Abstract
Sjögren’s syndrome (SjS) is a systemic autoimmune disease that primarily targets salivary and lacrimal glands. SjS affects 2–4 million people in the US alone and greatly affects the life quality of the afflicted individuals. Autoreactive effector T cells are central executors and orchestrators in the pathogenic processes of SjS by mediating target organ inflammation and destruction and by facilitating B cell responses and autoantibody production. A variety of cytokines that are produced by effector T cells or capable of directly affecting effector T cells are elevated in the target organs and circulations of SjS patients. The recent advancement in the understanding about the functions of these cytokines, achieved by using both human samples and mouse disease models, has generated great insights into the cytokine control of autoimmune responses in the SjS disease setting. In this review, we summarized the recent findings on the expression and functions of cytokines in this disease, with specific focus on those derived from T cells and/or directly affecting T cell responses.
Introduction
Sjögren’s syndrome (SjS) is a systemic autoimmune disease which primarily affects salivary and lacrimal glands. SjS affects 2–4 million people in the US alone, with 90% of the patients being women. SjS is characterized by progressive lymphocytic infiltration of salivary and lacrimal glands and generation of autoantibodies that include anti-SSA/Ro, SSB/La and other exocrine gland- specific autoantibodies, which together lead to impaired secretory function [1–3]. The primary clinical symptoms are xerostomia (dry mouth) and keratoconjunctivitis (dry eyes), which are presented as difficulty swallowing, chewing or talking, sandy or burning sensations in the eyes, dry or burning feelings at lips, nose and throat, and as a result, a higher incidence of dental caries. In addition, patients also often suffer from dryness of gastrointestinal tract, vagina, lung and skin, and from other extra-glandular symptoms such as chronic fatigue, fibromyalgia, muscle and joint pain, nephritis and peripheral neuropathy [1, 3, 4]. Finally, they have a much higher risk of developing B cell lymphoma than the general population and people with other autoimmune disorders [1, 3, 4]. SjS is usually chronic, progressive and at times debilitating, thereby greatly affecting the life quality of the patients. SjS can occur as primary SjS (pSjS) or secondary SjS, which is associated with other connective tissue diseases [2, 5]. The current diagnosis of SjS is conducted according to the Revised European-American Criteria for the Classification of SjS [6], which entail histological analysis of a minor salivary gland biopsy for lymphocytic infiltration, presence of serum anti-SSA and/or SSB autoantibodies, presence of oral and ocular symptoms, as well as oral and ocular tests for saliva and tear production.
Although the etiology of SjS remains elusive, accumulating evidence indicates that both genetic factors and environmental triggers, such as viral infections, sex hormone changes and tissue injuries, contribute to the initiation of autoimmune process in SjS [3, 7–10]. Both self-reactive T and B cells play crucial roles for the development and onset of SjS by driving exocrine gland inflammation and autoantibody production [3, 5, 11–13]. Cytokines are powerful orchestrators and effectors of the innate and adaptive immune responses. The differentiation of distinct effector T cells subsets, T helper (Th) 1, Th2, Th17 and T follicular helper (TFH) cells, are instructed or influenced by various cytokines. Each effector T cell subset in turn produces a group of signature cytokines, which execute specialized effects on target tissues or pathogens and often simultaneously propel the further differentiation and expansion of the same effector subset. Many cytokines have been shown to be elevated in the target organs and serum of SjS patients and mouse models of SjS, as discussed later in this review. Functional studies performed with mouse models of SjS that are deficient in specific cytokine genes demonstrate that IFN-γ, IL-4 and IL-17, signature cytokines for Th1, Th2 and Th17 effector cells, are all essential for the full development and onset of SjS by modulating the differentiation, expansion and function of self-reactive T and B cells and by directly affecting the homeostasis and biological activities of the target tissues [14–19]. In this review, we summarized the recent progress on the expression and functions of cytokines in the pathogenesis of SjS, with specific focus on the cytokines that are either produced by effector T cells or directly affecting T cell responses.
Th1-associated cytokines
1. IFN-γ
IFN-γ, the hallmark cytokine of Th1 and cytotoxic CD8 T cells, plays a pivotal role in cellular immunity and host defense against intracellular pathogens and tumor [20]. IFN-γ is produced predominantly by natural killer (NK) and natural killer T (NKT) cells as part of the innate immune response, and by Th1 cell and CD8+ cytotoxic T cells (CTL) during antigen-specific adaptive immune response. Among its plethora of biological effects, IFN-γ can activate macrophages and NK cells, enhance MHC expression and antigen presentation, induce expression of many chemokines and adhesion molecules in both immune and non-immune cells, promote differentiation of Th1 cells and induce apoptosis of various tissue and cell types [21, 22]. IFN-γ has been shown to play a pathogenic role in mouse models of type 1 diabetes [23, 24], systemic lupus erythematosus (SLE) [25, 26], rheumatoid arthritis (RA) [27] and dextran sodium sulfate-induced inflammatory bowel diseases (IBD) [28].
The salivary glands and saliva from patients with primary SjS exhibit elevated levels of IFN-γ compared to non-SjS subjects [29, 30]. Patients with primary SjS demonstrate an increased Th1 response over Th2 response in the salivary glands, saliva and serum, compared to individuals or the non-SjS sicca patients [31, 32]. Moreover, the Th1 response shows positive association with lymphocytic infiltration of the salivary glands [33]. In non-obese diabetic (NOD) mice, a widely used model for type 1 diabetes and secondary SjS, IFN-γ-deficiency abolishes multiple pre-immunological abnormalities of the salivary glands, thus preventing the subsequent tissue-specific autoimmunity and clinical onset of SjS [15]. Mechanistically, IFN-γ has been reported to induce death and secretory dysfunction in salivary gland cells as well as enhance the abilities of epithelial cells and antigen presenting cells (APCs) in the salivary glands to present antigens and activate T cells [5, 34, 35]. Furthermore, our own study and another report have shown that IFN-γ induces expression of chemokines CXCL9 and −10, both of which are ligands for CXCR3, in a human salivary gland cell line and in primary salivary gland epithelial cells from SjS patients [36]. Consistent with a potential role of CXCR3 ligands in SjS pathogenesis, CXCL9, −10 and −11 are significantly increased in salivary gland lesions and tears of SjS patients [29, 36, 37]. Antagonizing CXCL10 activity in MRL/lpr mice, a model of secondary SjS, during the early stage of disease development significantly reduces mononuclear cell infiltration of salivary glands [38]. These findings suggest that IFN-γ-CXCR3 ligand pathway may be a crucial mechanism by which IFN-γ promotes effector T cell recruitment to target tissues and initiates local autoimmune responses. Definitive in vivo studies, especially loss-of-function studies, are required to determine the functions of endogenous IFN-γ in the immunological phase of the SjS development and in the persistence of SjS after disease onset. Moreover, additional effects of this cytokine in SjS, such as those affecting macrophage and NK cell function and inducing additional chemokines, also await further investigation.
