Abstract
Introduction
Critical illnesses continue to be major causes of morbidity and mortality worldwide. Recent investigations show that stem cells may be beneficial as prognostic biomarkers and novel therapeutic strategies in these syndromes. This article reviews the use of stem cells in sepsis and acute lung injury as prognostic biomarkers and also as a potential for exogenous cell-based therapy.
Methods
A directed search of the medical literature was done using PubMed and OVID to evaluate topics related to pathophysiology of sepsis and acute lung injury, in addition to the characterization and utilization of stem cells in these diseases.
Conclusions
Stem cells have shown significant promise in the field of critical care medicine both for prognostication and treatment strategies. Although recent studies have been done to describe the mechanistic pathways of stem cells in critical illness, further investigation is necessary to fully delineate the mechanisms behind a stem cell’s immunomodulatory characteristics and its ability to mobilize and engraft in tissues.
Key Indexing Terms: Stem cells, Sepsis, ALI, Biomarkers, Cell-based therapy
Every year, critical illnesses affect millions of people worldwide and result in significant morbidity and mortality. Sepsis, the prototypical critical illness, is manifested clinically by a systemic inflammatory response to an infection and can progress with variable intensity to severe organ dysfunction and death.1 Acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), are the devastating causes of respiratory failure, which is one of the most common organ dysfunctions in sepsis. When characterized by disruption of the alveolar capillary membrane, ALI/ARDS can result in pulmonary edema and significant hypoxemia.2 Despite years of research and recent advances in therapeutic strategies for these 2 diseases,3–5 morbidity, mortality and healthcare expenditures remain high,6,7 whereas efforts to identify novel therapies and factors that predict survival have been unrevealing.
Complex pathophysiologic responses occur when a host responds to a systemic infection. Research has shown that sepsis8,9 and ALI/ARDS2,10,11 are characterized by proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, in addition to coexisting anti-inflammatory cytokines, which modulate the inflammatory response.12 Bronchoalveolar lavage studies in subjects with ALI/ARDS have even shown significant inflammation in radiographically spared regions of the lung.13 Understanding the pathogenesis of these syndromes has lead to aggressive attempts to identify the new pathogenetically important biomarkers and therapies. Both embryonic and adult tissue-derived stem cells have shown remarkable potential to repair and regenerate various organs, including the lungs.14 –16 Krause et al17 discovered in a landmark article that single bone marrow cells can self-renew in vivo and differentiate into hematopoietic progenitors and mature cell types, opening the door for cell-based therapy. In addition, stem cells are able to mitigate injury and inflammation through paracrine mechanisms14,18,19 and may even help prognosticate survival.20,21 Although there is still considerable controversy regarding a stem cell’s ability to engraft and repair injured tissue, these findings suggest that stem cells may offer novel approaches as prognostic biomarkers and cell-based therapies in critical illness. This review will focus on the utilization of adult tissue-derived stem cells in sepsis and ALI/ARDS.
EPIDEMIOLOGY AND PATHOPHYSIOLOGY OF SEPSIS AND ALI
Sepsis as a Prototypical Critical Illness
Sepsis is a significant public health problem, affecting more than 700,000 people every year in the United States. In the United States, sepsis is a leading cause of death in the intensive care unit (ICU), and it is the 10th leading cause of death overall.6,22,23 Sepsis is an acute inflammatory response to an infection, and the severity of the inflammatory response may cause organ dysfunction, which is a primary determinant of survival.1 The development of organ dysfunction, such as respiratory, hepatic or renal failure, is highly variable in patients with sepsis and cannot be predicted by clinical or physiologic variables.24–27
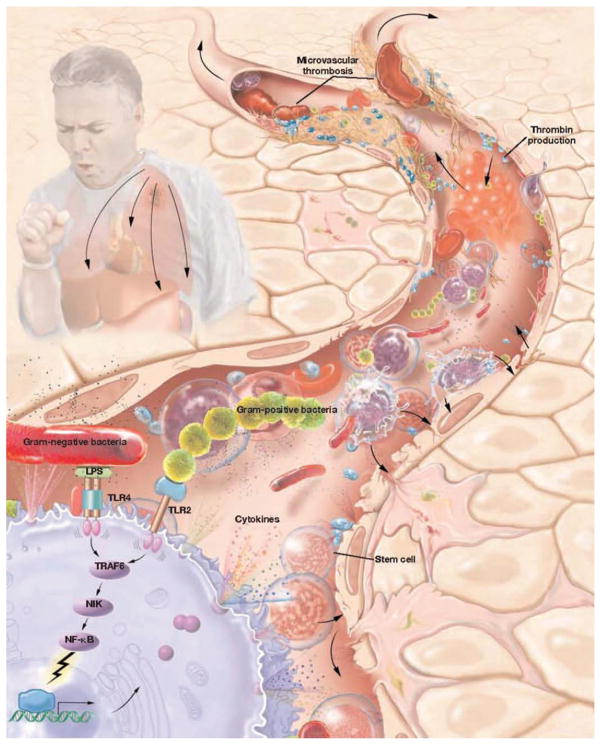
The pathophysiologic changes in the microvasculature significantly influence morbidity and mortality in sepsis (Figure 1).28 An infectious insult, classically described as bacterial endotoxin, initiates a pathophysiologic cascade involving circulatory disturbances, lactic acidosis and tissue necrosis, ultimately resulting in shock and death.29,30 Studies have shown that a number of cytokines are released by lipopolysaccharide (LPS)-activated inflammatory cells during the onset of an endotoxin response and are important for host immune defense and resolution of the inflammatory response as they interact with invading pathogens.31,32 Simultaneously, activation of anti-inflammatory pathways may lessen the inflammatory response.12 Low doses of endotoxin have been known to activate macrophages33 and release a variety of chemoattractant factors initiating signaling cascades. These systemic signals mobilize stem cells from the bone marrow, recruiting them to injured sites where they may differentiate into a variety of tissue-specific cell types,14,16,17,34 –36 and modify the immune response.37,38
FIGURE 1.
