Abstract
Aims
The aim of this study was to assess whether pericardial fat, intrathoracic fat, and visceral abdominal adipose tissue (VAT) are associated with the prevalence of cardiovascular disease (CVD).
Methods and results
Participants from the Framingham Heart Study Offspring cohort underwent abdominal and chest multidetector computed tomography to quantify volumes of pericardial fat, intrathoracic fat, and VAT. Relations between each fat depot and CVD were assessed using logistic regression. The analysis of 1267 participants (mean age 60 years, 53.8% women, 9.7% with prevalent CVD) demonstrated that pericardial fat [odds ratio (OR) 1.32, 95% confidence interval (CI) 1.11–1.57; P = 0.002] and VAT (OR 1.35, 95% CI 1.11–1.57; P = 0.003), but not intrathoracic fat (OR 1.14, 95% CI 0.93–1.39; P = 0.22), were significantly associated with prevalent CVD in age–sex-adjusted models and after adjustment for body mass index and waist circumference. After multivariable adjustment, associations were attenuated (P > 0.14). Only pericardial fat was associated with prevalent myocardial infarction after adjusting for conventional measures of adiposity (OR 1.37, 95% CI 1.03–1.82; P = 0.03).
Conclusion
Pericardial fat and VAT, but not intrathoracic fat, are associated with CVD independent of traditional measures of obesity but not after further adjustment for traditional risk factor. Taken together with our prior work, these findings may support the hypothesis that pericardial fat contributes to coronary atherosclerosis.
Keywords: Pericardial fat, Visceral abdominal fat, Cardiovascular disease, Framingham Heart Study, Epidemiology
Introduction
Obesity currently affects nearly one-third of the population in the industrialized world.1,2 Traditionally, anthropometric measures such as body mass index (BMI) or waist circumference (WC) have been used to quantify overall adiposity. However, regional fat depots may be of greater importance than overall adiposity.3–8 Several studies have highlighted pericardial fat and abdominal visceral adipose tissue (VAT) as unique, pathogenic fat depots.9–17 Abdominal VAT is the largest visceral fat depot in the human body with more than 10 times the volume of pericardial fat.18 It is significantly correlated with cardiovascular disease (CVD) risk factors, the metabolic syndrome,19,20 and systemic markers of inflammation.21 Therefore, VAT is hypothesized to have a systemic effect on atherosclerosis.
In contrast, pericardial fat is a smaller fat depot, but surrounds the coronary arteries and the myocardium and therefore may have a local (paracrine) effect on the development of coronary artery disease. Recently, we showed that pericardial fat is associated with metabolic risk factors.18 Furthermore, we demonstrated that pericardial fat is correlated with the presence of coronary artery calcification.18 Additional studies have identified an association between pericardial fat and the severity of coronary artery disease.22,23 Taken together, these findings support the hypothesis that the magnitude of perivascular fat tissue may be a determinant of the extent of atherosclerosis in the coronary arteries.
In contrast, the impact of intrathoracic fat on total CVD, because of its small volume and lack of close proximity to the coronary arteries, may be limited, despite its correlation with CVD risk factors. Therefore, the aim of this study was to assess the association of pericardial, intrathoracic, and visceral abdominal fat with the prevalence of CVD in the Offspring population of the Framingham Heart Multi-detector Computed Tomography (MDCT) Study. We hypothesized that pericardial fat, because it may have a local effect on the coronary arteries and abdominal VAT and a systemic effect on atherosclerosis as the largest visceral fat depot, are associated with total CVD. Further, we hypothesized that intrathoracic fat, which is a small fat depot not in local anatomic contact with the coronary arteries, would be less likely to be associated with CVD.
Methods
Study sample
Participants of this study were drawn from the Framingham Heart MDCT Study sample. Offspring participants underwent MDCT imaging between June 2002 and April 2005. Exclusion criteria were pregnancy, age <40 years for women and age <35 years for men, and weight >320 pounds. Study design has been described previously.24,25
Overall, 1422 subjects underwent CT scanning of the chest and abdomen between June 2002 and April 2005. Of these, 1342 had non-missing or interpretable pericardial fat, intrathoracic fat, and VAT measures, and 1320 of those attended the seventh examination cycle. Of the remaining 1320, 47 subjects were excluded due to a history of prior open heart surgery, and an additional six subjects were excluded due to a missing covariate profile, resulting in a total sample size of 1267 participants.