2. IL-12
IL-12, produced mainly by macrophages and dendritic cells (DCs), is a critical promoter for the differentiation of IFN-γ-producing T cells, both Th1 and effector CD8 T cells [39, 40]. IL-12 potently induces and facilitates cellular immune responses by promoting proliferation, cytotoxic activity and IFN-γ production from effector T cells and NK cells [22, 39, 40]. IL-12 treatment decreases the frequency of regulatory T cells (Tregs) and downregulates Foxp3 levels in these cells [41]. Hence, IL-12 engages multiple mechanisms to promote immune activation. Aberrantly enhanced IL-12 activity is implicated in the pathogenesis of many immune-mediated diseases, including psoriasis, Crohn’s disease, RA and type 1 diabetes [42–44].
IL-12 levels are elevated in the target organs of patients with SjS, with macrophages and DCs being the main cellular sources [45, 46]. A marked increase in IL-12 mRNA and protein levels is detected at the early stage of the disease development in mouse models of SjS compared to control mice [47, 48]. Moreover, thyroid gland-specific IL-12-transgenic mice with SJL genetic background exhibit SjS-like salivary gland inflammation, anti-SSB/La autoantibody production and salivary gland secretory dysfunction [49]. However, loss-of-function studies in mouse disease models are still needed to define the functional importance of endogenously produced IL-12 in the pathogenesis of SjS.
3. IL-18
IL-18, a member of IL-1 family of pro-inflammatory cytokines, mediates a variety of inflammatory responses in the context of inflammation, infections and autoimmunity through affecting both immune and non-immune cells and both innate and adaptive immune systems [50–52]. One major effect of IL-18 on adaptive immunity is to enhance IL-12-induced Th1 responses [51, 53]. IL-18 facilitates the development of autoimmune and inflammatory diseases, including pulmonary inflammatory disease, RA, type 1 diabetes and IBD [52–55]. IL-18 is detected in the serum, salivary glands and saliva of SjS patients and its levels correlate with the severity of the disease among the patients[56–58]. Moreover, expression of IL-18 by salivary gland-infiltrating macrophages and DCs shows positive correlation with degree of leukocyte infiltration and lymphoma development in patients with primary SjS [45]. Similarly to IL-12, the in vivo pathogenic role of IL-18 in SjS still awaits future determination.
4. TNF-α
Tumor necrosis factor-alpha (TNF-α) is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reactions such as fever, cell death, sepsis, cachexia and inflammation. It is produced by many cell types including Th1 cells and CD8 effector cells and implicated in the pathogenesis of various autoimmune and rheumatic diseases. SjS patients have higher salivary TNF-α level than non-SjS sicca patients [32]. TNF-α enhances the surface expression of Ro (SS-A) and La (SS-B) on human keratinocytes, two important autoantigens involved in SjS and SLE [59]. Moreover, in vitro studies showed that TNF-α, alone or cooperating with IFN-γ, induces apoptosis and secretory dysfunction of salivary gland cells [34, 60, 61]. However, despite these lines of evidence suggesting a role of TNF-α in SjS pathogenesis, the in vivo functional studies are still lacking. Furthermore, a randomized, double-blind and placebo-controlled clinical trial of infliximab, a neutralizing monoclonal antibody against TNF-α that showed promising therapeutic effects on a number of autoimmune or inflammatory disorders including SLE, did not demonstrate any efficacy in treating pSjS [62]. Therefore, TNF-α may not be an indispensable pathogenic factor for SjS or it may play a more complicated role than simply promoting the disease.
Th2-associated cytokines
1. IL-4
IL-4, originally characterized as an anti-inflammatory cytokine, is the hallmark cytokine of Th2 immune response. IL-4 amplifies Th2 differentiation, mediates asthma and allergy and provides critical stimulating signals for the growth and function of activated B cells [63, 64]. Meanwhile, IL-4 inhibits the activation of Th1 response and cellular immunity, which largely accounts for its anti-inflammatory function [65, 66]. Provision of exogenous IL-4 protects against development of type 1 diabetes and RA [67, 68]. However, IL-4 is required for the development of systemic autoimmune diseases SLE, in that it promotes target organ inflammation and pathologies and faciliates production of IgG1 and IgE gammaglobulins [25, 69].
IL-4 has been detected in the salivary glands of a portion of SjS patients, especially those with higher degree of B cell accumulation in the target organs [33, 70]. Deficiency of IL-4 or STAT6 gene in NOD and NOD.B10-H2b mice abolishes the production of IgG1-type anti-M3R antibody, the crucial autoantibody causing the secretory dysfunction and xerostomia [14, 17, 48]. Moreover, whereas sera from NOD.B10-H2b mice can cause salivary secretory dysfunction upon transfer to C57BL/6 mice, those from STAT6-deficient NOD.B10-H2b mice fail to do so [17]. IL-4 effect seems to be specifically targeting the anti-M3R of IgG1 isotype, as it does not affect anti-M3R of the other isotypes, nor does it affect overall salivary gland inflammation [14, 17, 48]. Hence, IL-4 plays an indispensable role in the pathogenic IgG1-type anti-M3R autoantibody, likely by affecting isotype switching process, and consequently facilitates the exocrine dysfunction in mouse models of SjS.