The pathophysiology of sepsis involves a complex set of interactions between inciting toxin, host immune response and inflammatory and coagulation pathways. LPS, lipopolysaccharide; TRAF6, TNF receptor-associated factor 6; NIK, nuclear factor κβ inducing kinase; NF-κβ, nuclear factor κβ. Adopted from Cribbs SK, Martin GS. Treating sepsis: an update on the latest therapies, part 1. Infect Med 2009;26:134–143.
ALI and ARDS
ALI and ARDS, severe forms of hypoxemic respiratory failure, are most frequently complications of sepsis.7 Patients with this disease often require extended mechanical ventilatory support and have a mortality of approximately 30% to 50%.7 Unfortunately, current treatment is largely supportive with lung protective ventilation and a conservative fluid strategy3,39 because there are no proven pharmacologic therapies to reduce the severity of lung injury or to improve the clinical outcomes.40– 42
Characterized by diffuse pulmonary infiltration, increased pulmonary capillary permeability and severe hypoxemia, ALI/ARDS can also result in damage of epithelial and endothelial surfaces, disruption of the alveolar-capillary barrier and flooding of alveolar spaces with fluid.2 In addition, endotoxemia itself can cause an acute systemic inflammatory response, which has been associated with lung edema, altered pulmonary function and deposition of inflammatory cells in the lungs.43 Experimental models have shown that pulmonary neutrophils increase activation of nuclear factor-κβ and produce increased amounts of proinflammatory cytokines.10,44 Pulmonary epithelial and endothelial cells of donor origin have been identified after hematopoietic stem cell and lung transplant in both murine and human studies,17,45,46 suggesting that stem cells once evoked from a cellular response have the unique ability to target the injured areas,16,32,45 to modulate the immune response and to repair the damaged tissue.47–50 These properties make bone marrow-derived stem cells attractive as cell-based therapy and as prognostic biomarkers for both sepsis and ALI/ARDS.
STEM CELL CLASSIFICATION
Stem cells have the capacity for extensive self-renewal with the potential to change into cells of multiple lineages.51 Individual stem cells self-generate and undergo continuous cell formation, leading to a succession of cells that have progressively less capacity for self-generation until ultimately a lineage-committed cell is formed. Until recently, the beneficial effects of stem cells were mostly attributed to their ability to incorporate into tissue (engraftment) and repair injured areas. Engraftment may still occur with some stem cells, but recent investigations propose that other mechanisms may be involved, including a stem cell’s ability to exert paracrine effects and generate the necessary signaling factors for tissue repair. These abilities may make stem cells uniquely suited to be prognostically and therapeutically important in critical illness.
A variety of stem cells have been isolated and characterized in the last several years. Although, currently, no uniform classification exists, stem cells have been broadly classified into adult-tissue derived and embryonic stem cells. Embryonic stem cells are derived from the inner cell mass of a developing blastocyst and are designated as pluripotent. Pluripotency gives these stem cells the ability to proliferate indefinitely without differentiation. However, this characteristic may present as a disadvantage for these cells have undergone unregulated growth leading to the formation of neoplasms.52–54 In contrast, adult tissue-derived stem cells, which will be discussed in this review, are also able to differentiate into a variety of adult tissues, but the fate of these cells seems to be somewhat restricted. Of these cells, mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) are perhaps the most widely studied.
Mesenchymal Stem Cells
MSCs have been isolated from multiple tissues,55,56 but the bone marrow is the best characterized source and much of the knowledge of MSCs is derived from studies involving bone marrow-derived MSCs (BMDMSCs). The small numbers present in the bone marrow necessitate expansion of these cells in vitro. Consensus minimal criteria to define human MSC include (1) selection for a plastic-adherent cell population in standard culture conditions; (2) expression of CD105, CD73 and CD90 and lack expression of CD45, CD34, CD14, CD11b, CD79α, CD19 or human leukocyte antigen-DR surface molecules and (3) ability to differentiate into adipocytes, osteocytes and chrondrocytes in vitro.57 In the lung, BMDMSCs are able to engraft as type I and type II alveolar epithelial cells, endothelial cells, fibroblasts and bronchial epithelial cells.14,19 MSCs have immunomodulatory characteristics that augment tissue repair. In animal models of lung injury, administration of these cells attenuates severity of the inflammatory response despite low levels of cell engraftment.14,16 In addition, MSCs seem to not be immunogenic having an innate ability to avoid detection by a recipient’s immune system.38 These cells are able to inhibit lymphocyte proliferation,47,58 expressing only intermediate levels of major histocompatibility complex (MHC) class I but not MHC class II,59 intimating that undifferentiated and differentiated MSCs may be suitable for transplantation even between MHC incompatible individuals (Figure 2).
