Abstract
The present study evaluated a buddy program designed to provide support for individuals with chronic fatigue syndrome (CFS). The intervention involved weekly visits by a student paraprofessional, who helped out with tasks that needed to be done in an effort to reduce some of the taxing demands and responsibilities that participants regularly encountered. This model of rehabilitation focused on avoiding overexertion in persons with CFS, aiming to avoid setbacks and relapses while increasing their tolerance for activity. Participants with CFS were randomly assigned to either a 4-month buddy intervention or a control condition. Posttest results showed that individuals who received a student buddy intervention had significantly greater reductions in fatigue severity and increases in vitality than individuals in the control condition. There were no significant changes between groups for physical functioning and stress. Buddy interventions that help patients with CFS reduce overexertion and possibly remain within their energy envelopes can be thought of as representing a different paradigm than nonpharmacologic interventions that focus only on increasing levels of activity through graded exercise.
Individuals with chronic fatigue syndrome (CFS) often experience difficulties both at work and in completing daily life activities (Yatanabe, Evengard, Natelson, Jason, & Kuratsune, 2008). Some patients with this illness quit or were forced to resign from their jobs because they were not able to adequately perform because of fatigue, cognitive problems, and other symptoms. Many struggle to complete basic life activities such as grocery shopping, cleaning, and cooking (Roesner et al., 2008). Patients often overexert themselves in an effort to catch up on responsibilities that they had not been able to complete while ill. Yet, completing these tasks can contribute to postexertional malaise, one of the hallmarks of this illness. Not having adequate support often leaves patients feeling discouraged and powerless. Social support could be crucial to enabling those with CFS to cope with the limitations imposed by their illness (Jason, Fennell, & Taylor, 2003).
One way to provide support to people with CFS is by developing volunteer care giving programs. Jason, Ferrari, Taylor, Slavich, and Stenzel (1996) found that helping with daily chores on a regular basis was perceived as one of the higher priority needs identified by a national sample of patients with CFS. Much of the controversy surrounding illness management for CFS centers on the uncertainty regarding an appropriate balance between rest and activity. One model for rehabilitation focuses on avoiding overexertion and underexertion, and thereby persons with CFS can avoid setbacks and relapses while increasing their tolerance for activity. Such a framework for treatment plans and illness management is based upon individualized assessments and tailored to the patient's situation. For example, patients identified as continually overexerting themselves are advised to conserve their energy resources so that long-term gains in tolerance to activity can be made. In other words, all persons with CFS should not necessarily increase or decrease their activity levels; instead, what is needed is the use of moderation and energy conservation, which has been referred to as the “Envelope Theory” (Jason, Melrose et al., 1999; Jason, Tryon et al., 1999). Hawk, Jason, and Torres-Harding (2007) have found that patients with CFS can rate their weekly level of available and expended energy with good interrater reliability.
In evaluating Envelope Theory, a time series study by Jason, Tryon, Frankenberry, and King (1997) found that energy expended, physical exertion, and mental exertion were positively related to actigraphy. In a second study, Jason, Melrose et al. (1999) and Jason, Tryon et al. (1999) found a positive significant relationship between current fatigue level and self-rated expended energy (i.e., the amount of energy that participants perceived they had used) two days prior. In a correlational study, Jason, Muldowney, and Torres-Harding (2008) found that the individuals with CFS experienced a range of negative symptoms and disability when they extend beyond their energy envelope.
Several clinical case studies have also supported Envelope Theory. Jason, Melrose et al. (1999) and Jason, Tryon et al. (1999) presented evidence that when patients with CFS kept their expended energy levels within the envelope of their perceived energy levels, fatigue was lower and perceived energy higher. Shlaes and Jason (1996) provided participants with CFS a Buddy/Mentor intervention, and those who were provided the intervention were able to conserve energy and experienced significant decreases in fatigue severity, while the control group experienced significant increases in fatigue severity. In addition, Pesek, Jason, and Taylor (2000) found that when participants with CFS were provided with a buddy to reduce activities and assist in identifying and reducing discrepancies between perceived and expended energy, overall fatigue severity as well as severity ratings for CFS symptoms decreased. Unfortunately, each of the studies above had relatively small sample sizes.
