Abstract
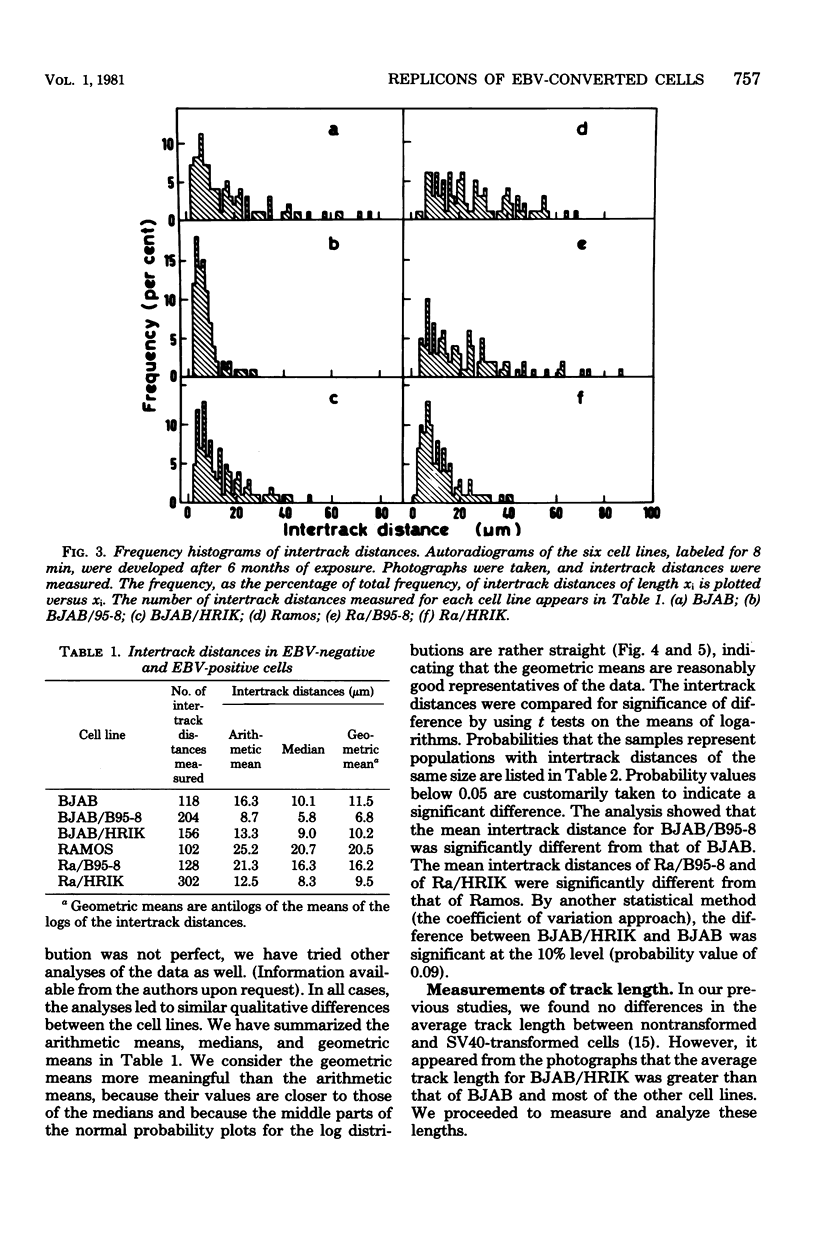
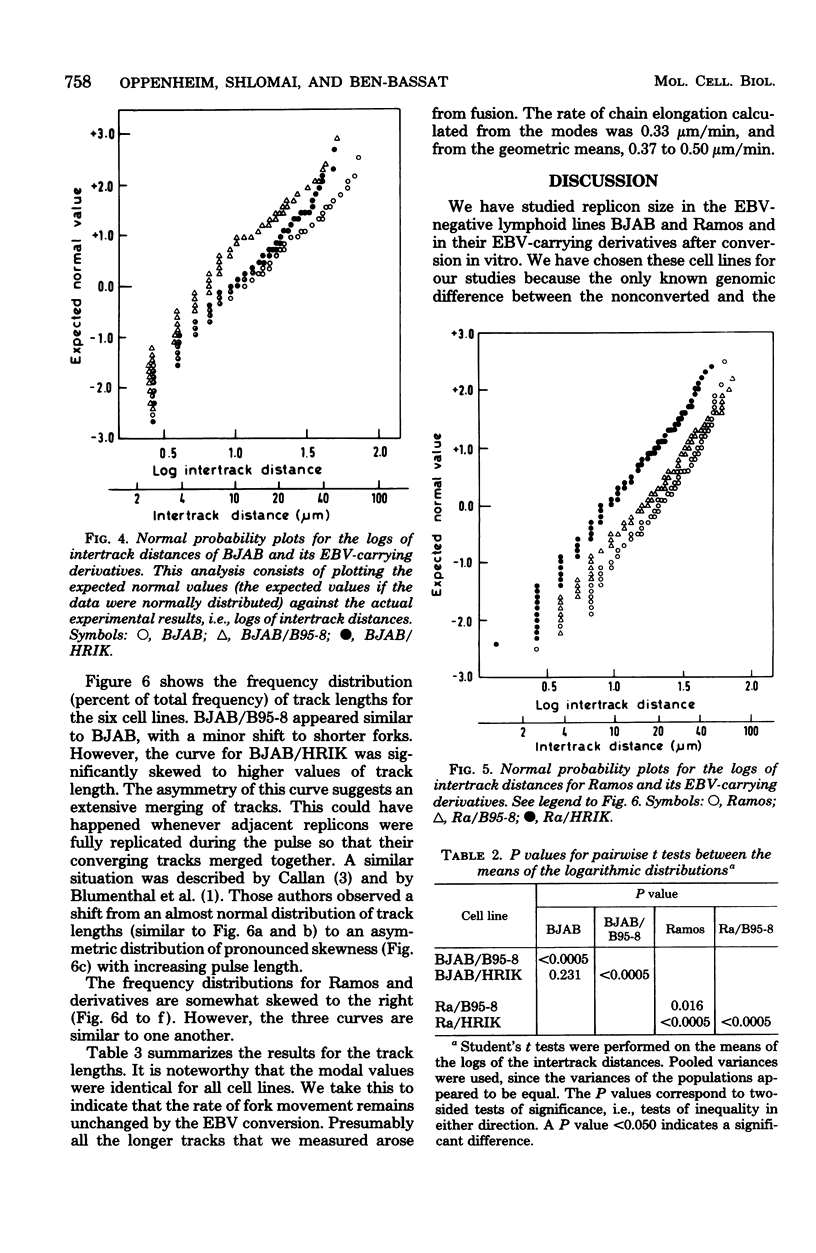
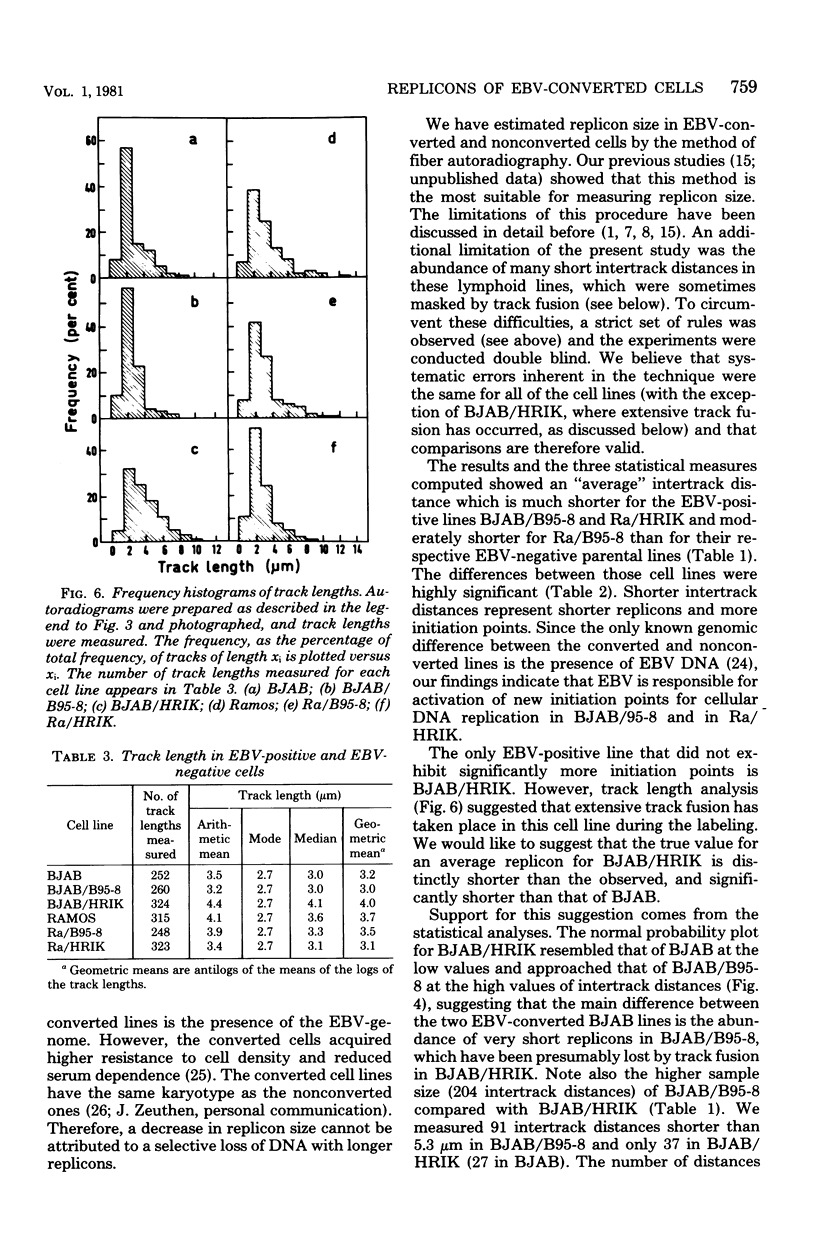
Replicon size was estimated in two Epstein-Barr virus (EBV)-negative human lymphoma lines, BJAB and Ramos, and four EBV-positive lines derived from the former ones by infection (conversion) with two viral strains, B95-8 and P3HR-1. Logarithmic cultures were pulse-labeled with [3H]thymidine, and the deoxyribonucleic acid was spread on microscopic slides and autoradiographed by the method of Huberman and Riggs. After developing, replication forks were visualized as silver grain tracks on the autoradiograms. Average replicon size was estimated by scoring the number of replication forks per constant length of deoxyribonucleic acid and by measuring distances between centers of adjacent tracks, followed by detailed statistical analyses. Three of the four EBV-converted cell lines, BJAB/B95-8, Ra/B95-8, and Ra/HRIK, were found to have significantly shorter replicons (41, 21, 54% shorter, respectively), i.e., more initiation points, than their EBV-negative parents. BJAB/HRIK had replicons which were only slightly shorter (11%) than those of BJAB. However, analysis of track length demonstrated that extensive track fusion occurred during the labeling of BJAB/ HRIK, implying that its true average replicon size is shorter than the observed value. The results indicate that in analogy to simian virus 40, EBV activates new initiation points for cellular DNA replication in EBV-transformed cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal A. B., Kriegstein H. J., Hogness D. S. The units of DNA replication in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:205–223. doi: 10.1101/sqb.1974.038.01.024. [DOI] [PubMed] [Google Scholar]