2. IL-13
IL-13 is originally characterized as a Th2 cytokine as it can be produced by these effector T cells and has overlapping effects with IL-4 on macrophages, B cells and inflammation [71–73]. IL-13 enhances the activities of many cell types, such as B cells, fibroblasts and mast cells, and as a result plays pivotal roles in allergic asthma, anti-helminth immunity and tissue fibrosis [71]. IL-13 levels are reported to be increased in patients with rheumatic disease RA and SLE and correlated with disease severity [74, 75]. IL-13 mRNA has been detected in the exocrine glands of SjS patients [33, 76]. A recent report demonstrates the presence of IL-13-producing T cells in salivary gland draining lymph nodes in Id3-deficient mice, a mouse model of primary SjS, but not in wild-type control mice [77]. Id3-deficient mice demonstrate an increase in mast cells in the salivary glands, which may contribute to the salivary gland dysfunction. Blockade of IL-13 activity in Id3-KO mice with a neutralizing antibody improves salivary gland secretory function, which is associated with a reduction of mast cells in the target organs [77]. This study has presented important evidence that IL-13 facilitates development of secretory dysfunction, possibly by affecting mast cells.
Th17-associated cytokines
1. IL-17A
IL-17, a potent pro-inflammatory cytokine that has been in the center of investigation in the field of autoimmunity and inflammation, is the hallmark cytokine produced by Th17 cells. It plays important roles in host defense against bacterial and fungal infections and has been shown to be the predominant pathogenic player in numerous autoimmune and inflammatory diseases [78–80]. IL-23, a member of IL-12 family of cytokines that comprises the p40 subunit of IL-12, has been shown to be a crucial cytokine for induction, stabilization and expansion of Th17 cells [39, 79, 81]. A rapidly growing knowledge in recent years about the pro-inflammatory functions of IL-17 and IL-23-Th17 pathway has greatly advanced our understanding about the fundamental mechanisms causing autoimmunity and chronic inflammation, once attributed mainly to Th1 cells [39, 78–81]. An increase in IL-17 and IL-23 levels was reported in patients of various autoimmune or inflammatory diseases, including multiple sclerosis, RA, Crohn’s disease and psoriasis, and an essential role of IL-23-IL-17 pathway in the development and onset of these diseases has been demonstrated in the corresponding mouse models [39, 78–83].
Elevated IL-17 and IL-23 levels are reported in salivary gland and serum of patient with primary SjS [84–87]. Moreover, immunohistochemistry analysis has shown IL-17 and IL-23 proteins and their receptors within lymphocytic infiltrates and ductal areas in salivary glands of SjS patients [84, 86, 88]. In addition, IL-17-producing-CD3+CD4−CD8− T cells are also expanded in the peripheral blood and salivary gland infiltrates in patients with SjS [89]. Inhibition of IL-17 activity by adenovirus-mediated expression of a soluble IL-17R:Fc fusion protein impedes the development of SjS-like disease in C57BL/6.NOD-Aec1Aec2 mice, a model of primary SjS, as indicated by diminished tissue- inflammation and autoantibody production and improved secretory function [19] . Conversely, adenovirus-mediated overexpression of IL-17 in non-SjS-prone C57BL/6 mice induces the development of SjS-like disease based on multiple disease parameters [18], including tissue-inflammation and antinuclear autoantibody production. Interestingly, overexpression of IL-17 results in the activation of B cell antibody production, the underlying mechanisms for which requires future investigation. Collectively, these findings define a crucial pathogenic function of IL-17 in SjS and identify it as a potential therapeutic target for this disease.
2. IL-22
IL-22 is an IL-10 family cytokine member that is expressed by Th17 and NK cells, among others [78, 90]. Recent findings have shown important functions of IL-22 in autoimmunity, inflammation and epithelial cell and mucosal tissue homeostasis [78, 90, 91], with both tissue-protective effects and pro-inflammatory effects reported in different disease conditions [78, 90, 91]. Serum levels of IL-22 were significantly elevated in patients with SjS and correlated with levels of autoantibodies and rheumatoid factor, suggesting a possible role of IL-22 in the development of this disease [92]. Moreover, in the salivary glands of patients with SjS, IL-22 mRNA and protein are both increased, with Th17 and NK cells as the main producers of this cytokine [93]. These findings suggest a potential role of IL-22 in SjS pathogenesis, which remains to be assessed by functional studies using mouse models and human samples.
Other cytokines
1. IL-21
IL-21 is a T cell-derived, pleiotropic cytokine belonging to the common cytokine receptor γ chain (γc)-dependent cytokine family [94, 95]. IL-21 is produced by TFH, Th17 and NKT cells, with its receptor expressed on T cells and various types of immune cells and non-hematopoietic cells [94–96]. IL-21 can directly promote plasma cell differentiation and enhance germinal center B cell response, potentiate Th17 cell differentiation and enhance effector and memory CD8 T cell survival and function [79, 81, 96, 97]. Rapidly growing evidence in recent years has identified IL-21 as a crucial new player in the pathogenesis of a number of autoimmune diseases, including IBD, SLE, type 1 diabetes and psoriasis [98, 99]. IL-21 also has anti-inflammatory and immune-suppressive effect by promoting the generation of IL-10-producing type 1 regulatory T (Tr1) cells [100, 101].
Patients with SjS have significantly elevated serum IL-21 levels, which are positively correlated with levels of IgG1. Immunohistochemistry analyses show that lymphocytic foci in the salivary glands from SjS patients express high levels of IL-21 compare to the controls [102]. Moreover, IL-21-producing T cells are detected in salivary glands in SjS patients [103]. A recent report shows that local suppression of IL-21 in submandibular glands, achieved by lentivirus mediated expression of IL-21 shRNA, reduces target organs inflammation and improves salivary gland secretory function and thus impedes the development of SjS in NOD mice [104]. The effect of IL-21 is associated with an impaired TFH response, which might result in subsequent diminished B cell antibody production. Thus, IL-21 is a newly characterized pathogenic player in SjS, with direct impact on both B and T cells.