Recently, Jason, Benton, Torres-Harding, and Muldowney (in press) and Jason, Porter, Herrington, Sorenson, and Kubow (2009) compared two groups of patients who participated in a nonpharmacologic intervention trial. Some were able to keep expended energy close to available energy and others were not successful at this task. Those who were able to stay within their energy envelope had significant improvements in physical functioning and fatigue severity. Findings suggest that helping patients with CFS maintain appropriate energy expenditures in coordination with available energy reserves can help improve functioning over time.
The present study evaluated the Buddy Program at the Center for Community Research at DePaul University. This 4-month program was developed to provide social support to those with CFS. The helpers were college students and the patients were individuals diagnosed with CFS. The theoretical framework of this rehabilitation program involved the Envelope Theory, whereby patients were provided the support of a college student to reduce overexertion and exhaustion doing daily living tasks. The study's hypothesis is that participants in the program would have significant positive changes over time on measures of fatigue, vitality, stress, and physical functioning compared with a nonintervention control group.
Methods
Participants
Thirty individuals, 5 males, and 25 females served as participants. Participants' average age was 57.6 years. Regarding ethnicity, 83.3% were Caucasian and 16.7% were other. As for marital status, 23.3% were married, 23.3% were never married, and 53.3% were divorced, separated or widowed. In terms of work status, 53.3% were on disability, 33.3% were unemployed, and 13.3% were working part-time or full-time. In terms of education, 66.6% had earned a standard college degree, and 33.3% had partial college or high school/GED or less. Participants were individuals that were diagnosed with CFS using theFukuda et al. (1994) criteria and the recruited participants felt that they could benefit from having the assistance of a volunteer buddy. Participants were recruited through Chicago area specialists, Chicago support groups, and the Chicago-based CFS newsletter.
Student Buddies
Fifteen buddies were recruited for the intervention. The role of the 15 student buddies was to provide support to their assigned 15 participants. The student buddies, undergraduate students at DePaul University, agreed to spend 2 hours per week visiting a participant at their home. They received course credit for their participation. Emotional support was provided through offering empathy, trust, listening, understanding, and concern. Any form of direct help provided functional support. Students offer this type of social support by working on a variety of household tasks during their visits such as organizing files, writing letters, creating photo albums, and helping their assigned participants monitor their energy levels. The participants defined the role of the student buddies and their individual needs. For example, while one participant may have needed more social support, another participant may have needed more functional support. This assistance was intended to help participants avoid overexertion, thereby avoiding setbacks and relapses, while increasing their tolerance for activity. The buddies did not use their time with the participants to observe and coach the patient in Envelope Theory, but rather they used their time to do specific tasks each week that were too physically demanding for the participants.
The student buddies were required to attend 4 hours of training over a 2-week period. There were two meetings, each lasting 2 hours. In addition, there were subsequent 1-hour weekly meetings throughout the 4-month duration of the program. Training included theoretical articles on the Envelope Theory, personal stories about people with CFS, empathetic listening training, and role-playing. Difficulties that might be encountered (such as eventual termination of the relationship, the need to work around participants' schedules, etc.) were discussed. After students completed the training, they were matched with participants. The student buddies had a background in psychology or social work.
Student buddies were matched with participants based on the participants' particular needs and interests that they included on an initial request form. The participants also included information of which the student buddy needed to be aware, such as any health problems, allergies, or sensitivities. The student buddies as well as the participants were asked about what they wished to gain from being a part of the program. Matches were also made by geographical location of both the student buddy and the participant.