The study was approved by the Institutional Review Boards of the Boston University Medical Center and the Massachusetts General Hospital. All subjects provided written informed consent.
Multi-detector computed tomography imaging protocol
Participants underwent radiographic assessment of their chest and abdomen in the supine position within one procedure using an eight-slice MDCT scanner (LightSpeed Ultra, General Electric, Milwaukee, WI, USA). The thoracic scan was performed during inspiratory breath hold, with an average scan length of 18 s (tube voltage 120 kVp, tube current 320 mA <220 lbs and 400 mA >220 lbs, gantry rotation time 500 ms, temporal resolution 330 ms). Image acquisition was prospectively triggered with the centre of the acquisition at 70% of the R-R-interval. Images were reconstructed with a slice thickness of 2.5 mm without overlap and a field of view of 25 cm. On average, 48 contiguous slices were taken for volume coverage from the carina to the diaphragm. For the abdominal scan, 25 contiguous slices were reconstructed with a slice thickness of 5 mm without overlap, starting 150 mm above the upper edge of S1, and a field of view of 35 cm (tube voltage: 120 kVp, tube current 320 mA <220 lbs and 400 mA >220 lbs, gantry rotation time 500 ms, pitch 1.33).
Fat tissue measurements
Pericardial fat, total thoracic fat, and VAT volumes were assessed using a dedicated workstation (Aquarius 3D Workstation, TeraRecon, San Matteo, CA, USA). Fat volumes were measured by a semi-automatic segmentation technique. The reader was required to manually trace a region of interest. Within the region of interest, fat was defined as pixels within a window of −195 to −45 Houndsfield units (HU) and a window centre of −120 HU. Pericardial fat volume was defined as any adipose tissue located within the pericardial sac. Total thoracic fat volume was defined as any adipose tissue located within the thorax from the level of the right pulmonary artery to the diaphragm and from the chest wall to the descending aorta in addition to fat inside the pericardial sac (Figure 1). For VAT, the muscular abdominal wall was manually traced to separate VAT from the subcutaneous fat. Inter- and intra-observer reproducibilities were excellent for VAT (ICC ≥ 0.99),19 pericardial fat (ICC 0.95), and total thoracic fat (ICC 0.98).18 Intrathoracic fat was defined as the difference between total thoracic fat and pericardial fat in order to create a unique fat depot that was non-overlapping with the pericardial fat compartment. This is in contrast to our prior work in which intrathoracic fat referred to total thoracic fat.
Figure 1.
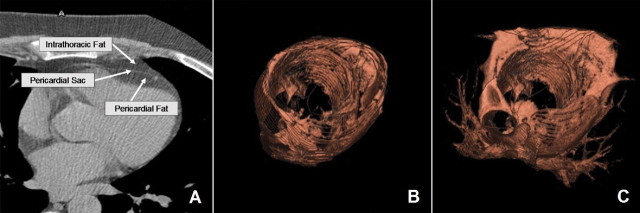
Pericardial fat and intrathoracic fat in an axial image, showing that the pericardial sac was defined as the border between pericardial and intrathoracic fat (A) and three-dimensional reconstructions of pericardial fat (B) and total thoracic fat (C). Intrathoracic fat was calculated by subtracting pericardial fat from total thoracic fat. For this subject, pericardial fat volume was 153 cm3, intrathoracic fat volume was 287 cm3, and VAT volume was 1865 cm3.