FIGURE 2.
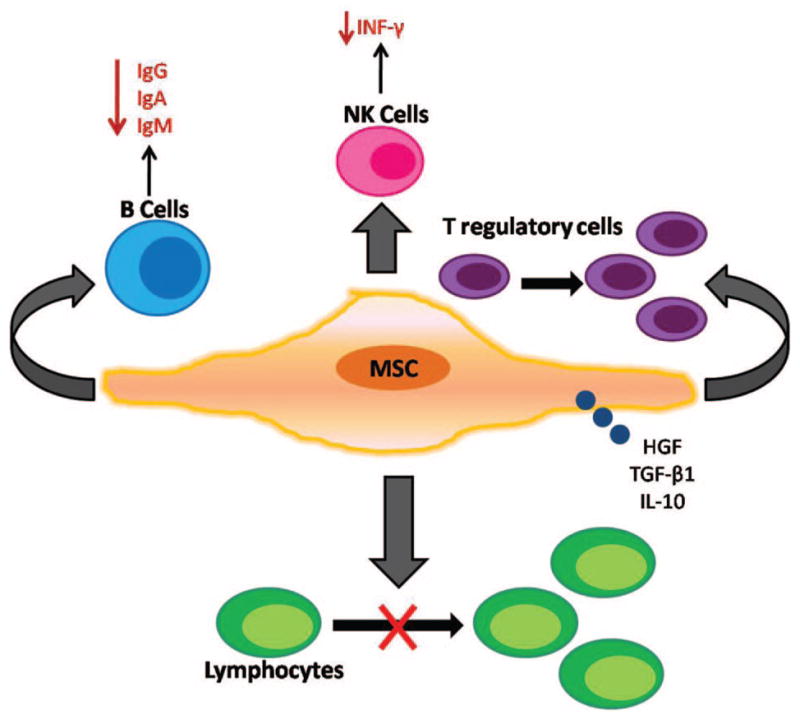
Bone marrow-derived mesenchymal stem cells (BMDMSCs) organize a complex array of mechanisms that regulate T-cell proliferation including secretion of soluble effector molecules and growth factors [hepatocyte growth factor (HGF), transforming growth factor (TGF)-β1 and interleukin (IL)-10] which inhibit lymphocyte proliferation. At high concentrations, BMDMSCs inhibit B-cell proliferation, immunoglobulin secretion and interferon (INF)-γ production in stimulated natural killer (NK) cells. In addition, MSCs increase proliferation of T-regulatory cells by increasing TGF-β1 production.
Endothelial Progenitor Cells
In addition to MSCs, EPCs have also been studied in sepsis and ALI/ARDS. These cells were first discovered in 1997 when Asahara et al60 purified a population of cells that displayed properties of both endothelial cells and progenitor cells. They hypothesized that the peripheral blood contained cells capable of trafficking toward ischemic sites and differentiating into mature ECs, which they termed “endothelial progenitor cells.”60 Progenitor cells are different than stem cells in that they are farther along the pathway of differentiation compared with true stem cells, committed to forming 1 specific cell type. Since their discovery, EPCs have been extensively studied as biomarkers of cardiovascular disease and as cell-based therapy for the repair of damaged vasculature.61,62 However, similar to MSCs, EPC investigators continue to speculate on an alternate mechanism besides engraftment, given the lack of evidence of long-term engraftment into newly formed vessels.63,64
In contrast to MSCs, the phenotypic characterization of EPCs is controversial for there is no unique marker in animals or humans to define an EPC. The peripheral circulation often contains other circulating endothelial cells (CECs), which confound accurate identification of EPCs. Several culture methods have been described,60,62,65,66 but, typically, the initial population in a cell culture is heterogeneous, making it difficult to determine the precursor cell that gives rise to EPCs. There is an alternative method known as fluorescence-activated cell sorting, which can potentially identify more homogenous EPCs using specific cell surface antigens.67 Human circulating EPCs have typically been identified as cells that express CD34, CD133 or vascular endothelial growth factor receptor (VEGFR)-2.60,68 However, these cell surface antigens are also expressed on human hematopoietic stem cells,69 making it difficult to differentiate between EPCs, CECs and other hematopoietic cells.
STEM CELLS AS PROGNOSTIC BIOMARKERS
The last several years have seen a significant increase in the amount of preclinical and clinical data regarding the predictive potential of stem cells in critical illness.