Procedure
Before the intervention started, all participants completed a consent form and a brief assessment battery that took less than 25 minutes to complete. All 30 participants completed the questionnaire, and then 15 participants were randomly assigned to the experimental (E) group and 15 to the control (C) group. The E group received a student buddy directly after completing a baseline battery of questionnaires. The C group did not receive an intervention for 4 months after their baseline assessment. After the C group completed their posttesting, they were provided a buddy intervention. Each buddy pairing lasted for 4 months. Buddies scheduled meetings for 2 hours a week. In other words, each participant was supposed to obtain 32 hours of assistance over the 4 months. Because sometimes participants were sick and could not be visited, meetings sometimes did not occur over a week. However, all participants received at least 16 hours of assistance over the 4 months. We did not find any significant relationship between the number of hours in the program and any outcome measures, and this supports the possibility that all participants did receive a minimum threshold intervention. After a 4-month period, all 30 participants completed the questionnaire again. Upon the completion of the study, those in the C group received a student buddy for 4 months.
Measures
Medical Outcomes Study-Short Form-36 (MOS-SF-36)
The MOS-SF-36 identifies health concepts as perceived by the individual (Ware & Sherbourne, 1992). A higher score indicates better health or less impact of health on functioning. Test construction studies for the SF-36 (McHorney, Ware, & Raczek, 1993; McHorney, Ware, Lu, & Sherbourne, 1994) have shown adequate internal consistency, significant discriminate validity among subscales, and substantial differences between patient and nonpatient populations in the pattern of scores. The SF-36 has also indicated sufficient psychometric properties as a measure of functional status in samples having CFS (Buchwald, Pearlman, Umali, Schmaling, & Katon, 1996). The MOS physical functioning and vitality scales were used in the present study to assess physical functioning and vitality. The MOS physical functioning scale was utilized in the present investigation as it has been used in several other CFS outcomes studies (Deale, Husain, Chalder, & Wessely, 2001). In addition, White, Sharpe, Chalder, DeCesare, and Walwyn (2007) have recommended using the physical function subscale of the MOS-SF-36 as a primary outcome measure for CFS trials. The internal consistency reliability coefficient for the vitality scale is .87, and examples of items include “Do you feel pep?” and “Do you have a lot of energy?” Higher scores indicate more positive physical functioning and vitality.
Fatigue Severity Scale (FSS)
Krupp, LaRocca, Muir-Nash, and Steinberg's (1989) FSS was used to measure fatigue. This scale includes 9 items rated on 7-point scales and is sensitive to different aspects and gradations of fatigue severity. Most items in the FSS are related to behavioral consequences of fatigue. Sample questions include “I am easily fatigued” and “Exercise brings on my fatigue.” The FSS is also a practical measure because of its brevity and ease of administration and scoring. Previous findings have demonstrated the utility of the FSS (Krupp et al.) to discriminate between individuals with CFS, multiple sclerosis (MS), and primary depression (Pepper, Krupp, Friedberg, Doscher, & Coyle, 1993). In addition, the FSS (Krupp et al.) was normed on a sample of individuals with MS, systemic lupus erythematosus (SLE), and healthy controls. Taylor, Jason, and Torres (2000) found this scale to be an accurate and comprehensive measure of fatigue severity and functional disability for individuals with CFS-like symptomatology. Higher scores indicate more fatigue.
Perceived Stress Scale (PSS)
The PSS is a 4-item revised version of a previous 14-item measure of global perceived stress. The time period that this instrument measured was the previous month (Cohen, Kamarck, & Mermelstein, 1983). The brief version of this scale has a coefficient alpha reliability of .72, Higher scores indicate more stress.
Statistical Tests
Chi-squares and t-tests were used to test differences between conditions on demographic variables. For the four dependent variables, time, condition, and interaction effects were examined using repeated measures analysis of variance (ANOVA). For variables with significant interaction effects, baseline means were compared with conditions, and posttreatment means were compared with conditions using t-tests.
Results
There were no significant differences between the E and C groups in terms of demographic variables including race, education, marital status, and work status. (See Table 1.) There also were no significant baseline differences between the E and C groups on the dependent variables. (See Table 2.)