Risk factor and covariate assessment
Risk factors and covariates were measured at the seventh examination cycle (1998–2001). BMI was defined as weight (in kilograms) divided by the square of height (in metres). WC was measured at the level of the umbilicus. Fasting plasma glucose, total and high-density cholesterol, and triglycerides were measured on fasting morning samples. Diabetes was defined as fasting plasma glucose level of ≥126 mg/dL or treatment with either insulin or a hypoglycaemic agent. Hypertension was defined as systolic blood pressure above 140 mmHg, diastolic blood pressure above 90 mmHg, or treatment. Participants were considered current smokers if they had smoked at least one cigarette per day within the previous year. Alcohol use was defined as a consumption of more than seven drinks per week for women and more than 14 dinks per week for men. Women were considered menopausal if their periods had stopped for ≥1 year.
CVD included coronary heart disease (CHD, defined as recognized or unrecognized myocardial infarction, stable or unstable angina pectoris, and coronary insufficiency), stroke (defined as atherothrombotic infarction, transient ischaemic attack, cerebral embolism, intracerebral haemorrhage, and subarachnoid haemorrhage), intermittent claudication (defined as the presence of exertional calf discomfort that was relieved with rest), and congestive heart failure (CHF, defined as the presence of two major criteria or one major and two minor criteria according to the Framingham Heart Study criteria for the diagnosis of CHF26). All suspected CVD events were previously adjudicated by a panel of three Framingham investigators after review of all available Framingham Heart Study examination records, hospitalization records, and physician notes, as described previously in detail.27
Statistical analysis
Pericardial fat, intrathoracic fat, and visceral abdominal fat were normally distributed. Associations between the fat tissues were assessed using the Pearson's correlation coefficient. Associations between the amount of each fat depot and the prevalence of CVD were assessed using multivariable logistic regression. First, all fat depots were standardized to a mean of 0 and a standard deviation of 1 to facilitate direct comparisons of effect sizes across different fat depots. For models examining CVD as an outcome, we made the following covariate adjustments: (i) age and sex adjustment; (ii) age, sex, BMI, and WC adjustment; (iii) age, sex, BMI, WC, and multivariable adjustment, including systolic blood pressure, hypertension treatment, total cholesterol/HDL cholesterol, lipid-lowering therapy, diabetes, smoking, alcohol use, menopausal status, and hormone replacement therapy. Separate logistic regression analyses were preformed for each fat tissue. To assess for a potential clustering of the data due to subjects from the same family, we determined the P-values for the association of pericardial fat, intrathoracic fat, and VAT with overall CVD using general estimate equations (GEEs). For the GEE, we assumed the exchangeable compound symmetry correlation structure between members of the same nuclear family, and robust standard errors were used. In GEE, we observed similar P-values and statistical significance for the same models as using generalized linear models. For the three fat variables of interest (pericardial fat, intrathoracic fat, and VAT), we assess if there was a significant quadratic trend. For all three variables, the quadratic trend was not significant (P > 0.14).
In secondary analyses, we tested the association of pericardial fat and VAT with CHD, myocardial infarction, and stroke. Because of the relatively small number of events (myocardial infarction, n = 39 and stroke, n = 19), these models were limited to the following covariate adjustments: (i) age and sex; (ii) age, sex, BMI, and WC.
Sex interaction was tested in all models. SAS version 9.13 was used for all computations. A two-tailed P-value of <0.05 was considered statistically significant.
Results
Overall, 1267 participants (mean age: 60 ± 9 years, 53.8% women) were included in this evaluation. Mean pericardial fat volume was 124 ± 50 cm3, mean intrathoracic fat volume was 115 ± 63 cm3, and mean visceral abdominal fat volume was 2091 ± 1099 cm3. Detailed sample characteristics are shown in Table 1. The three fat depots were highly correlated (Pearson's correlation coefficient: 0.68 for pericardial fat vs. intrathoracic fat, 0.63 for pericardial fat vs. VAT, and 0.74 for intrathoracic fat vs. VAT; P < 0.001 for all).
Table 1.
