2.0 | NR | [23] |
| APEX AMI (pexelizumab) | 2004–06 | PCI | Ant/large inf < 6 hrs | 5745/2885 | 1m 3.9 3m 4.5 |
1m 4.0/3.4 3m 4.8/3.5 |
[24] |
| FINESSE (facilitated PCI) | 2002–06 | PCI | All < 6 hrs | 2452/806 | 3m 4.5 | 3m 2.2/6.8 | [25] |
| TYPHOON (sirolimus stent) | 2003–04 | PCI | All < 12 hrs | 712/357 | 12m2.2 | NR | [26] |
| COMMA (pexelizumab) | 2000–02 | PCI | All < 6 hrs | 814/271 | 6d 3.7 1m 4.8 3m 5.9 6m 7.4 |
6d 3.0/5.2 3m 4.8/5.2 |
[27] |
| COMPLY (pexelizumab) | 2000–02 | lytic | All < 6 hrs | 920/307 | 6d 5.9 1m 8.1 3m 9.4 6m 10.4 |
6d 3.3/3.9 3m 8.1/4.2 |
[12] |
| AMISTAD-II (adenosine) | 1999–2000 | either | Ant < 6 hours | 2118/703 | 6m 11.8 | In hosp 4.0/NR 6m rehosp 4.3% |
[11] |
Abbreviations: PCI – percutaneous coronary intervention, lysis – thrombolytic therapy, ant – anterior, large inf – inferior with reciprocal ST changes in anterior leads, hrs – hours, TIMI – thrombolysis in myocardial infarction flow grade, CHF – congestive heart failure, shock – cardiogenic shock, d – days, m – months, NR – not reported, in hosp – during initial hospitalization, rehosp – rehospitalization
A number of treatment advances may have contributed to superior outcomes including: a) reduced total ischemic time as reflected by substantial improvements in door to balloon or door to needle times [13, 14]; b) increased use of primary PCI over primary thrombolysis, c) improvements in the use of anti-platelet agents such as early aspirin administration, larger loading doses of clopidogrel, and increased use of glycoprotein IIb IIIa inhibitors; and d) increased use of beta-blockers, angiotensin converting enzyme inhibitors, and lipid lowering agents.
Whatever the cause, the steady decrease in event rates presents three important challenges to clinical trial design. First, larger numbers of patients must be enrolled to reach statistical significance for a given endpoint. Second, treatments to enhance myocardial salvage may only provide measurable clinical benefit to those patients with large areas of myocardium at risk. Third, consideration should be given to the prevalence and clinical practices in each country that is included in the trial; changes in clinical practice are adopted at a different pace throughout the world, increasing the challenge in a multi-national trial.
2. Translating idealized animal models to the realities of clinical practice inevitably introduces new variables and therefore new risks to the development program
Patients are heterogeneous in terms of ischemic time, location of coronary blockage, underlying health of the myocardium, baseline medications, response to drugs, age, concomitant diseases such as diabetes mellitus, the country where the patient resides and a number of other variables. Certain variables such as advanced age, the presence of diabetes mellitus, longer duration of ischemia, reperfusion prior to drug exposure, and chronic administration of atorvastatin have been shown to attenuate the response to cardioprotective agents [15–17]. Conversely, concomitant medications such as morphine may have their own cardioprotective effects, thereby masking the benefits of drugs under study [18]. Therefore, it seems prudent to incorporate such clinical variables into preclinical models and to utilize the results to guide the design of subsequent clinical trials. For example, since the preclinical data indicated maximal efficacy of δV1-1 when it was administered prior to reperfusion, perhaps a trial including only patients with TIMI flow 0/1 is more likely to demonstrate maximal benefit.
3. Can STEMI patients still benefit from acute drug intervention to salvage myocardium?
Certainly, preventing STEMI-associated death and heart failure remains important to the health of individual patients and to help contain our rising health care expenditures. Coronary artery disease remains the largest cause of mortality worldwide [19]. The incidence of congestive heart failure continues to rise and ischemic damage remains a leading cause [20]. If we are to succeed in future trials in this large unmet need, we must incorporate lessons from the past into our trial designs.
Based on recent studies, we suggest the following strategies to enhance the likelihood of successful trials:
-
Include only those STEMI patients who will benefit the most (i.e. those who have a lot of myocardium to lose). Subjects presenting with uncomplicated inferior infarcts generally have a small area of myocardium at risk and can be expected to have low rates of death and heart failure. There is little room for improvement. We suggest selecting for the following characteristics at presentation:
First infarct
Large anterior infarct or new onset heart failure
Ischemic time less than 6 hours
Ensure strategies for drug dosing and route of administration are optimized to provide maximum efficacy (e.g., local delivery and dwelling time for the drug at the ischemic region.)
When calculating sample size, establish rigorous event rates for your control patients and expect these rates to be better in your study than they were in prior studies. We anticipate further reductions in door to balloon time and adjunct therapies will continue to lower clinical event rates.