Table 1.
Buddy Demographics
| Control |
Experimental significant |
|||
|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |
| Age | 15 | 58.33(9.35) | 15 | 56.8(16.11) |
| N | % | N | % | |
| Gender | ||||
| Female | 12 | 80.0 | 13 | 86.7 |
| Male | 3 | 20.0 | 2 | 13.3 |
| Race | ||||
| White | 13 | 86.7 | 12 | 80.0 |
| Other | 2 | 13.3 | 3 | 20.0 |
| Martial status | ||||
| Married | 3 | 20.0 | 4 | 26.7 |
| Never married | 3 | 20.0 | 4 | 26.7 |
| Separated/widowed | 9 | 60.0 | 7 | 46.6 |
| Divorced | ||||
| Education | ||||
| High school degree/partial training | 5 | 33.3 | 5 | 33.3 |
| College degree/Grad/professional | 10 | 66.7 | 10 | 66.7 |
| Work status | ||||
| On disability | 9 | 60.0 | 7 | 46.6 |
| Unemployed | 5 | 33.3 | 5 | 33.3 |
| Work part-time or full-time | 1 | 6.6 | 3 | 20.0 |
Table 2.
Mean Differences Between Groups on Measures
| Control |
Experimental significant |
|||
|---|---|---|---|---|
|
N = 15 |
N = 15 |
|||
| Baseline | 4 Months | Baseline | 4 Months | |
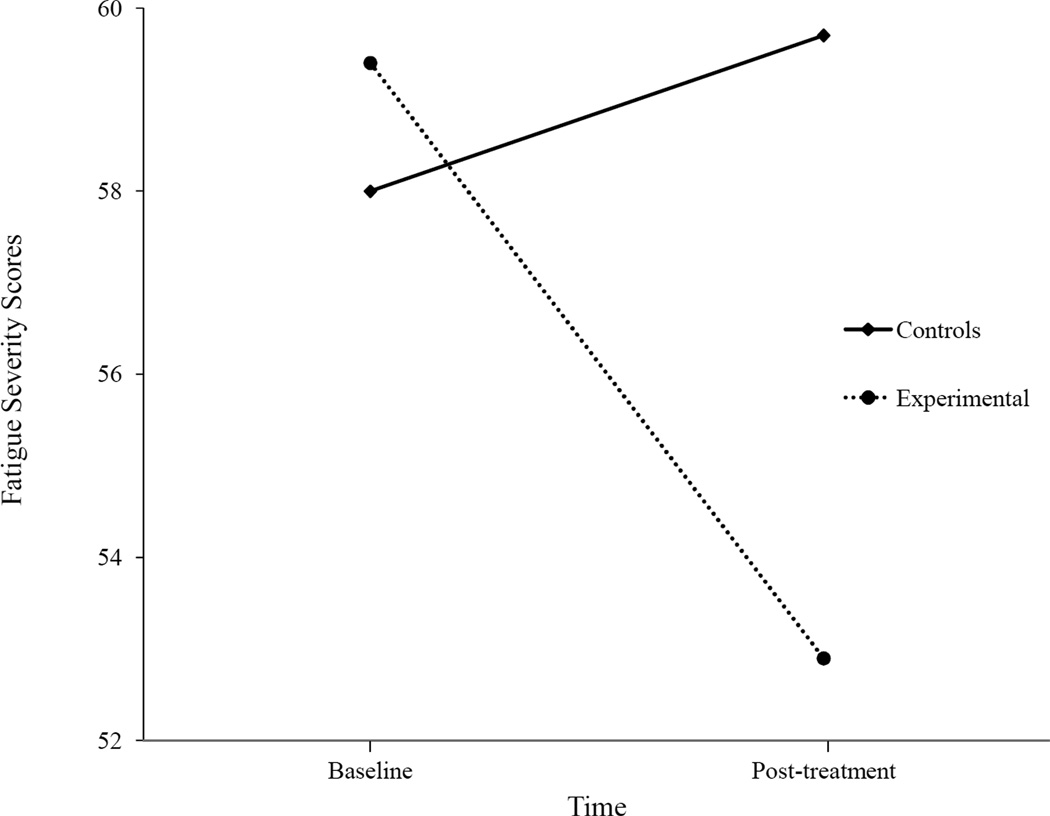
| FSS (Krupp)* | 58.0 (3.5) | 59.4 (3.7)a | 59.7 (3.8) | 52.9 (10.5)a |
| Perceived stress | 13.6 (2.4) | 12.9 (2.1) | 12.7 (2.1) | 12.7 (1.8) |
| Physical functioning | 36.0 (29.9) | 29.7 (24.9) | 31.2 (13.1) | 36.1 (14.10) |
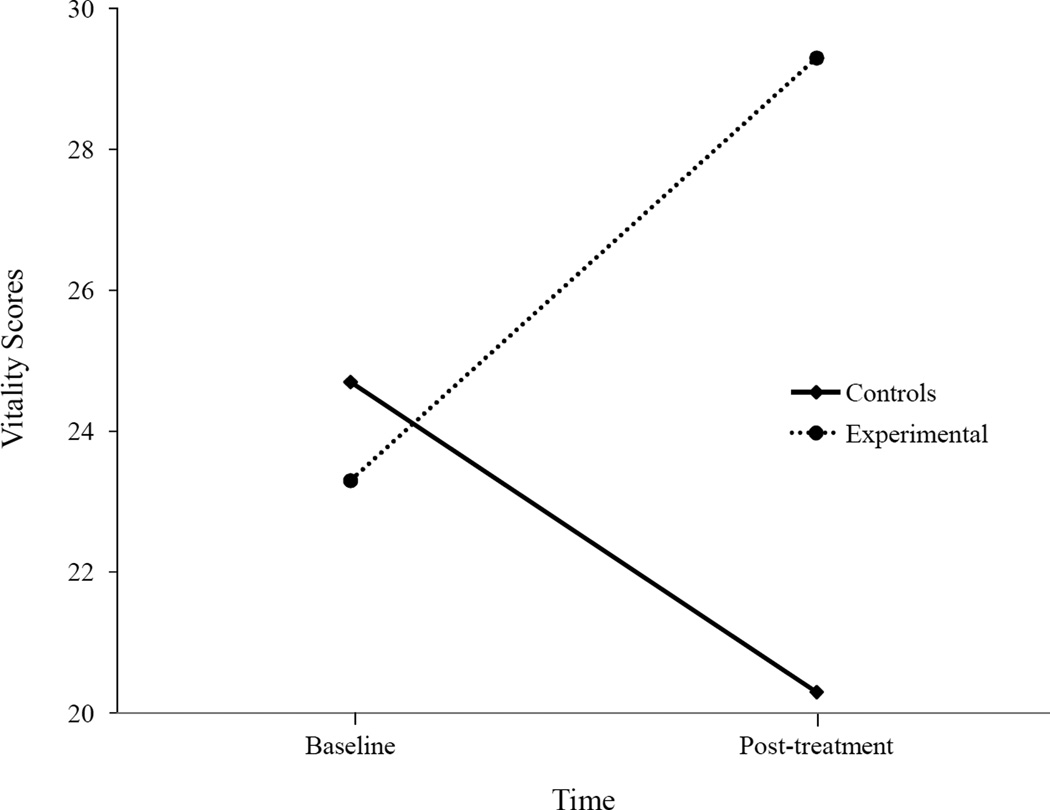
| Vitality | 24.7 (9.7) | 20.3 (10.1)b | 23.3 (10.8) | 29.3 (13.9)b |
Note. FSS = Fatigue Severity Scale. Similar letter subscripts across rows indicate significant differences in means.
Difference is statistically significant at the p ≤ .05 level.