Study sample characteristics
| Characteristic | n = 1267 |
|---|---|
| Age (years) | 60 ± 9 |
| Women (%) | 53.8 (682) |
| Body mass index (kg/m2) | 28.2 ± 5.1 |
| Waist circumference (cm) | 94.2 ± 13.7 |
| Pericardial fat (cm3) | 124 ± 50 |
| Intrathoracic fat (cm3) | 115 ± 63 |
| Visceral abdominal fat (cm3) | 2091 ± 1099 |
| Systolic blood pressure (mmHg) | 126 ± 18 |
| Hypertensive treatment (%) | 29.1 (369) |
| HDL cholesterol (mg/dL) | 53.2 ± 16.0 |
| Total cholesterol (mg/dL) | 201 ± 36 |
| Lipid-lowering treatment (%) | 18.4 (233) |
| Diabetesa (%) | 9.8 (124) |
| Smoking (%) | |
| Current | 10.2 (129) |
| Former | 51.9 (657) |
| Never | 38.0 (481) |
| Alcohol useb (%) | 16.3 (207) |
| Post-menopausal (%) | 82.7 (564) |
| Hormone replacement therapy (%) | 36.7 (250) |
| All CVD (%) | 9.7 (123) |
| CHD (%) | 6.3 (80) |
| Myocardial infarction (%) | 3.1 (39) |
| Stroke (%) | 1.5 (19) |
| CHF (%) | 0.2 (3) |
| Intermittent claudication (%) | 1.9 (24) |
Data presented as mean ± standard deviation for continuous traits or per cent (n) for dichotomous traits. CVD, cardiovascular disease; CHD, coronary heart disease; HDL, high density lipoprotein; CVD, cardiovascular disease; CHF, congestive heart failure.
aDefined as fasting plasma glucose ≥126 mg/dL or treatment with either insulin or a hypoglycaemic agent.
bDefined as more than 14 drinks per week (men) or more than seven drinks per week (women).
Fat depots and the association with cardiovascular disease
In an age- and sex-adjusted model, both pericardial fat and VAT but not intrathoracic fat were significantly associated with CVD (Table 2). The association of pericardial fat and visceral abdominal fat remained statistically significant after further adjustment for BMI and WC, but was attenuated after multivariable adjustment. There was no statistically significant difference in the magnitude of the associations between pericardial fat and VAT with CVD (P = 0.99 for the model adjusted for age and sex).
Table 2.
Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease per standard deviation of fat tissuea
| Models for all three exposures | Pericardial fat |
VAT |
Intrathoracic fat |
|||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Age and sex | 1.32 (1.11–1.57) | 0.002 | 1.35 (1.11–1.57) | 0.003 | 1.14 (0.93–1.39) | 0.22 |
| Age, sex, BMI, and WC | 1.31 (1.08–1.59) | 0.006 | 1.46 (1.11–1.92) | 0.007 | 1.08 (0.85–1.59) | 0.54 |
| Age, sex, BMI, WC, and multivariable adjustmentb | 1.17 (0.95–1.45) | 0.14 | 1.23 (0.92–1.63) | 0.16 | 0.96 (0.75–1.24) | 0.76 |
BMI, body mass index; WC, waist circumference; VAT, visceral abdominal fat; CI, confidence interval.
aAll fat depots have been standardized to a mean of 0 and standard deviation of 1 to facilitate comparisons across depots.
bIncludes systolic blood pressure, hypertension treatment, total cholesterol/HDL, lipid-lowering therapy, diabetes, smoking, alcohol use, menopausal status, and hormone replacement therapy.
Visceral fat tissues and the association with coronary heart disease, myocardial infarction, and stroke
After observing a significant association of pericardial fat and VAT with overall CVD, we performed secondary analysis to further investigate the association of both fat tissues with CHD and myocardial infarction as an outcome variable that may be influenced by a potential local effect of pericardial fat and stroke as an outcome variable that is influenced by a systemic effect of VAT but is not as likely to be influenced by pericardial fat.
Both pericardial fat and VAT were significantly associated with the prevalence of CHD in an age- and sex-adjusted model and after further adjustment for BMI and WC. However, associations were stronger for pericardial fat than for VAT.
In an age- and sex-adjusted model, both pericardial fat and VAT were significantly associated with the prevalence of myocardial infarction. This association remained significant for pericardial fat after further adjustment for BMI and WC, but was not significant for VAT.