Prognostic Implications of Stem Cells in Sepsis
A variety of stem cells have been investigated for their prognostic capabilities in sepsis (Table 1). In 2001, Mutunga et al70 found a significant increase in CECs in a small group of patients with sepsis and septic shock compared with ICU controls or healthy people. CECs were identified by indirect immunofluorescence, using antibodies to von Willebrand factor and VEGFR KDR. Increasing numbers of CECs were found even in patients with sepsis without shock, suggesting that endothelial damage preceded the development of organ failure.70 In addition, the number of CECs was higher in patients who died of septic shock compared with survivors, supporting the view that the magnitude of increase in circulating cell numbers in sepsis maybe related to vascular injury severity.70 This study provided direct evidence for endothelial dysfunction in humans during septic shock. A few years later, Tsaganos et al71 studied 44 patients with ventilator-associated pneumonia and sepsis, a more homogenous population than previous studies. They observed that time of survival was decreased for patients with ≥310/μL of CD34/CD45 cells on day 1 compared with those patients with <310/μL of cells, a similar result to Mutunga et al70 in that survival was associated with fewer number of circulating CD34/CD45 progenitor cells.
TABLE 1.
Stem cells as prognostic biomarkers in patients with sepsis and ALI/ARDS
| Author | Cell type | Disease | Clinical outcome | P |
|---|---|---|---|---|
| Mutunga et al70 | Endothelial cells | Sepsis | Positive correlation with mortality | 0.026 |
| Burnham et al20 | Endothelial progenitor cells (colony-forming unit) | ALI/ARDS | Positive correlation with survival | <0.04 |
| Tsaganos et al71 | CD34/CD45 cells | Ventilator-associated pneumonia and sepsis | Positive correlation with mortality | 0.022 |
| Rafat et al21 | Endothelial progenitor cells (FACS) | Sepsis | Positive correlation with survival | 0.0001 |
| Burnham et al72 | Endothelial progenitor cells (colony-forming unit) | Sepsis and ALI/ARDS | Positive correlation with survival | 0.06 |
| Cribbs et al (unpublished) | Endothelial progenitor cells (colony-forming unit) | Sepsis | Positive correlation with improved organ dysfunction | <0.05 |
ALI/ARDS, acute lung injury/acute respiratory distress syndrome; FACS, fluorescence-activated cell sorting.
Studies evaluating EPCs specifically in sepsis have only emerged in the last few years. Rafat et al21 measured levels of circulating EPCs in a cohort of 32 patients within 48 hours after sepsis onset. EPCs were identified by fluorescence-activated cell sorting using antibodies against CD34, CD133 and VEGFR-2. They found that the number of circulating EPCs was significantly higher in patients with sepsis versus nonseptic ICU patients and healthy controls,21 comparable with the previous studies. This number remained increased even 5 days after the initial sampling, and high numbers of circulating EPCs correlated with high serum levels of VEGF, granulocyte monocyte colony-stimulating factor and erythropoietin.21 However, in contrast to previous studies, Rafat et al21 found that sepsis survivors had significantly greater number of EPCs than non-survivors, suggesting that not only vascular damage induces release of stem cells in circulation but also circulating stem cells may predict clinical outcome in critically ill patients. Similarly, Burnham et al recently studied EPC in a cohort of patients with sepsis and ALI. EPC was assessed by a colony-forming unit (CFU) cell culture assay in which peripheral blood mononuclear cells were isolated using Ficoll density gradient centrifugation and cultured in EPC media. Analysis revealed that those with CFU count ≥48 per volume had an overall better survival compared with those with CFU count <48.72
Recently, our group at Emory University studied EPCs in a cohort of patients with sepsis as a biomarker for organ dysfunction, a major determinant of mortality in sepsis (manuscript submitted). EPCs were assessed by a CFU cell culture assay in which peripheral blood mononuclear cells were isolated using Ficoll density gradient centrifugation and cultured in EPC media. In this study, EPC CFU numbers were significantly lower in patients with sepsis compared with ICU and healthy control subjects (P = 0.0005). Furthermore, total Sepsis-Related Organ Failure Assessment score decreased as EPC CFU counts increased for 65 survivors and 21 nonsurvivors (P = 0.04) and for 53 patients without shock and 33 patients with shock (P = 0.0025). Hence, the number of circulating EPCs inversely associated with the severity of organ dysfunction, irrespective of shock and mortality in sepsis. This study shows that the measurement of EPC CFUs is a promising novel prognostic biomarker for organ dysfunction in patients with sepsis and supports the hypothesis that mobilization of EPC CFU counts from the bone marrow may contribute to repair of the vascular endothelium and to injured organs.