2. IL-10
IL-10, initially classified as a Th2 cytokines, is a potent anti-inflammatory cytokine which suppresses both innate and adaptive immune response [105, 106]. IL-10 inhibits the maturation, function and cytokine production of antigen presenting cells and also can directly suppress T cell differentiation into pro-inflammatory Th1 and Th17 subsets [106–108]. Consequently, IL-10 prevents the development of IBD and restrains the development of RA and experimental autoimmune encephalomyelitis, and limits tissue damage in response to certain microbial infections and chemical stimulants [107, 109, 110]. IL-10 can be produced by macrophages, Tr1 cells, Tregs and various effector T cell subsets [105, 106]. IL-10 has certain stimulatory effects on B cells, promoting their survival and proliferation and enhancing plasma cell generation, antibody production and isotype switching [107, 111, 112].
IL-10 levels are elevated in the saliva of SjS patients and correlated with severity of xerophthalmia and xerostomia [113]. Increased serum IL-10 levels are reported in patients with primary SjS, which are positively associated with the levels of IgG1 and immune cell-infiltration [114]. These lines of evidence suggest that IL-10, despite of its well-documented immune-suppressive effects, may play a pathogenic role in SjS. Indeed, transgenic expression of IL-10 in salivary and lacrimal glands leads to SjS-like lymphocytic infiltration and enhanced apoptosis of glandular cells, which are underpinned by the effect of IL-10 to upregulate Fas ligand expression in CD4 T cells [115]. IL-10-deficiency in NOD mice markedly reduces both insulitis and sialadenitis [116]. The pro-inflammatory properties of IL-10 in SjS may be attributed to additional mechanisms, such as upregulation of ICAM-1 expression in target tissues and acquisition of pro-inflammatory function in the presence of excess IFN-α [117, 118]. These mechanisms have been demonstrated in the cases of type 1 diabetes and SLE, in which IL-10 exerts pathogenic effects, likely in a fashion that depends on the stage of the disease and the cell types that produce IL-10 [117, 118]. Future investigations are warranted to thoroughly delineate the complex function of IL-10 in the pathogenesis of SjS.
Model for the role of cytokine network in SjS pathogenesis
As described in this review, findings from human SjS patients and various in vitro and in vivo studies using mouse SjS models have provided evidence implicating the functions of a number of T cell-derived or T cell-affecting cytokines in the development and onset of this disease. Among them, IL-4, IL-13, IFN-γ, IL-17, IL-21 and IL-10 have been convincingly shown to play distinct but essential roles in the pathogenic processes of this disease through in vivo loss-of-function studies using mouse SjS models. The roles of many other cytokines, including IL-12, IL-23, IL-18, TNF-α and IL-22, are strongly suggested but remain to be determined by in vivo loss-of-function studies. We propose a model delineating the specific functions and contributions of some of these cytokines in various pathogenic events leading to the onset of SjS (illustrated in Fig. 1). Th1 cytokine IFN-γ and TNF-α are crucial for the initial induction of salivary gland tissue apoptosis, auto-antigen release and activation of APCs and exocrine gland epithelial cells. Moreover, IFN-γ and TNF-α induces production of various chemokines, such as macrophage chemotactic protein, CXCR3 ligands and CCL20 [119] from exocrine gland cells and APCs, which initiate and reinforce the recruitment of macrophages, Th1, Th17 and other T cell subsets to the exocrine glands. IL-17 produced by Th17 cells in the exocrine glands induces or potentiates production of inflammation cytokines, such as IL-1 and TNF-α, and chemokines, such as MIP3α, from exocrine gland cells. It also induces production of matrix metalloproteinases from tissue cells to enhance tissue damage. Moreover, IL-17 may also directly affect B cell responses. At a later phase of SjS development, IL-4 produced by Th2 cells provides crucial help to B cells in their activation, proliferation and isotype switch to produce IgG1 type anti-M3R antibodies that contribute to secretory dysfunction of salivary gland cells. Th2-derived IL-13 facilitates the secretory dysfunction, possibly by enhancing the proliferation and activation of mast cells and histamine release from these cells. However, how mast cells promote the impairment of secretory dysfunction requires further characterization. Also around this phase or at an even later stage, IL-21 produced by TFH cells or other effector T cell subsets enhances the germinal center (GC) B cell responses to worsen the secretory function, and further positively reinforce the TFH response. IL-21 may also have additional effects on T cell responses. In such a fashion, the coordinated effects of multiple T cell cytokines mediate distinct pathogenic events, propelling the development of various tissue pathologies and leading to the eventual onset of SjS (Fig. 1).
Figure 1.
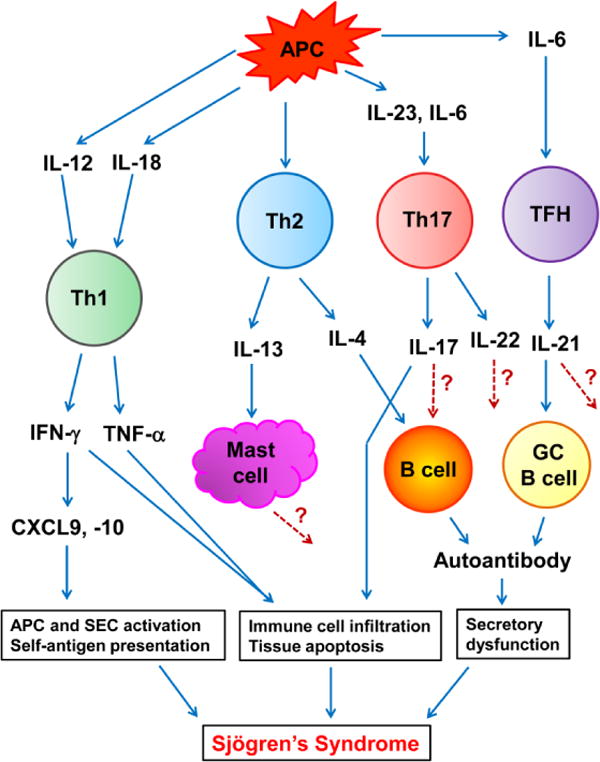
Model for the role of T cell cytokine network in SjS pathogenesis
Many aspects proposed in this model need to be tested in future by in vivo functional studies in mouse models and by in vitro functional studies using T cells from SjS patients. Furthermore, the precise effects and the stage-dependent functions of many cytokines, the underlying mechanisms for the effects of these cytokines and the interactions, cross-regulation and redundancy among cytokines and Th subsets in the development and persistence of SjS are either completely uncharacterized or only partially delineated, and therefore await in-depth investigations in future.