For the FSS scores, there was a significant interaction effect, F(1,28) = 8.63, p = .01 (see Fig. 1), but no significant time, F(1,28) = 3.59, p = .07, or condition effects F(1,28) = 1.98, p = .17. There were no significant E versus C differences at baseline for the FSS scores t(28) = −1.24, p = .22, but at the posttesting, the E scores were significantly lower than the C scores t(17.6) = 2.29, p = .04. Participants who received a student buddy intervention experienced significantly less fatigue over time than the C group.
Figure 1.
Fatigue Severity means between groups over time.
For vitality scores, there was a significant interaction effect F(1,28) = 5.47, p = .03 (see Fig. 2), but there was no significant time F(l,28) = .14, p = .71 or condition effect F(1,28) = 1.22, p = .28. There were no significant baseline differences in vitality scores between the E and C condition t(28) = .36, p = .73, but there were significant posttest differences in scores for E and C groups t(28) = −2.02, p = .05. Those who received the E condition experienced significantly more vitality over time than those in the C condition.
Figure 2.
Vitality means between groups over time.
There was no significant interaction effects for physical functioning F(1, 28) = 3.70, p = .06, nor were there significant time effects F(l, 28) = .06, p = .82 or condition effects F(l, 28) = .01, p = .91. For the stress variable, there were no significant interaction effects F(l, 27) = .49, p = .49, time effects F(1, 27) = .49, p = .49 or condition effects F(1, 27) = .90, p = .35.
Discussion
The E condition led to significant positive changes in fatigue levels and vitality over time. While the C group either stayed the same or decreased fatigue severity and vitality, the E group evidenced an 11% decrease in fatigue (with FSS scores decreasing from 59.7 to 52.9) and a 20% increase in vitality (with MOS vitality scale scores increasing from 23.3 to 29.3). These types of changes are impressive and clinically meaningful, but it should be noted that post test scores for the E group are still considerably below the norms of healthy groups (Krupp et al., 1989; McHorney et al., 1994).
It is of interest that the intervention was effective with fatigue and vitality, but it was not with physical functioning and stress. These findings suggest that efforts to reduce overextertion might be particularly effective in reducing fatigue and increasing vitality, as the participants have been spared from doing some of the tasks that make them feel overextended and exhausted. Although reducing these states might be of particular helpfulness to patients, this intervention does not appear to reduce the functional limitations and stress that is experienced by the patients.
Assistance with daily activities, such as grocery shopping or housework, could have reduced overexertion and exhaustion, thus allowing participants to feel more energy (vitality) and less fatigue. This study endorsed an approach involving helping patients with CFS stay within their energy envelope (Jason, Melrose et al., 1999; Jason, Tryon et al., 1999). This Envelope Theory has certain similarities with pacing (Goudsmit, 2001). These approaches do not unilaterally increase activity for all patients. For example, the Envelope Theory recommends that patients with CFS pace their activity according to their available energy resources (Jason, Melrose et al., 1999; Jason, Tryon et al., 1999). In this approach, the phrase “staying within the envelope” is used to designate a comfortable range of energy expenditure in which an individual avoids both overexertion and underexertion, maintaining an optimal level of activity over time. The key is to not overexpend energy supplies or consistently go outside the envelope of available energy. In addition, it is important to avoid under-exertion, which might involve increasing activity for some individuals. Rather than a cure, this approach focuses on improving the ability of patients to cope with this illness.
The buddies provided the participants an opportunity to confide in another person, and this emotional support may have also helped the participants increase vitality and energy (Antoni et al., 1994). In fact, emotional support might have been one of the primary mediators of change. Future research that assesses the efficacy of energy conservation should compare a control group given only emotional support in the weekly visits compared to a treatment group offered both emotional and physical/task support during weekly visits.