Prognostic Implications of Stem Cells in ALI
Recent advancements with stem cells have also been made in the area of ALI/ARDS (Table 1). A few years ago, Burnham et al hypothesized that an increased number of circulating EPCs in the first 72 hours of ALI would be beneficial. They found that patients with ALI/ARDS had significantly greater EPC CFU counts compared with the healthy controls.20 In addition, patients with ALI with an EPC CFU count ≥35 had >28-day survival compared with those patients with an EPC CFU count <35, concluding that adequate mobilization of EPCs from bone marrow in ALI/ARDS could contribute to repair and recovery of damaged pulmonary endothelium,20 conferring a survival benefit. Furthermore, EPC counts were similarly increased in the 44% of subjects with septic shock.20
THERAPEUTIC POTENTIAL OF STEM CELLS
An assortment of clinical trials have used stem cells in diseases such as cardiovascular diseases,49,61 heart failure,73 pulmonary hypertension,74 graft-versus-host disease15 and cerebrovascular disease.75 However, until recently, MSCs and EPCs had not been extensively studied in critical illness.
Cell-Based Therapy in Sepsis
Many studies evaluating cell therapy in sepsis have been done regarding BMDMSCs (Table 2). Xu et al19 hypothesized that BMDMSCs would inhibit the acute inflammatory response from endotoxin, effectively protecting the lung from ALI. They found that BMDMSCs delivered intravenously to mice after systemic administration of endotoxin suppressed systemic inflammation.19 Lungs from animals receiving endotoxin but not BMDMSCs showed vascular congestion and increased cellularity, attributable mostly to neutrophils, whereas those receiving BMDMSCs plus endotoxin were devoid of these changes and resembled control animals.19 In addition, BMDMSCs moderated the increase in proinflammatory cytokines but did not alter serum concentrations of the anti-inflammatory cytokine IL-10.19
TABLE 2.
Preclinical therapeutic studies of stem cells in animal models of sepsis and ALI/ARDS
| Author | Cell type | Disease | Clinical outcome with stem cells |
|---|---|---|---|
| Rojas et al16 | Bone marrow-derived MSC | Bleomycin-induced ALI | Histological decrease in lung injury Increase in GM-CSF and G-CSF Decrease in proinflammatory cytokines |
| Gupta et al14 | Bone marrow-derived MSC | Endotoxin-induced ALI | Histologic decrease in lung injury Increase survival Decrease in excess lung water and BAL protein Decrease in proinflammatory cytokines Increase in anti-inflammatory cytokine |
| Xu et al19 | Bone marrow-derived MSC | Endotoxin-induced ALI | Histologic decrease in lung injury Decrease in excess lung water Decrease in proinflammatory cytokines Increase in anti-inflammatory cytokines |
| Kahler et al45 | Bone marrow-derived EPC (cultured in vitro) | ALI | Integration of EPC into tissue of injured lung |
| Ortiz et al85 | Bone marrow-derived MSC and MSC-conditioned medium | Bleomycin-induced ALI | Decrease in proinflammatory cytokines Decrease in BAL protein |
| Zhao et al86 | Bone marrow-derived MSC | Bleomycin-induced ALI | Histologic decrease in lung injury Decrease in laminin and hyaluronan in BAL Decrease in proinflammatory cytokines |
| Lam et al81 | Peripheral blood EPC (cultured in vitro) | ALI | Histologic decrease in lung injury Decrease in endothelial dysfunction in pulmonary artery Decrease in excess lung water |
| Nemeth et al76 | Bone marrow-derived MSC | Sepsis | Improve survival and organ dysfunction Decrease in proinflammatory cytokines Increase in anti-inflammatory cytokines |
| Gonzalez-Rey et al78 | Adipose-derived MSC | Sepsis | Histologic and clinical decrease in colitis Improve survival and organ dysfunction Decrease in proinflammatory cytokines Increase in anti-inflammatory cytokines |
| Lee et al83 | Human allogeneic MSC and human MSC-conditioned medium | ALI | Histologic decrease in lung injury Decrease in excess lung water Restored alveolar fluid clearance |
| Mei et al79 | Bone marrow-derived MSC | Sepsis | Histologic decrease in lung injury Decrease in BAL protein Improve survival in setting of antibiotics Improved organ dysfunction Decrease in proinflammatory cytokines |
| Gene plus cell therapy | |||
| Mei et al84 | Syngeneic MSC with and without ANGPT1 gene | Endotoxin-induced ALI | Histologic decrease in lung injury Decrease in inflammatory cells in BAL Near complete reversal of increased lung permeability with gene transfection |
| Xu et al87 | Bone marrow-derived MSC with and without Ang1 gene | Endotoxin-induced ALI | Further histologic decrease in lung injury with gene transfection vs. MSC alone Further decrease excess lung water and BAL protein vs. MSC alone Further decrease in proinflammatory cytokines |
GM-CSF, granulocyte monocyte colony-stimulating factor; MSC, mesenchymal stem cell; ALI, acute lung injury; EPC, endothelial progenitor cell; BAL, bronchoalveolar lavage.