- Callan H. G. DNA replication in the chromosomes of eukaryotes. Cold Spring Harb Symp Quant Biol. 1974;38:195–203. doi: 10.1101/sqb.1974.038.01.023. [DOI] [PubMed] [Google Scholar]
- Callan H. G. Replication of DNA in the chromosomes of eukaryotes. Proc R Soc Lond B Biol Sci. 1972 Apr 18;181(1062):19–41. doi: 10.1098/rspb.1972.0039. [DOI] [PubMed] [Google Scholar]
- Clements G. B., Klein G., Povey S. Production by EBV infection of an EBNA-positive subline from an EBNA-negative human lymphoma cell line without detectable EBV DNA. Int J Cancer. 1975 Jul 15;16(1):125–133. doi: 10.1002/ijc.2910160114. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Evenson D., Staiano-Coico L., Sharpless T., Melamed M. R. Relationship between RNA content and progression of lymphocytes through S phase of cell cycle. Proc Natl Acad Sci U S A. 1979 Jan;76(1):358–362. doi: 10.1073/pnas.76.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R. Eucaryotic DNA: organization of the genome for replication. Cell. 1978 Oct;15(2):317–325. doi: 10.1016/0092-8674(78)90001-6. [DOI] [PubMed] [Google Scholar]
- Hand R., Tamm I. Initiation of DNA replication in mammalian cells and its inhibition by reovirus infection. J Mol Biol. 1974 Jan 15;82(2):175–183. doi: 10.1016/0022-2836(74)90339-8. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Luka J., Zeuthen J. Transformation induced by Epstein-Barr virus and the role of the nuclear antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):253–261. doi: 10.1101/sqb.1980.044.01.029. [DOI] [PubMed] [Google Scholar]