STEMI remains a major cause of death and disability [13, 14], leading to 450,000 deaths from MI each year in the US alone [22]. While our successes in reducing mortality over the past decade are a cause to celebrate, demonstrating benefits from new therapies will be increasingly difficult because of the lower clinical event rates. Yet, there is still room for improvement. Thoughtful translation from the lab to the clinic, careful patient selection to maximize clinical effect, and rigorous attention to study design will be essential as new therapies such as δV1-1 are advanced to the clinic.
References
- 1.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98:11114–9. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inagaki K, Hahn HS, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation. 2003;108:869–75. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 3.Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, et al. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation. 2003;108:2304–7. doi: 10.1161/01.CIR.0000101682.24138.36. [DOI] [PubMed] [Google Scholar]
- 4.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 5.Ikeno F, Inagaki K, Rezaee M, Mochly-Rosen D. Impaired perfusion after myocardial infarction is due to reperfusion-induced deltaPKC-mediated myocardial damage. Cardiovasc Res. 2007;73:699–709. doi: 10.1016/j.cardiores.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279:47985–91. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 7.Sivaraman V, Hausenloy DJ, Kolvekar S, Hayward M, Yap J, Lawrence D, et al. The divergent roles of protein kinase C epsilon and delta in simulated ischaemia-reperfusion injury in human myocardium. J Mol Cell Cardiol. 2009;46:758–64. doi: 10.1016/j.yjmcc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, et al. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation. 2008;117:886–96. doi: 10.1161/CIRCULATIONAHA.107.759167. [DOI] [PubMed] [Google Scholar]
- 9.Miyaji Y, Walter S, Chen L, Kurihara A, Ishizuka T, Saito M, et al. Distribution of KAI-9803, a novel {delta}PKC inhibitor, after intravenous administration to rats. Drug Metab Dispos. 2011 doi: 10.1124/dmd.111.040725. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Lincoff AM. American College Cardiology - Late Breaking Clinical Trial. 2011. Selective Inhibition of Delta Protein Kinase C to Reduce Infarct Size after Primary Percutaneous Intervention for Acute Myocardial Infarction: The PROTECTION-AMI Phase IIb Clinical Trial. [Google Scholar]
- 11.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–80. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 12.Mahaffey KW, Granger CB, Nicolau JC, Ruzyllo W, Weaver WD, Theroux P, et al. Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: the COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation. 2003;108:1176–83. doi: 10.1161/01.CIR.0000087404.53661.F8. [DOI] [PubMed] [Google Scholar]
- 13.Daneault B, Do DH, Maltais A, Berube S, Harvey R, Gervais A, et al. Reduction of Delays in Primary Percutaneous Coronary Intervention. Can J Cardiol. 2011 doi: 10.1016/j.cjca.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Muller UM, Eitel I, Eckrich K, Erbs S, Linke A, Mobius-Winkler S, et al. Impact of minimising door-to-balloon times in ST-elevation myocardial infarction to less than 30 min on outcome: an analysis over an 8-year period in a tertiary care centre. Clin Res Cardiol. 2011;100:297–309. doi: 10.1007/s00392-010-0242-7. [DOI] [PubMed] [Google Scholar]
- 15.Mensah K, Mocanu MM, Yellon DM. Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: a potential role for phosphatase and tensin homolog deleted on chromosome ten? J Am Coll Cardiol. 2005;45:1287–91. doi: 10.1016/j.jacc.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Tani M, Honma Y, Hasegawa H, Tamaki K. Direct activation of mitochondrial K(ATP) channels mimics preconditioning but protein kinase C activation is less effective in middle-aged rat hearts. Cardiovasc Res. 2001;49:56–68. doi: 10.1016/s0008-6363(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 17.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–4. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 18.Huhn R, Heinen A, Weber NC, Schlack W, Preckel B, Hollmann MW. Ischaemic and morphine-induced post-conditioning: impact of mK(Ca) channels. Br J Anaesth. 2010;105:589–95. doi: 10.1093/bja/aeq213. [DOI] [PubMed] [Google Scholar]
- 19.Who Fact Sheets. 2008 http://www.who.int/mediacentre/factsheets/fs310/en/index.html.
- 20.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 21.Gu YL, Kampinga MA, Wieringa WG, Fokkema ML, Nijsten MW, Hillege HL, et al. Intracoronary versus intravenous administration of abciximab in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention with thrombus aspiration: the comparison of intracoronary versus intravenous abciximab administration during emergency reperfusion of ST-segment elevation myocardial infarction (CICERO) trial. Circulation. 2010;122:2709–17. doi: 10.1161/CIRCULATIONAHA.110.002741. [DOI] [PubMed] [Google Scholar]
- 22.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, et al. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 2011;305:1863–72. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–30. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr, O’Neill WW, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 25.Ellis SG, Tendera M, de Belder MA, van Boven AJ, Widimsky P, Janssens L, et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N Engl J Med. 2008;358:2205–17. doi: 10.1056/NEJMoa0706816. [DOI] [PubMed] [Google Scholar]
- 26.Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carrie D, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355:1093–104. doi: 10.1056/NEJMoa062006. [DOI] [PubMed] [Google Scholar]
- 27.Granger CB, Mahaffey KW, Weaver WD, Theroux P, Hochman JS, Filloon TG, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation. 2003;108:1184–90. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]