More recently, MSCs have been evaluated in other murine models of sepsis, including cecal ligation and puncture (CLP). Mice given MSCs at the time of surgery for CLP lived longer than those untreated.76 In particular, the beneficial effect was seen when cells were injected 24 hours before or 1 hour after CLP. Examining organ dysfunction, this group found that kidney function (measured by serum creatinine and renal tubular injury scores) and liver dysfunction (measured by glycogen storage and transaminases) were markedly improved in the treated mice.76 Similar to others, the authors also found that TNF-α and IL-6 concentrations were reduced in treated mice, whereas anti-inflammatory cytokine IL-10 levels started to increase 3 hours postcell infusion. In addition, treatment of MSCs significantly reduced peritoneal, renal and liver vascular permeability seen with CLP surgery. The authors also examined whether MSCs might reprogram other immune cells to help mediate this response. They discovered that although lymphocyte populations of T, B and natural killer (NK) cells did not mediate the effect of MSCs on CLP, MSCs were no longer effective in mice lacking monocytes, macrophages or IL-10 suggesting that injected MSCs may interact with circulating immune cells to reprogram the host immune response.76 Their experiments show that the beneficial effect could be due to increased release of prostaglandin E2 from MSCs acting on the EP2 and EP4 receptors of the macrophages, stimulating production and release of IL-10,76 an effect seen in previous studies.77 Similarly, Gonzalez-Rey et al78 discovered that infusion of human adipose-derived MSC ameliorated severity of dextran sulfate sodium-induced colitis, increasing survival in mice. These MSC decreased inflammatory cytokines and increased IL-10, protecting animals from severe sepsis.78 A recently published study treating peritoneal sepsis in mice also demonstrated that intravenous BMDMSCs reduced mortality in septic mice given antibiotics, even when compared with control mice given antibiotics.79 MSCs alone significantly reduced pulmonary and systemic cytokine levels in addition to enhancing bacterial clearance.79
Cell-Based Therapy in ALI
Various investigators have demonstrated the ability of stem cells to repair damaged lung epithelium and endothelium16,32,45,80 and have correlated these cells to clinical outcomes (Table 2). Kahler et al45 demonstrated that EPCs are integrated into the endothelial tissue of a transplanted lung suffering from ALI, whereas virtually no cells were found in the healthy lung, illustrating that EPCs home to areas of vascular injury and may be important in the repair process. Lam et al81 studied the effects of autologous EPCs transplantation on pulmonary endothelial regeneration in a rabbit model of ALI and showed that endothelial dysfunction in the pulmonary artery was significantly attenuated in rabbits treated with EPCs. These cells potentiated the relaxation response to acetylcholine in pulmonary arteries, reducing lung water content and hyaline membrane formation, proposing that the therapeutic benefits of EPCs in ALI were most likely derived from an effect on re-endothelialization of the damaged pulmonary artery wall and alveolar-capillary membrane. Most recently, Mao et al82 isolated EPCs from male donor bone marrow and expanded them in vitro before infusing them into female rats with LPS-induced ALI. Using Y-chromosome in situ hybridization and reverse transcription polymerase chain reaction to confirm engraftment, they found that rats receiving EPCs had reduced pulmonary edema, hemorrhage and hyaline membrane formation in addition to an increased survival rate (44% versus 81%, P = 0.03) compared with saline-treated controls.82 EPCs were not detected in lungs without LPS, again implying that injured lung cells produced chemoattractant factors that allow EPCs to mobilize to the injury site. Furthermore, anti-inflammatory cytokines were increased in EPC-treated rats.82 These findings suggest that EPC benefits arise not only from repair of damaged endothelium but also from improving the inflammatory milieu.
The immunologic tolerance of BMDMSCs, along with their anti-inflammatory characteristics, makes them potentially attractive for cell-based therapy in ALI/ARDS as well. Several animal studies have shown that exogenously administered BMDMSCs augment tissue repair. These effects seem to be mediated by soluble factors produced by BMDMSCs for levels of engraftment seem to be too low to account for their protective effects.14,19 Administration of BMDMSCs attenuated the acute inflammatory response to LPS and protected the lung from injury and pulmonary edema in several preclinical models of ALI,14,16,19,83–85 even conferring a survival benefit.14 Xu et al19 showed by histologic examination that infusion of BMDMSCs completely attenuated the neutrophil infiltration in the lung between 6 and 48 hours, the time frame when LPS is known to cause structural alterations in the lung. Further investigation showed that BMDMSCs migrated in response to LPS-treated lungs when placed in a coculture environment, again suggesting that chemoattractant factors from the injured lung instigated BMDMSCs chemotaxis.19 Gupta et al14 found that intrapulmonary delivery of MSCs improved survival and attenuated endotoxin-induced ALI in mice compared with control mice that received saline (80% versus 42%; P < 0.01). Mice given MSCs trended toward lower excess lung water at 24 hours with a significant difference at 48 hours (P < 0.01) versus those mice that received the fibroblast cell line or nonviable MSCs. In addition, there was significant histological improvement in the severity of lung injury after MSCs administration despite a level of engraftment of <5% at 48 hours after injury. The beneficial effects with MSCs seem to be mediated by a shift from a proinflammatory to an anti-inflammatory response to endotoxin, an effect also noted by Xu et al.19