- Lenoir G., Berthelon M. C., Favre M. C., de-Thé G. Characterization of Epstein-Barr virus antigens. I. Biochemical analysis of the complement-fixing soluble antigen and relationship with Epstein-Barr virus-associated nuclear antigen. J Virol. 1976 Feb;17(2):672–674. doi: 10.1128/jvi.17.2.672-674.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Siegert W., Klein G. Solubilization of the Epstein-Barr virus-determined nuclear antigen and its characterization as a DNA-binding protein. J Virol. 1977 Apr;22(1):1–8. doi: 10.1128/jvi.22.1.1-8.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y., Avila J., Saral R. The semiautonomous replicon: a molecular model for the oncogenicity of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):17–24. doi: 10.1101/sqb.1974.039.01.005. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Oppenheim A. Initiation points for DNA replication in nontransformed and simian virus 40-transformed Chinese hamster lung cells. Cell. 1977 Aug;11(4):859–869. doi: 10.1016/0092-8674(77)90297-5. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Miller G. Human lymphoblastoid cell lines and Epstein-Barr virus: a review of their interrelationships and their relevance to the etiology of leukoproliferative states in man. Yale J Biol Med. 1971 Jun;43(6):358–384. [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Luka J., Falk L., Klein G. Detection of a nuclear, EBNA-type antigen in apparently EBNA-negative Herpesvirus papio (HVP)-transformed lymphoid lines by the acid-fixed nuclear binding technique. Int J Cancer. 1977 Dec 15;20(6):941–946. doi: 10.1002/ijc.2910200618. [DOI] [PubMed] [Google Scholar]