Only VAT but not pericardial fat was associated with stroke in an age- and sex-adjusted model. The association of VAT with stroke remained statistically significant after further adjustment for BMI and WC (Table 3).
Table 3.
Association of pericardial fat and visceral abdominal fat with coronary heart disease, myocardial infarction, and stroke per standard deviation of fat tissuea
| Pericardial fat |
VAT |
||||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| CHD | Age and sex adjusted | 1.97 (1.32–2.94) | 0.0009 | 1.34 (1.09–1.66) | 0.006 |
| Age, sex, BMI, and WC adjusted | 1.92 (1.23–3.02) | 0.004 | 1.36 (1.02–1.82) | 0.04 | |
| Myocardial infarction | Age and sex adjusted | 1.48 (1.15–1.90) | 0.002 | 1.55 (1.14–2.11) | 0.005 |
| Age, sex, BMI, and WC adjusted | 1.37 (1.03–1.82) | 0.03 | 1.38 (0.91–2.08) | 0.13 | |
| Stroke | Age and sex adjusted | 1.43 (0.99–2.07) | 0.06 | 1.82 (1.18–2.80) | 0.006 |
| Age, sex, BMI, and WC adjusted | 1.29 (0.85–1.98) | 0.23 | 1.83 (1.01–3.30) | 0.046 | |
CHD, coronary heart disease; BMI, body mass index; WC, waist circumference; VAT, visceral abdominal fat; CI, confidence interval.
aAll fat depots have been standardized to a mean of 0 and standard deviation of 1 to facilitate comparisons across depots.
There was no significant sex interaction for any fat depot (P > 0.07 for all age- and sex-adjusted models).
Discussion
In this cross-sectional study, we examined associations of pericardial fat, intrathoracic fat, and VAT with CVD in a community-based sample and found that pericardial fat and VAT were both associated with CVD in age- and sex-adjusted models. These associations remained significant after adjustment for BMI and WC.
The finding that pericardial fat, a very small fat depot, is associated with CVD supports the hypothesis that pericardial fat may have a paracrine role in the pathogenesis of CVD. In contrast, the strong association of VAT with CVD is likely due to powerful associations between VAT and CVD risk factors, including hypertension, dyslipidaemia, and diabetes, that we and others have reported previously, suggesting that VAT has systemic effects as a pathogenic fat depot. These hypotheses are supported by our finding that pericardial fat is predominantly associated with CHD and myocardial infarction, whereas only VAT is associated with stroke. However, the lacking association of pericardial fat with stroke may be caused by a smaller number of strokes compared with myocardial infarction (MI). The finding that pericardial fat and VAT were similarly associated with overall CVD may indicate that the potential paracrine effect of pericardial fat and the systemic effect of VAT might be of comparable effect size.
However, associations were attenuated upon adjustment for CVD risk factors, suggesting that ultimately relations between fat depots and CVD are due to shared risk factors. Also, we cannot rule out that several findings may be influenced by diminished statistical power to detect modest effect sizes. Further, the association between pericardial fat, VAT, and CVD events may be mediated via shared risk factors in the pathogenesis between fat depots and actual events.
Notably, we observed different associations to CVD of pericardial fat, intrathoracic fat, and VAT, despite their close correlation.
In the context of the current literature
Our finding that pericardial fat is associated with the prevalence of CVD and myocardial infarction after adjustment for age and sex and traditional measures of obesity are supported by our prior work, demonstrating a significant association of pericardial fat volume with traditional risk factors and the presence of coronary artery calcium.18 Likewise, Taguchi et al.22 found a significant association between pericardial fat volume and the prevalence of coronary artery disease in non-obese Japanese subjects. In a study of 203 participants from Korea, a close association between epicardial fat thickness and the severity of coronary artery disease was found.23 Overall, our results are consistent with the hypothesis that perivascular fat may be associated with local vascular injury. In this context, our observation that VAT and stroke are strongly associated warrants further exploration.