This group has also tested the ability of human allogeneic MSCs to resolve pulmonary edema in an ex vivo perfused human lung preparation injured by endotoxin.88 After Escherichia coli endotoxin-induced ALI, treatment with either allogeneic human MSCs or human MSC-conditioned medium reduced pulmonary edema, improved lung endothelial barrier integrity and normalized alveolar epithelial fluid transport.88 In addition, histological examination showed a reduction in inflammatory cell infiltration and septal thickening. Previous animal studies have shown that alterations in proinflammatory and anti-inflammatory cytokines may account for some of these beneficial effects,14 but anti-inflammatory cytokines were not increased in this ex vivo human model. However, levels of monocytes and neutrophils were significantly lower compared with their previous animal model,14 leading the authors to believe that these low levels may represent an early anti-inflammatory effect or lung recovery.88 In addition, the authors also tested the ability of MSCs to secrete keratinocyte growth factor (KGF), which has shown to reduced ALI in small animal models of pulmonary edema.89,90 They found that MSCs pre-treated with KGF siRNA resulted in an 80% reduction in therapeutic effect, which was restored by the addition of recombinant KGF. This suggests that secreted KGF is an important paracrine soluble factor that mediates the effect of MSCs on alveolar fluid clearance.88
CONCLUSION
Considerable progress has been made in the last decade regarding management strategies in critical illnesses, specifically sepsis and ALI/ARDS. Fluid resuscitation in severe sepsis and septic shock has hit a new milestone,5 whereas the use of lung protective ventilation with tidal volume and plateau pressure limits has reduced mortality substantially in ALI/ARDS.3 However, despite these recent advances, more work needs to be done to (1) further improve morbidity, it is now apparent that survivors of ARDS suffer neuropsychiatric problems and muscular weakness, which can be permanent leading to long-term disability91,92 and (2) reduce the cost of healthcare, as our growing aging population continues to rise, so do the most common diseases requiring ICU care, severe sepsis and ALI.7,93 Stem cells have shown significant promise in this field, both as biomarkers to prognosticate organ dysfunction and mortality and as novel therapies to treat organ failures, including ALI. Whether these mechanisms are based on paracrine effects or direct tissue engraftment remain to be elucidated, but our better understanding of disease pathogenesis and stem-cell pathobiology suggests tremendous potential for growth in this area.
References
- 1.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 5.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 6.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 7.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 8.Abraham E, Wunderink R, Silverman H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–41. [PubMed] [Google Scholar]
- 9.Mira JP, Cariou A, Grall F, et al. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA. 1999;282:561–8. doi: 10.1001/jama.282.6.561. [DOI] [PubMed] [Google Scholar]
- 10.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 suppl):S195–9. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly SC, Haslett C, Reid PT, et al. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997;3:320–3. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 12.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 13.Pittet JF, Mackersie RC, Martin TR, et al. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997;155:1187–205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 15.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 16.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 20.Burnham EL, Taylor WR, Quyyumi AA, et al. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–60. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 21.Rafat N, Hanusch C, Brinkkoetter PT, et al. Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit Care Med. 2007;35:1677–84. doi: 10.1097/01.CCM.0000269034.86817.59. [DOI] [PubMed] [Google Scholar]
- 22.Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–21. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 23.Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–42. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 24.Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–74. [PubMed] [Google Scholar]
- 25.Finfer S, Bellomo R, Lipman J, et al. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med. 2004;30:589–96. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 26.Padkin A, Goldfrad C, Brady AR, et al. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med. 2003;31:2332–8. doi: 10.1097/01.CCM.0000085141.75513.2B. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 28.Cribbs SK, Martin GS. Treating sepsis: an update on the latest therapies, part 1. Infect Med. 2009;26:134–43. [Google Scholar]
- 29.Thomas L. The physiological disturbances produced by endotoxins. Annu Rev Physiol. 1954;16:467–90. doi: 10.1146/annurev.ph.16.030154.002343. [DOI] [PubMed] [Google Scholar]
- 30.Thomas L, Zweifach BW, Benacerraf B. Mechanisms in the production of tissue damage and shock by endotoxins. Trans Assoc Am Physicians. 1957;70:54–63. [PubMed] [Google Scholar]
- 31.Fujita M, Kuwano K, Kunitake R, et al. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int Arch Allergy Immunol. 1998;117:202–8. doi: 10.1159/000024011. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172:1266–72. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 33.Lentsch AB, Czermak BJ, Bless NM, et al. Essential role of alveolar macrophages in intrapulmonary activation of NF-kappaB. Am J Respir Cell Mol Biol. 1999;20:692–8. doi: 10.1165/ajrcmb.20.4.3414. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 38.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 39.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 40.Gerlach H, Keh D, Semmerow A, et al. Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med. 2003;167:1008–15. doi: 10.1164/rccm.2108121. [DOI] [PubMed] [Google Scholar]
- 41.Laterre PF, Wittebole X, Dhainaut JF. Anticoagulant therapy in acute lung injury. Crit Care Med. 2003;31(4 suppl):S329–36. doi: 10.1097/01.CCM.0000057912.71499.A5. [DOI] [PubMed] [Google Scholar]
- 42.Sprung CL, Caralis PV, Marcial EH, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137–43. doi: 10.1056/NEJM198411013111801. [DOI] [PubMed] [Google Scholar]
- 43.Rojas M, Woods CR, Mora AL, et al. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–41. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 44.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. [DOI] [PubMed] [Google Scholar]