At a theoretical level, it is important to speculate about how a buddy intervention might lead to positive changes among patients with CFS. It is at least possible that centrally mediated kindling and oxidative stress might lead to immune, autonomic, and neuroendocrine dysfunction (Jason, Benton et al., 2009; Jason, Porter et al., 2009). Kindling involves prolonged stimulation of the limbic-hypothalamic-pituitary axis, resulting in a lowered threshold for activation. Once this system is charged by either high-intensity stimulation (e.g., due to an acute viral infection) or chronically repeated low-intensity stimulation (e.g., through repeated chemical exposure), it can sustain a high level of arousal with little or no external stimulus. Kindling might result in excessive arousal in patients with CFS, which could lead to more excitatory postsynaptic receptors and decrease inhibitory presynaptic receptors. Such a theory has implications for treatment as the goal might involve helping patients normalize neuroendocrine-immune functioning (Van Houdenhove, Van Den Eede, & Luyten, 2009; Jason, Benton et al., 2009), or using pharmacologic drugs that are agonists to central sympathetic outflow (Wyller, Eriksen, & Malterud, 2009). Buddy programs might help patients reduce overexertion, and thereby reduce limbic stimulation and arousal. In other words, the buddy program can be thought of as a different paradigm than those nonpharmacologic interventions that focus only on increasing levels of activity through graded exercise.
There were several limitations in the study, including small sample size and no follow-up assessments. There was also a large variability in the amount of contact time with participants (i.e., ranging from 16 hours to 32 hours), although this was not related to outcomes. In addition, the current study did not have an attention control group. This is a serious limitation in the study as the delivery of emotional support is a common attentional control condition, and the buddy group almost certainly contained this component. There were also only four dependent measures, but given the small sample, it was important to control the number of variables to reduce chance findings. The fact that significant effects were found on two of the four dependent variables suggests that these types of buddy interventions might have considerable promise for helping patients with CFS. However, more research is needed with larger samples and long-term follow-up before more definitive conclusions can be made.
Acknowledgments
The authors appreciate the financial assistance provided by the National Institute of Allergy and Infectious Diseases (grant numbers AI36295 and AI49720). We also thank the following people for their help on the project: Carole Howard, Patrick Holiday, Gail Schoebacher, Mary Thompson, Sandy Reyes, Samra Cheema, Gina Delucca, Blair Coleman, Kimberly Korkowski, and Laura Thomas.
Contributor Information
Leonard A. Jason, DePaul University
Nicole Roesner, DePaul University.
Nicole Porter, DePaul University.
Brittany Parenti, DePaul University.
Jennifer Mortensen, Michigan State University.
Lindsay Till, Northwestern University.
References
- Antoni H, Brickman A, Lutgendorg S, Filmas N, Imia-Fins A, Ironson, et al. Psychosocial correlates of illness burden in chronic fatigue syndrome. Clinical Infectious Disease. 1994;18((1)):73–80. doi: 10.1093/clinids/18.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. American Journal of Medicine. 1996;101((4)):364–370. doi: 10.1016/S0002-9343(96)00234-3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarack T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: A 5-year follow-up study. American Journal of Psychiatry. 2001;158:2038–2042. doi: 10.1176/appi.ajp.158.12.2038. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Annals of Internal Medicine. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Goudsmit E. Measuring the quality of trials of treatments for chronic fatigue syndrome. Journal of the American Medical Association. 2001;286:3078–3079. doi: 10.1001/jama.286.24.3078. [DOI] [PubMed] [Google Scholar]
- Hawk C, Jason LA, Torres-Harding S. Reliability of a chronic fatigue syndrome questionnaire. Journal of Chronic Fatigue Syndrome. 2007;13:41–66. [Google Scholar]