Conclusion
Cytokines are crucial effectors and modulators of the autoimmune responses that lead to the onset of SjS. They are also promising targets for treatment and prevention of this disease. The recent advancement in our understanding about the functions of SjS-associated cytokines, achieved by utilizing both human samples and mouse models, will guide future studies that elucidate the regulation of cytokine expression and interaction between different cytokines in the context of SjS, and fully determine the precise pathological effects of these cytokines in the development, progression and systemic manifestation of SjS. These accomplishments will provide crucial insights and tools for the development of better diagnostic criteria and more effective and specific therapeutic strategies to combat this prevalent, high-impact autoimmune disease.
References
- 1.Fox PC. Autoimmune diseases and sjogren’s syndrome: An autoimmune exocrinopathy. Ann N Y Acad Sci. 2007;1098:15–21. doi: 10.1196/annals.1384.003. [DOI] [PubMed] [Google Scholar]
- 2.Lee BH, Tudares MA, Nguyen CQ. Sjogren’s syndrome: An old tale with a new twist. Arch Immunol Ther Exp (Warsz) 2009;57:57–66. doi: 10.1007/s00005-009-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of sjogren’s syndrome. Nat Rev Rheumatol. 2010;6:529–37. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- 4.Theander E, Manthorpe R, Jacobsson LT. Mortality and causes of death in primary sjogren’s syndrome: A prospective cohort study. Arthritis Rheum. 2004;50:1262–9. doi: 10.1002/art.20176. [DOI] [PubMed] [Google Scholar]
- 5.Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in sjogren’s syndrome: Contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol. 2007;32:252–64. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 6.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, et al. Classification criteria for sjogren’s syndrome: A revised version of the european criteria proposed by the american-european consensus group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolov NP, Illei GG. Pathogenesis of sjogren’s syndrome. Curr Opin Rheumatol. 2009;21:465–70. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triantafyllopoulou A, Moutsopoulos H. Persistent viral infection in primary sjogren’s syndrome: Review and perspectives. Clin Rev Allergy Immunol. 2007;32:210–4. doi: 10.1007/s12016-007-8004-7. [DOI] [PubMed] [Google Scholar]
- 9.Ohyama Y, Carroll VA, Deshmukh U, Gaskin F, Brown MG, et al. Severe focal sialadenitis and dacryoadenitis in nzm2328 mice induced by mcmv: A novel model for human sjogren’s syndrome. J Immunol. 2006;177:7391–7. doi: 10.4049/jimmunol.177.10.7391. [DOI] [PubMed] [Google Scholar]
- 10.Stojanovich L, Marisavljevich D. Stress as a trigger of autoimmune disease. Autoimmunity reviews. 2008;7:209–13. doi: 10.1016/j.autrev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Cohen PL. The t cell in sjogren’s syndrome: Force majeure, not spectateur. J Autoimmun. 2012;39:229–33. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa I, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates sjogren’s syndrome in id3 knockout mice. Immunology. 2007;122:73–9. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Dai M, Zhuang Y. A t cell intrinsic role of id3 in a mouse model for primary sjogren’s syndrome. Immunity. 2004;21:551–60. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Brayer JB, Cha S, Nagashima H, Yasunari U, Lindberg A, et al. Il-4-dependent effector phase in autoimmune exocrinopathy as defined by the nod.Il-4-gene knockout mouse model of sjogren’s syndrome. Scand J Immunol. 2001;54:133– 40. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 15.Cha S, Brayer J, Gao J, Brown V, Killedar S, et al. A dual role for interferon-gamma in the pathogenesis of sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60:552–65. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 16.Roescher N, Tak PP, Illei GG. Cytokines in sjogren’s syndrome: Potential therapeutic targets. Ann Rheum Dis. 2010;69:945–8. doi: 10.1136/ard.2009.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen CQ, Gao JH, Kim H, Saban DR, Cornelius JG, et al. Il-4-stat6 signal transduction-dependent induction of the clinical phase of sjogren’s syndromelike disease of the nonobese diabetic mouse. J Immunol. 2007;179:382–90. doi: 10.4049/jimmunol.179.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen CQ, Yin H, Lee BH, Carcamo WC, Chiorini JA, et al. Pathogenic effect of interleukin-17a in induction of sjogren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res Ther. 2010;12:R220. doi: 10.1186/ar3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. Il17: Potential therapeutic target in sjogren’s syndrome using adenovirus-mediated gene transfer. Laboratory investigation; a journal of technical methods and pathology. 2011;91:54–62. doi: 10.1038/labinvest.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glimcher LH, Murphy KM. Lineage commitment in the immune system: The t helper lymphocyte grows up. Genes & development. 2000;14:1693–711. [PubMed] [Google Scholar]
- 21.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–50. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12:597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, et al. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:13844–9. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicoletti F, Zaccone P, Di Marco R, Di Mauro M, Magro G, et al. The effects of a nonimmunogenic form of murine soluble interferon-gamma receptor on the development of autoimmune diabetes in the nod mouse. Endocrinology. 1996;137:5567–75. doi: 10.1210/endo.137.12.8940385. [DOI] [PubMed] [Google Scholar]
- 25.Peng SL, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–46. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theofilopoulos AN, Koundouris S, Kono DH, Lawson BR. The role of ifn-gamma in systemic lupus erythematosus: A challenge to the th1/th2 paradigm in autoimmunity. Arthritis research. 2001;3:136–41. doi: 10.1186/ar290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boissier MC, Chiocchia G, Bessis N, Hajnal J, Garotta G, et al. Biphasic effect of interferon-gamma in murine collagen-induced arthritis. Eur J Immunol. 1995;25:1184–90. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- 28.Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, et al. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–8. doi: 10.1111/j.1365-2249.2006.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boumba D, Skopouli FN, Moutsopoulos HM. Cytokine mrna expression in the labial salivary gland tissues from patients with primary sjogren’s syndrome. British journal of rheumatology. 1995;34:326–33. doi: 10.1093/rheumatology/34.4.326. [DOI] [PubMed] [Google Scholar]