- 45.Kahler CM, Wechselberger J, Hilbe W, et al. Peripheral infusion of rat bone marrow derived endothelial progenitor cells leads to homing in acute lung injury. Respir Res. 2007;8:50. doi: 10.1186/1465-9921-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suratt BT, Cool CD, Serls AE, et al. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:318–22. doi: 10.1164/rccm.200301-145OC. [DOI] [PubMed] [Google Scholar]
- 47.Rasmusson I, Uhlin M, Le Blanc K, et al. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887–93. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 48.Ringden O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–6. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 49.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 50.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–7. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCulloch EA, Till JE. Perspectives on the properties of stem cells. Nat Med. 2005;11:1026–8. doi: 10.1038/nm1005-1026. [DOI] [PubMed] [Google Scholar]
- 52.Almstrup K, Sonne SB, Hoei-Hansen CE, et al. From embryonic stem cells to testicular germ cell cancer—should we be concerned? Int J Androl. 2006;29:211–8. doi: 10.1111/j.1365-2605.2005.00643.x. [DOI] [PubMed] [Google Scholar]
- 53.Lensch MW, Schlaeger TM, Zon LI, et al. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell. 2007;1:253–8. doi: 10.1016/j.stem.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Pierce GB, Jr, Dixon FJ, Jr, Verney EL. Teratocarcinogenic and tissue-forming potentials of the cell types comprising neoplastic embryoid bodies. Lab Invest. 1960;9:583–602. [PubMed] [Google Scholar]
- 55.Dicker A, Le Blanc K, Astrom G, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–90. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 56.Krampera M, Sartoris S, Liotta F, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 57.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 58.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 59.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 60.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 61.Assmus B, Schachinger V, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 62.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 63.Badorff C, Dimmeler S. Neovascularization and cardiac repair by bone marrow-derived stem cells. Handb Exp Pharmacol. 2006:283–98. [PubMed] [Google Scholar]
- 64.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434–9. doi: 10.1161/01.res.87.6.434. [DOI] [PubMed] [Google Scholar]
- 66.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 67.Prater DN, Case J, Ingram DA, et al. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141–9. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 68.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 69.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–46. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mutunga M, Fulton B, Bullock R, et al. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med. 2001;163:195–200. doi: 10.1164/ajrccm.163.1.9912036. [DOI] [PubMed] [Google Scholar]
- 71.Tsaganos T, Giamarellos-Bourboulis EJ, Kollias S, et al. Kinetics of progenitor hematopoietic stem cells in sepsis: correlation with patients survival? BMC Infect Dis. 2006;6:142. doi: 10.1186/1471-2334-6-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burnham EL, Mealer M, Gaydos J, et al. Acute lung injury but not sepsis is associated with increased colony formation by peripheral blood mononuclear cells. Am J Respir Cell Mol Biol. 2010;43:326–33. doi: 10.1165/rcmb.2009-0015OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Assmus B, Fischer-Rasokat U, Honold J, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOP-CARE-CHD Registry. Circ Res. 2007;100:1234–41. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 74.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–71. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 75.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 76.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shinomiya S, Naraba H, Ueno A, et al. Regulation of TNFalpha and interleukin-10 production by prostaglandins I(2) and E(2): studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochem Pharmacol. 2001;61:1153–60. doi: 10.1016/s0006-2952(01)00586-x. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 79.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–57. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 80.Yamada M, Kubo H, Ishizawa K, et al. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005;60:410–3. doi: 10.1136/thx.2004.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lam CF, Liu YC, Hsu JK, et al. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology. 2008;108:392–401. doi: 10.1097/ALN.0b013e318164ca64. [DOI] [PubMed] [Google Scholar]
- 82.Mao M, Wang SN, Lv XJ, et al. Intravenous delivery of bone marrow-derived endothelial progenitor cells improves survival and attenuates lipopolysaccharide-induced lung injury in rats. Shock. 2010;34:196–204. doi: 10.1097/SHK.0b013e3181d49457. [DOI] [PubMed] [Google Scholar]
- 83.Lee SH, Jang AS, Kim YE, et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir Res. 2010;11:16. doi: 10.1186/1465-9921-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mei SH, McCarter SD, Deng Y, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao F, Zhang YF, Liu YG, et al. Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc. 2008;40:1700–5. doi: 10.1016/j.transproceed.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 87.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 88.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E.coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franco-Montoya ML, Bourbon JR, Durrmeyer X, et al. Pulmonary effects of keratinocyte growth factor in newborn rats exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L965–76. doi: 10.1152/ajplung.00136.2009. [DOI] [PubMed] [Google Scholar]
- 90.Yano T, Deterding RR, Simonet WS, et al. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol. 1996;15:433–42. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- 91.Davidson TA, Caldwell ES, Curtis JR, et al. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–60. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 92.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 93.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]