- Jason LA, Benton M, Torres-Harding S, Muldowney K. The impact of energy modulation on physical functioning and fatigue severity among patients with ME/CFS. Patient Education and Counseling. doi: 10.1016/j.pec.2009.02.015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason LA, Fennell P, Taylor RR, editors. Handbook of chronic fatigue syndrome. New York: John Wiley & Sons, Inc.; 2003. [Google Scholar]
- Jason LA, Ferrari JR, Taylor RR, Slavich SP, Stenzel CL. A national assessment of the service, support, and housing preferences by persons with chronic fatigue syndrome: Toward a comprehensive rehabilitation program. Evaluation and the Health Professions. 1996;19:194–207. doi: 10.1177/016327879601900204. [DOI] [PubMed] [Google Scholar]
- Jason L, Melrose H, Lerman A, Burroughs V, Lewis K, King C, et al. Managing chronic fatigue syndrome: Overview and case study. AAOHN Journal. 1999;47:17–21. [PubMed] [Google Scholar]
- Jason LA, Muldowney K, Torres-Harding S. The energy envelope theory and Myalgic Encephalomyelitis/chronic fatigue syndrome. AAOHN Journal. 2008;56:189–195. doi: 10.3928/08910162-20080501-06. [DOI] [PubMed] [Google Scholar]
- Jason LA, Porter N, Herrington J, Sorenson M, Kubow S. Kindling and oxidative stress as contributors to Myalgic Encephalomyelitis/chronic fatigue syndrome. Manuscript submitted for publication. 2009 [PMC free article] [PubMed] [Google Scholar]
- Jason LA, Tryon W, Frankenberry E, King C. Chronic Fatigue Syndrome: Relationships of self-ratings and actigraphy. Psychological Reports. 1997;81:1223–1226. doi: 10.2466/pr0.1997.81.3f.1223. [DOI] [PubMed] [Google Scholar]
- Jason LA, Tryon WW, Taylor RR, King C, Frankenberry EL, Jordan KM. Monitoring and assessing symptoms of chronic fatigue syndrome; Use of time series regression. Psychological Reports. 1999;85:121–130. doi: 10.2466/pr0.1999.85.1.121. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patents with multiple sclerosis and systemic Lupus erythematosus. Archives of Neurology. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Lu AW, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- Pepper CM, Krupp LB, Friedberg F, Doscher C, Coyle PK. A comparison of neuropsychiatric characteristics in chronic fatigue syndrome, multiple sclerosis, and major depression. The Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5:200–205. doi: 10.1176/jnp.5.2.200. [DOI] [PubMed] [Google Scholar]
- Pesek J, Jason L, Taylor R. An empirical investigation of the envelope theory. Journal of Human Behavior in the Social Environment. 2000;3:59–77. [Google Scholar]
- Roesner N, Porter N, Holiday P, Delucca G, Robinson A, Walano N, et al. An alternative way to help people with ME/CFS. Bulletin of IACFS/ME. 2008;16:12–21. [Google Scholar]
- Shlaes JL, Jason LA. A buddy/mentor program for PWCs. The CFIDS Chronicle, Winter. 1996:21–25. [Google Scholar]
- Taylor RR, Jason LA, Torres A. Fatigue rating scales: An empirical comparison. Psychological Medicine. 2000;30:849–856. doi: 10.1017/s0033291799002500. [DOI] [PubMed] [Google Scholar]
- Van Houdenhove BV, Van Den Eede F, Luyten P. Does hypothalamic-pituitary-adrenal axis hypofunction in chronic fatigue syndrome reflect a ‘crash’ in the stress system? Medical Hypothesis. 2009;72:701–705. doi: 10.1016/j.mehy.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Ware J, Sherbourne C. The MOS 36-item short-from health survey: Conceptual framework and item selection. Medical Care. 1992:473–483. [PubMed] [Google Scholar]
- White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R. Protocol for the PACE trial: A randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise, as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy. BMC Neurology. 2007;7:6. doi: 10.1186/1471-2377-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyller VB, Eriksen HR, Malterud K. Can sustained arousal explain the chronic fatigue syndrome. Behavioral and Brain Functions. 2009;5:10. doi: 10.1186/1744-9081-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatanabe Y, Evengard B, Natelson BH, Jason LA, Kuratsune H. Fatigue science for human health. Tokyo: Springer; 2008. [Google Scholar]