- 30.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mrna expression in salivary gland biopsies of sjogren’s syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]
- 31.van Woerkom JM, Kruize AA, Wenting-van Wijk MJ, Knol E, Bihari IC, et al. Salivary gland and peripheral blood t helper 1 and 2 cell activity in sjogren’s syndrome compared with non-sjogren’s sicca syndrome. Ann Rheum Dis. 2005;64:1474–9. doi: 10.1136/ard.2004.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang EH, Lee YJ, Hyon JY, Yun PY, Song YW. Salivary cytokine profiles in primary sjogren’s syndrome differ from those in non-sjogren sicca in terms of tnf-alpha levels and th-1/th-2 ratios. Clinical and experimental rheumatology. 2011;29:970–6. [PubMed] [Google Scholar]
- 33.Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, et al. The th1/th2 cytokine balance changes with the progress of the immunopathological lesion of sjogren’s syndrome. Clin Exp Immunol. 2002;128:562–8. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland par-c10 cell line. American journal of physiology Cell physiology. 2008;295:C1191–201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin d1 prevents tnf-alpha-mediated disruption of salivary epithelial formation. American journal of physiology Cell physiology. 2012;302:C1331–45. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced t cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (cxcl10) and monokine induced by interferon-gamma (cxcl9), in the salivary gland lesions of patients with sjogren’s syndrome. Arthritis Rheum. 2002;46:2730–41. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 37.Yoon KC, Park CS, You IC, Choi HJ, Lee KH, et al. Expression of cxcl9, −10, −11, and cxcr3 in the tear film and ocular surface of patients with dry eye syndrome. Investigative ophthalmology & visual science. 2010;51:643–50. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasegawa H, Inoue A, Kohno M, Muraoka M, Miyazaki T, et al. Antagonist of interferon-inducible protein 10/cxcl10 ameliorates the progression of autoimmune sialadenitis in mrl/lpr mice. Arthritis Rheum. 2006;54:1174–83. doi: 10.1002/art.21745. [DOI] [PubMed] [Google Scholar]
- 39.Vignali DA, Kuchroo VK. Il-12 family cytokines: Immunological playmakers. Nat Immunol. 2012;13:722–8. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Garra A, Murphy KM. From il-10 to il-12: How pathogens and their products stimulate apcs to induce t(h)1 development. Nat Immunol. 2009;10:929–32. doi: 10.1038/ni0909-929. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J, Zhao J, Perlman S. Differential effects of il-12 on tregs and non-treg t cells: Roles of ifn-gamma, il-2 and il-2r. PLoS One. 2012;7:e46241. doi: 10.1371/journal.pone.0046241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boniface K, Blom B, Liu YJ, de Waal Malefyt R. From interleukin-23 to t-helper 17 cells: Human t-helper cell differentiation revisited. Immunol Rev. 2008;226:132–46. doi: 10.1111/j.1600-065X.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li R, Zheng X, Popov I, Zhang X, Wang H, et al. Gene silencing of il-12 in dendritic cells inhibits autoimmune arthritis. Journal of translational medicine. 2012;10:19. doi: 10.1186/1479-5876-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trembleau S, Penna G, Gregori S, Giarratana N, Adorini L. Il-12 administration accelerates autoimmune diabetes in both wild-type and ifn-gamma-deficient nonobese diabetic mice, revealing pathogenic and protective effects of il-12-induced ifn-gamma. J Immunol. 2003;170:5491–501. doi: 10.4049/jimmunol.170.11.5491. [DOI] [PubMed] [Google Scholar]
- 45.Manoussakis MN, Boiu S, Korkolopoulou P, Kapsogeorgou EK, Kavantzas N, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of sjogren’s syndrome: Correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–88. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- 46.Szodoray P, Alex P, Brun JG, Centola M, Jonsson R. Circulating cytokines in primary sjogren’s syndrome determined by a multiplex cytokine array system. Scand J Immunol. 2004;59:592–9. doi: 10.1111/j.0300-9475.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 47.Yanagi K, Haneji N, Hamano H, Takahashi M, Higashiyama H, et al. In vivo role of il-10 and il-12 during development of sjogren’s syndrome in mrl/lpr mice. Cellular immunology. 1996;168:243–50. doi: 10.1006/cimm.1996.0072. [DOI] [PubMed] [Google Scholar]
- 48.Gao J, Killedar S, Cornelius JG, Nguyen C, Cha S, et al. Sjogren’s syndrome in the nod mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. 2006;26:90–103. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Vosters JL, Landek-Salgado MA, Yin H, Swaim WD, Kimura H, et al. Interleukin-12 induces salivary gland dysfunction in transgenic mice, providing a new model of sjogren’s syndrome. Arthritis Rheum. 2009;60:3633–41. doi: 10.1002/art.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis BK, Wen H, Ting JP. The inflammasome nlrs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dinarello CA. Il-18: A th1-inducing, proinflammatory cytokine and new member of the il-1 family. The Journal of allergy and clinical immunology. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 52.Kawayama T, Okamoto M, Imaoka H, Kato S, Young HA, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443–9. doi: 10.1089/jir.2012.0029. [DOI] [PubMed] [Google Scholar]
- 53.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both th1 and th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 54.Volin MV, Koch AE. Interleukin-18: A mediator of inflammation and angiogenesis in rheumatoid arthritis. J Interferon Cytokine Res. 2011;31:745–51. doi: 10.1089/jir.2011.0050. [DOI] [PubMed] [Google Scholar]
- 55.Oikawa Y, Shimada A, Kasuga A, Morimoto J, Osaki T, et al. Systemic administration of il-18 promotes diabetes development in young nonobese diabetic mice. J Immunol. 2003;171:5865–75. doi: 10.4049/jimmunol.171.11.5865. [DOI] [PubMed] [Google Scholar]
- 56.Bulosan M, Pauley KM, Yo K, Chan EK, Katz J, et al. Inflammatory caspases are critical for enhanced cell death in the target tissue of sjogren’s syndrome before disease onset. Immunol Cell Biol. 2009;87:81–90. doi: 10.1038/icb.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becker H, Pavenstaedt H, Willeke P. Emerging treatment strategies and potential therapeutic targets in primary sjogren’s syndrome. Inflamm Allergy Drug Targets. 2010;9:10–9. doi: 10.2174/187152810791292935. [DOI] [PubMed] [Google Scholar]