Potential mechanisms
The specific composition and metabolic activity of visceral fat tissues such as pericardial fat and VAT are widely recognized as differing from subcutaneous fat. Visceral fat tissues have smaller adipocyte size,28 higher protein content,29 high rate of fatty acid incorporation,30 and fast insulin-induced fatty acid breakdown29 and secrete several pro- and anti-inflammatory mediators and cytokines such as adiponectin, interleukin-6, and TNF-α.10–16,21,31 The amount of adiponectin, a stabilizer of the inhibitor of NF-κB released from pericardial fat,11 decreases with an increased amount of fat.32 The decrease in adiponectin enhances the activity of NF-κB, which leads to an increase in TNF-α and hence to a local increase of inflammation.11 A mismatch of pro- and anti-inflammatory mediators and cytokines secreted by pericardial fat is suspected to have a local influence on the underlying coronary arteries. Increased CD45 mRNA expression in the pericardial fat of subjects with coronary artery disease, representing elevated macrophage infiltration,13 and an increase in mast cells in the adventitia of coronary lesions14 have been observed. The hypothesis of an impact on local inflammation of pericardial fat and its role in the pathogenesis of atherosclerosis of the coronary arteries are supported by our findings.
Despite the relative size, VAT differentiates from pericardial fat in blood supply and drainage. Intra-abdominal mesenteric fat (VAT) has a circulatory communication path to the liver via the portal circulation and thus may be highly associated with insulin resistance of the liver and hepatic production of inflammatory factors such as high sensitivity-C-reactive protein. VAT is associated with metabolic risk factors,5,15,16,31,33–36 traditional CVD risk factors,19 and systemic inflammatory markers.21 These associations further emphasize the importance of VAT as a mediator of systemic CVD risk factors.
In contrast, intrathoracic and pericardial fat depots are substantially smaller than VAT and are unlikely to release substances that can be detected systemically. Therefore, their hypothesized role is more likely to be paracrine via their local effect on inflammation in the underlying tissue.10,13,14,21
Implications
Together with our previous findings of visceral fat tissues being associated with risk factors and vascular calcification,18,19 these findings suggest that visceral fat depots are associated with CVD. Further research is warranted to establish the incremental value of fat measurements to traditional CV risk factors and the causal relationship between pericardial fat and VAT and the development of CVD.
Strength and limitations
The strengths of our study include a community-based sample not selected for adiposity-related traits. Fat volumes were quantified using a highly reproducible volumetric CT-based measure. Limitations of our study include the predominantly white Framingham Offspring Study, hence generalization to other ethnic groups is uncertain. Also, we were only able to assess prevalent CVD and by definition could not assess the relation between fat depots and fatal CVD. Given the cross-sectional study design, we cannot establish causality. Although our data are plausible with the biological hypothesis, a cause-and-effect relationship between pericardial fat and myocardial infarction cannot be established in this cross-sectional study. However, our results are consistent with our previous data, describing a cross-sectional association of pericardial fat and coronary artery calcium in subjects without known CVD. We included individuals with CHF as outcomes and recognize that these patients may have an underlying pathology different from CAD. However, there were only three participants with CHF: one patient had angina pectoris about 2 years after the CHF occurred; one patient had an MI about 10 years before the CHF occurred, and the third patient only had CHF. Thus, it is unlikely to have affected our results. Further, we excluded all participants who had previously undergone cardiac bypass surgery, due to the unreliability of pericardial fat in this setting. Therefore, we have excluded participants with the most informative physiology and biased our results towards the null. A further limitation of our study is the diminished statistical power to detect modest effect sizes. Thus, our findings that need to be confirmed in studies with larger numbers of CVD events, including prospective studies, are warranted to determine whether metabolic fat depots are independently associated with CVD after adjustment for traditional CVD risk factors.
Conclusion
Pericardial fat and VAT, but not intrathoracic fat, are associated with CVD independent of traditional measures of obesity. However, none of these fat depots are independently associated with CVD after further adjustment for traditional risk factors. Taken together with our prior work, these findings may support the hypothesis that pericardial fat contributes to coronary atherosclerosis but needs to be confirmed in larger studies.
