Abstract
Innate immunity to viral infection is initiated within the infected cells through the recognition of unique viral signatures by pattern recognition receptors (PRRs) that mediate the induction of potent antiviral factor, type I interferons (IFNs). Infection with RNA viruses is recognized by the members of the retinoic acid inducible gene I (RIG-I)-like receptor (RLR) family in the cytosol. Our recent study demonstrates that IFN production in response to RNA viral ligands is increased in the absence of autophagy. The process of autophagy functions as an internal clean-up crew within the cell, shuttling damaged cellular organelles and long-lived proteins to the lysosomes for degradation. Our data show that the absence of autophagy leads to the amplification of RLR signaling in two ways. First, in the absence of autophagy, mitochondria accumulate within the cell leading to the build up of mitochondrial associated protein, IPS-1, a key signaling protein for RLRs. Second, damaged mitochondria that are not degraded in the absence of autophagy provide a source of reactive oxygen species (ROS), which amplify RLR signaling in Atg5 knockout cells. Our study provides the first link between ROS and cytosolic signaling mediated by the RLRs, and suggests the importance of autophagy in the regulation of signaling emanating from mitochondria.
In infected cells, cytosolic PRRs are used to sense viral ligands within the cytosol and mount an appropriate innate immune response. The RLR family, consisting of RIG-I and melanoma differentiation-associated gene 5 (MDA5), recognize RNA viruses within the cytosol and induce the expression of potent antiviral factors such as type I interferons (IFN) and proinflamatory cytokines. Activated RLRs signal through the adaptor protein IFN-beta promoter stimulator 1 (IPS-1) also known as MAVS, Cardif, or VISA, which requires localization to the mitochondria for its function (Fig. 1). IPS-1 activates two major signaling pathways leading to the induction of proinflammatory cytokines via activation of the IKK kinase complex and NFκB, and the induction of IFNs through the IKK-related kinases TBK1 and IKKε, which activate IRF3.
Figure 1. The mitochondria as a point of intersect between autophagy and RLR signaling.
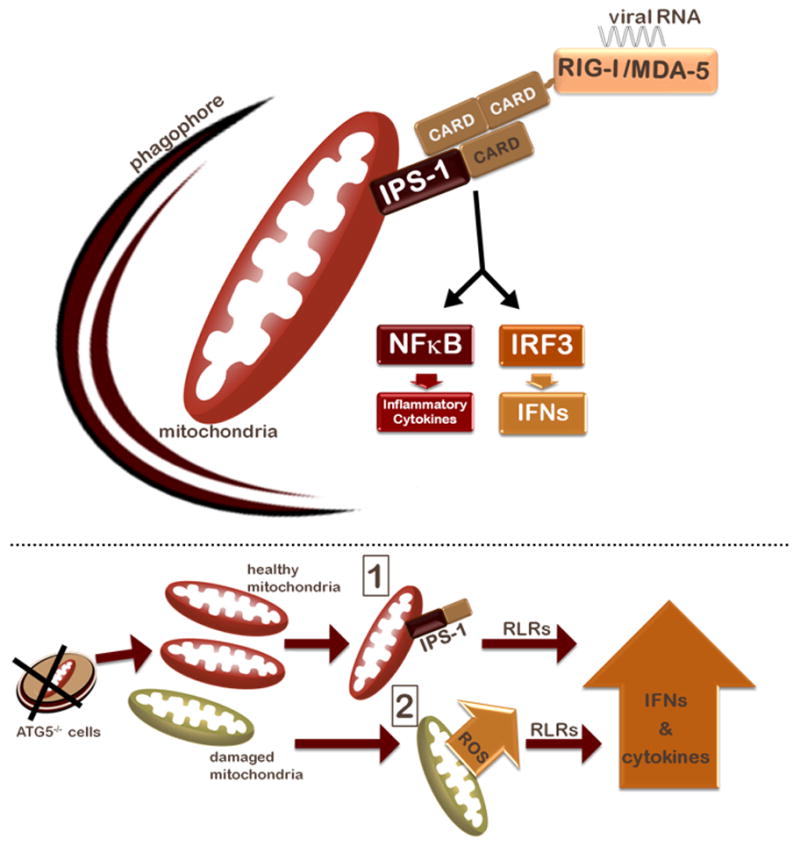
Top: a schematic representation of RLR signaling and mitochondrial clearance by autophagy. Bottom: two possibilities are illustrated for how the absence of autophagy and the resultant mitochondrial accumulation results in increased IFN and inflammatory cytokine signaling in response to RLR stimulation. First, mitochondrial accumulation results in increased levels of the mitochondrial protein IPS-1, which results in subsequent amplification of IFN and inflammatory cytokine signaling by RLRs. Second, levels of mitochondria-associated ROS rise as damaged and potentially leaky mitochondria fail to be cleared, potentiating RLR signaling.