- Ohno S., Luka J., Klein G., Daniel M. D. Detection of a nuclear antigen in Herpesvirus ateles-carrying marmoset lines by the acid-fixed nuclear binding technique. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2042–2046. doi: 10.1073/pnas.76.4.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Luka J., Lindahl T., Klein G. Identification of a purified complement-fixing antigen as the Epstein-Barr-virus determined nuclear antigen (EBNA) by its binding to metaphase chromosomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1605–1609. doi: 10.1073/pnas.74.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A., Horowitz A. T. No activation of new initiation points for deoxyribonucleic acid replication in BALB/c 3T3 cells transformed by Kirsten sarcoma virus. Mol Cell Biol. 1981 Aug;1(8):763–768. doi: 10.1128/mcb.1.8.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A., Martin R. G. Initiation points for DNA replication in nontransformed and simian virus 40-transformed BALB/c 3T3 cells. J Virol. 1978 Jan;25(1):450–452. doi: 10.1128/jvi.25.1.450-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Comparison between growth characteristics of an Epstein--Barr virus (EBV)-genome-negative lymphoma line and its EBV-converted subline in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3518–3520. doi: 10.1073/pnas.72.9.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz M., Klein G. Epstein-Barr virus (EBV)-induced change in the saturation sensitivity and serum dependence of established, EBV-negative lymphoma lines in vitro. Virology. 1976 Apr;70(2):570–573. doi: 10.1016/0042-6822(76)90302-0. [DOI] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Oncogenic Herpes viruses. Biochim Biophys Acta. 1975 Mar 20;417(1):25–53. doi: 10.1016/0304-419x(75)90007-4. [DOI] [PubMed] [Google Scholar]