Funding
This work was supported by the National Heart, Lung, and Blood Institute's Framingham Heart Study (N01-HC-25195). A.A.M. is supported by a grant from the German National Academic Foundation. G.A.R. is supported by a grant from the CAPES, Brazil.
Conflict of interest: none declared.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 5.Poirier P, Despres JP. Waist circumference, visceral obesity, and cardiovascular risk. J Cardiopulm Rehabil. 2003;23:161–169. doi: 10.1097/00008483-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 7.Lissner L, Bjorkelund C, Heitmann BL, Seidell JC, Bengtsson C. Larger hip circumference independently predicts health and longevity in a Swedish female cohort. Obes Res. 2001;9:644–646. doi: 10.1038/oby.2001.85. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto WY, Jablonski KA, Bray GA, Kriska A, Barrett-Connor E, Haffner S, Hanson R, Hill JO, Hubbard V, Stamm E, Pi-Sunyer FX. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes. 2007;56:1680–1685. doi: 10.2337/db07-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vos AM, Prokop M, Roos CJ, Meijs MF, van der Schouw YT, Rutten A, Gorter PM, Cramer MJ, Doevendans PA, Rensing BJ, Bartelink ML, Velthuis BK, Mosterd A, Bots ML. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J. 2008;29:777–783. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 10.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 11.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 12.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 13.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laine P, Kaartinen M, Penttila A, Panula P, Paavonen T, Kovanen PT. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99:361–369. doi: 10.1161/01.cir.99.3.361. [DOI] [PubMed] [Google Scholar]
- 15.Yatagai T, Nagasaka S, Taniguchi A, Fukushima M, Nakamura T, Kuroe A, Nakai Y, Ishibashi S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 16.Saijo Y, Kiyota N, Kawasaki Y, Miyazaki Y, Kashimura J, Fukuda M, Kishi R. Relationship between C-reactive protein and visceral adipose tissue in healthy Japanese subjects. Diabetes Obes Metab. 2004;6:249–258. doi: 10.1111/j.1462-8902.2003.0342.x. [DOI] [PubMed] [Google Scholar]
- 17.Kremen J, Dolinkova M, Krajickova J, Blaha J, Anderlova K, Lacinova Z, Haluzikova D, Bosanska L, Vokurka M, Svacina S, Haluzik M. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91:4620–4627. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 18.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 19.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 20.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 21.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, Masuda Y. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 23.Jeong JW, Jeong MH, Yun KH, Oh SK, Park EM, Kim YK, Rhee SJ, Lee EM, Lee J, Yoo NJ, Kim NH, Park JC. Echocardiographic epicardial fat thickness and coronary artery disease. Circ J. 2007;71:536–539. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 24.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 25.Shurtleff D. Some characteristics related to the incidence of cardiovascular disease and death: Framingham Study, 18-year follow-up: section 30. In: Kannel W, Fordon T, editors. The Framingham Study: and Epidemiological Investigation of Cardiovascular Disease. Washington, DC: Department of Health, Education, and Welfare; 1973. DHEW publication No. NIH 74-599. [Google Scholar]
- 26.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(Suppl. 4A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 27.Cupples L, D'Agostino R, Kiely D. The Framingham Heart Study, Section 35. An Epidemiological Investigation of Cardiovascular Disease Survival Following Cardiovascular Events: 30 Year Follow-up. Bethesda, MD: National Heart, Lung and Blood Institute; 1988. [Google Scholar]
- 28.Sons HU, Hoffmann V. Epicardial fat cell size, fat distribution and fat infiltration of the right and left ventricle of the heart. Anat Anz. 1986;161:355–373. [PubMed] [Google Scholar]
- 29.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989;94:225–232. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 30.Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14:1013–1022. [PubMed] [Google Scholar]
- 31.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 32.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 33.Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract. 2006;3:35–42. doi: 10.1038/ncpcardio0380. [DOI] [PubMed] [Google Scholar]
- 35.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]