- 58.Bikker A, van Woerkom JM, Kruize AA, Wenting-van Wijk M, de Jager W, et al. Increased expression of interleukin-7 in labial salivary glands of patients with primary sjogren’s syndrome correlates with increased inflammation. Arthritis Rheum. 2010;62:969–77. doi: 10.1002/art.27318. [DOI] [PubMed] [Google Scholar]
- 59.Dorner T, Hucko M, Mayet WJ, Trefzer U, Burmester GR, et al. Enhanced membrane expression of the 52 kda ro(ss-a) and la(ss-b) antigens by human keratinocytes induced by tnf alpha. Ann Rheum Dis. 1995;54:904–9. doi: 10.1136/ard.54.11.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pauley KM, Gauna AE, Grichtchenko II, Chan EK, Cha S. A secretagogue-small interfering rna conjugate confers resistance to cytotoxicity in a cell model of sjogren’s syndrome. Arthritis Rheum. 2011;63:3116–25. doi: 10.1002/art.30450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamachi M, Kawakami A, Yamasaki S, Hida A, Nakashima T, et al. Regulation of apoptotic cell death by cytokines in a human salivary gland cell line: Distinct and synergistic mechanisms in apoptosis induced by tumor necrosis factor alpha and interferon gamma. The Journal of laboratory and clinical medicine. 2002;139:13–9. doi: 10.1067/mlc.2002.120648. [DOI] [PubMed] [Google Scholar]
- 62.Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, et al. Inefficacy of infliximab in primary sjogren’s syndrome: Results of the randomized, controlled trial of remicade in primary sjogren’s syndrome (tripss) Arthritis Rheum. 2004;50:1270–6. doi: 10.1002/art.20146. [DOI] [PubMed] [Google Scholar]
- 63.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The il-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 64.Paul WE, Zhu J. How are t(h)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, et al. Inhibition of th1 development mediated by gata-3 through an il-4-independent mechanism. Immunity. 1998;9:745–55. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 66.Murphy KM, Reiner SL. The lineage decisions of helper t cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 67.Rapoport MJ, Jaramillo A, Zipris D, Lazarus AH, Serreze DV, et al. Interleukin 4 reverses t cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Roon JA, van Roy JL, Gmelig-Meyling FH, Lafeber FP, Bijlsma JW. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996;39:829–35. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]
- 69.Nakajima A, Hirose S, Yagita H, Okumura K. Roles of il-4 and il-12 in the development of lupus in nzb/w f1 mice. J Immunol. 1997;158:1466–72. [PubMed] [Google Scholar]
- 70.Ohyama Y, Nakamura S, Matsuzaki G, Shinohara M, Hiroki A, et al. Cytokine messenger rna expression in the labial salivary glands of patients with sjogren’s syndrome. Arthritis Rheum. 1996;39:1376–84. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- 71.Wynn TA. Il-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 72.McKenzie AN, Culpepper JA, de Waal Malefyt R, Briere F, Punnonen J, et al. Interleukin 13, a t-cell-derived cytokine that regulates human monocyte and b-cell function. Proc Natl Acad Sci U S A. 1993;90:3735–9. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (il)-4-independent induction of immunoglobulin (ig)e, and perturbation of t cell development in transgenic mice expressing il-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spadaro A, Rinaldi T, Riccieri V, Taccari E, Valesini G. Interleukin-13 in autoimmune rheumatic diseases: Relationship with the autoantibody profile. Clinical and experimental rheumatology. 2002;20:213–6. [PubMed] [Google Scholar]
- 75.Xu Z, Chen Y. Determination of serum interleukin-13 and nerve growth factor in patients with systemic lupus erythematosus and clinical significance. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2005;25:360–1. doi: 10.1007/BF02828168. [DOI] [PubMed] [Google Scholar]
- 76.Villarreal GM, Alcocer-Varela J, Llorente L. Differential interleukin (il)-10 and il-13 gene expression in vivo in salivary glands and peripheral blood mononuclear cells from patients with primary sjogren’s syndrome. Immunology letters. 1996;49:105–9. doi: 10.1016/0165-2478(95)02490-5. [DOI] [PubMed] [Google Scholar]
- 77.Mahlios J, Zhuang Y. Contribution of il-13 to early exocrinopathy in id3−/− mice. Molecular immunology. 2011;49:227–33. doi: 10.1016/j.molimm.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ouyang W, Kolls JK, Zheng Y. The biological functions of t helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Awasthi A, Kuchroo VK. Th17 cells: From precursors to players in inflammation and infection. International immunology. 2009;21:489–98. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory th17 cells. Ann N Y Acad Sci. 2008;1143:188–211. doi: 10.1196/annals.1443.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Z, O’Shea JJ. Th17 cells: A new fate for differentiating helper t cells. Immunologic research. 2008;41:87–102. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- 82.Geremia A, Jewell DP. The il-23/il-17 pathway in inflammatory bowel disease. Expert review of gastroenterology & hepatology. 2012;6:223–37. doi: 10.1586/egh.11.107. [DOI] [PubMed] [Google Scholar]
- 83.Nakajima K. Critical role of the interleukin-23/t-helper 17 cell axis in the pathogenesis of psoriasis. The Journal of dermatology. 2012;39:219–24. doi: 10.1111/j.1346-8138.2011.01458.x. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in sjogren’s syndrome: Findings in humans and mice. Arthritis Rheum. 2008;58:734–43. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of il-18 and th17 cells in salivary glands of patients with sjogren’s syndrome, and amplification of il-17-mediated secretion of inflammatory cytokines from salivary gland cells by il-18. J Immunol. 2008;181:2898–906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 86.Mieliauskaite D, Dumalakiene I, Rugiene R, Mackiewicz Z. Expression of il-17, il-23 and their receptors in minor salivary glands of patients with primary sjogren’s syndrome. Clinical & developmental immunology. 2012;2012:187258. doi: 10.1155/2012/187258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miletic M, Stojanovic R, Pajic O, Bugarski D, Mojsilovic S, et al. Serum interleukin-17 & nitric oxide levels in patients with primary sjogren’s syndrome. The Indian journal of medical research. 2012;135:513–9. [PMC free article] [PubMed] [Google Scholar]