Autophagy is a highly conserved process by which cytosolic constituents can be engulfed and degraded in the lysosomes. A previous report by Jounai et al. indicated that the ATG12-ATG5 heterodimeric complex, which plays a key role in autophagosome formation, negatively regulates the RLR pathway by directly binding to RIG-I, MDA-5 and IPS-1 and inhibiting their association. The question remained as to whether the classical process of autophagy was involved in the regulation of RLR signaling. To examine whether the autophagy process regulates RLR signaling, we focused on the interface between autophagy and mitochondria. Autophagy provides the major pathway for clearance of damaged mitochondria, via a process known as mitophagy. Given that the key adaptor protein of RLRs, IPS-1, is on the mitochondrial membrane, we hypothesized that autophagy could regulate RLR signaling either by maintenance of overall mitochondrial mass and/or integrity. Indeed, we observed both increased levels of mitochondrial mass and detected higher percentages of cells that have accumulated damaged mitochondria in the Atg5-deficient cells. This led to higher expression of IPS-1 and thus enhanced signaling via RLRs. However, the increase in IPS-1 alone was unable to account for the dramatic amplification of RLR signaling in Atg5−/− cells. As mitochondria are the major producers of reactive oxygen species (ROS) as a byproduct of oxidative respiration, the lack of clearance of damaged and potentially leaky mitochondria could provide a dangerous source of ROS within the cell, which could in turn affect RLR signaling. Our data indeed indicated that not only was the mitochondrial ROS level increased in the absence of autophagy, but also chemically inducing elevated levels of mitochondria-associated ROS resulted in increased IFN production upon RLR stimulation, and that antioxidant treatment subsequently reduced RLR signaling in ATG5-deficient MEFs and macrophages. These results revealed the importance of autophagy in the clearance of mitochondria, and provided the first evidence for ROS-mediated control of RLR signaling.
A key unanswered question is, what are the possible targets of oxidation, which could potentiate RLR signaling in this way? It is possible that a negative regulator of RLR signaling might become inactivated upon oxidation. It is also possible that oxidation enables the activity of a positive regulator of RLR signaling. Additionally, it is conceivable that proteins involved in sensing ROS levels with in the cell could be involved in RLR signaling in ways that remain to be identified. Identification of the molecular mechanism by which ROS amplify RLR signaling will provide important insights into the oxidative regulation of this key antiviral signaling pathway.
It is critical that inflammatory responses are carefully coordinated so as to provide effective defense against viral infections while inflicting minimal damage to the host. Over-activation of RLR signaling could lead to detrimental levels of inflammation or have other immunopathological consequences that are harmful to the host. An intriguing idea that stems from our study is the possibility that the localization of this antiviral signaling pathway to the mitochondria could centralize cellular stress signaling to one organelle that can facilitate a coordinated response. Recently, two additional proteins involved in cytosolic antiviral signaling have also been found to be localized on the mitochondria. It is tempting to speculate that the functional implication of RLR signaling occurring at the mitochondrial face allows for integration with other cellular stresses that could be caused by viral infection to generate a heightened antiviral response.
A broader implication of our study relates to diseases in which elevated ROS and/or reduced autophagy are associated. Viral infection of individuals with a reduced capacity to undergo autophagy or those who suffer from aberrant ROS levels might result in elevated RLR-induced cytokines. While this may result in more rapid viral clearance, such heightened cytokine levels might predispose these individuals to extensive tissue damage and increased risk of triggering an autoimmune reaction. Autophagy decreases with aging, and there are a great variety of disease states that involve increased ROS levels, including many of the late-onset diseases. Future studies are necessary to understand the ramifications of the loss of autophagy, elevated ROS levels and RLR stimulation in human diseases and aging.