- 88.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with sjogren’s syndrome immunopathogenesis. Am J Pathol. 2009;175:1167–77. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alunno A, Bistoni O, Bartoloni E, Caterbi S, Bigerna B, et al. Il-17-producing cd4-cd8- t cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary sjogren’s syndrome. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201511. [DOI] [PubMed] [Google Scholar]
- 90.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 91.Sonnenberg GF, Fouser LA, Artis D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by il-22. Nat Immunol. 2011;12:383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 92.Lavoie TN, Stewart CM, Berg KM, Li Y, Nguyen CQ. Expression of interleukin-22 in sjogren’s syndrome: Significant correlation with disease parameters. Scand J Immunol. 2011;74:377–82. doi: 10.1111/j.1365-3083.2011.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciccia F, Guggino G, Rizzo A, Ferrante A, Raimondo S, et al. Potential involvement of il-22 and il-22-producing cells in the inflamed salivary glands of patients with sjogren’s syndrome. Ann Rheum Dis. 2012;71:295–301. doi: 10.1136/ard.2011.154013. [DOI] [PubMed] [Google Scholar]
- 94.Spolski R, Leonard WJ. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 95.Spolski R, Leonard WJ. The yin and yang of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin Immunol. 2008;20:295–301. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of t follicular helper cells. J Exp Med. 2012;209:1241–53. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yi JS, Cox MA, Zajac AJ. Interleukin-21: A multifunctional regulator of immunity to infections. Microbes Infect. 2010;12:1111–9. doi: 10.1016/j.micinf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarra M, Franze E, Pallone F, Monteleone G. Targeting interleukin-21 in inflammatory diseases. Expert opinion on therapeutic targets. 2011;15:695–702. doi: 10.1517/14728222.2011.561319. [DOI] [PubMed] [Google Scholar]
- 99.Monteleone G, Sarra M, Pallone F. Interleukin-21 in t cell-mediated diseases. Discovery medicine. 2009;8:113–7. [PubMed] [Google Scholar]
- 100.Spolski R, Leonard WJ. Il-21 is an immune activator that also mediates suppression via il-10. Crit Rev Immunol. 2010;30:559–70. doi: 10.1615/critrevimmunol.v30.i6.50. [DOI] [PubMed] [Google Scholar]
- 101.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res. 2010;30:381–8. doi: 10.1089/jir.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kang KY, Kim HO, Kwok SK, Ju JH, Park KS, et al. Impact of interleukin-21 in the pathogenesis of primary sjogren’s syndrome: Increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res Ther. 2011;13:R179. doi: 10.1186/ar3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, et al. A subset of interleukin-21+ chemokine receptor ccr9+ t helper cells target accessory organs of the digestive system in autoimmunity. Immunity. 2011;34:602–15. doi: 10.1016/j.immuni.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 104.Liu H, Liu G, Gong L, Zhang Y, Jiang G. Local suppression of il-21 in submandibular glands retards the development of sjogren’s syndrome in non-obese diabetic mice. J Oral Pathol Med. 2012;41:728–35. doi: 10.1111/j.1600-0714.2012.01175.x. [DOI] [PubMed] [Google Scholar]
- 105.O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of il-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–31. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 106.Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory t cells (tr1) in autoimmunity. Semin Immunol. 2011;23:202–8. doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 108.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. Il-10 directly suppresses cd4 but not cd8 t cell effector and memory responses following acute viral infection. Proc Natl Acad Sci U S A. 2010;107:3018–23. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 110.Roers A, Siewe L, Strittmatter E, Deckert M, Schluter D, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced t cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004;200:1289–97. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beebe AM, Cua DJ, de Waal Malefyt R. The role of interleukin-10 in autoimmune disease: Systemic lupus erythematosus (sle) and multiple sclerosis (ms) Cytokine & growth factor reviews. 2002;13:403–12. doi: 10.1016/s1359-6101(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 112.Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center b cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–8. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bertorello R, Cordone MP, Contini P, Rossi P, Indiveri F, et al. Increased levels of interleukin-10 in saliva of sjogren’s syndrome patients. Correlation with disease activity. Clinical and experimental medicine. 2004;4:148–51. doi: 10.1007/s10238-004-0049-9. [DOI] [PubMed] [Google Scholar]
- 114.Perrier S, Serre AF, Dubost JJ, Beaujon G, Plazonnet MP, et al. Increased serum levels of interleukin 10 in sjogren’s syndrome; correlation with increased igg1. The Journal of rheumatology. 2000;27:935–9. [PubMed] [Google Scholar]
- 115.Saito I, Haruta K, Shimuta M, Inoue H, Sakurai H, et al. Fas ligand-mediated exocrinopathy resembling sjogren’s syndrome in mice transgenic for il-10. J Immunol. 1999;162:2488–94. [PubMed] [Google Scholar]
- 116.Rajagopalan G, Kudva YC, Sen MM, Marietta EV, Murali N, et al. Il-10-deficiency unmasks unique immune system defects and reveals differential regulation of organ-specific autoimmunity in non-obese diabetic mice. Cytokine. 2006;34:85–95. doi: 10.1016/j.cyto.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 117.Balasa B, La Cava A, Van Gunst K, Mocnik L, Balakrishna D, et al. A mechanism for il-10-mediated diabetes in the nonobese diabetic (nod) mouse: Icam-1 deficiency blocks accelerated diabetes. J Immunol. 2000;165:7330–7. doi: 10.4049/jimmunol.165.12.7330. [DOI] [PubMed] [Google Scholar]
- 118.Sharif MN, Tassiulas I, Hu Y, Mecklenbrauker I, Tarakhovsky A, et al. Ifn-alpha priming results in a gain of proinflammatory function by il-10: Implications for systemic lupus erythematosus pathogenesis. J Immunol. 2004;172:6476–81. doi: 10.4049/jimmunol.172.10.6476. [DOI] [PubMed] [Google Scholar]
- 119.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, et al. Induction of il-17+ t cell trafficking and development by ifn-gamma: Mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–41. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]


