Loss of neurons is typically the readout used for studies of neuropathological conditions ranging from stroke and traumatic brain injury to adult-onset neurodegenerative diseases such as Alzheimer’s and Parkinson’s[1]. One result is that studies of neurodegenerative disease often become descriptions of progressive neuronal cell death, and many therapeutic strategies focus on preventing cell death [2, 3]. Unfortunately, successes in reducing or preventing neuronal cell death have not translated to effective treatments for any neurodegenerative disease [4–6]. The problem is that neurodegenerative diseases are not caused by cell death signaling pathways. Although apoptotic pathways will eventually be activated and neurons lost as the disease progresses, the clinical symptoms of neurodegenerative diseases reflect abnormalities in synaptic function and the loss of synaptic connections, rather than the loss of neurons. Conflating the shared molecular mechanisms of cell death with unique disease-specific pathogenic mechanisms of neurodegeneration may be interfering with the search for effective therapies.
Although the final common steps in cell death pathways (i.e., nuclear fragmentation, etc.) are shared between neuronal and nonneuronal cells[7, 8], the sequence of events leading to cell death in neurons may include steps not found in nonneuronal cells[9]. Neuron-specific features may involve an extended period of time, often months or years, in contrast with the rapid progression of apoptosis observed in non-neuronal cells[10]. These initial neuron-specific steps manifest as a loss of synaptic function, a process initiated at a considerable distance from the neuronal perikaryon. Complicating the situation further, components of cell death signaling pathways can play roles in neurons unrelated to cell death, such as regulating aspects of synaptic function and plasticity[11–13].
Neuron-specific cell death features
A brief consideration of some unique aspects of cell death in neurons may be useful. Both extrinsic and mitochondrial-based pathways of apoptotic cell death can be observed in neurons[8, 10, 14], depending on the triggering stimulus. The initiators of cell death may be as diverse as failure to establish trophic relationships with a target cell, stroke and traumatic brain injury, or neurodegenerative disease, regardless of whether they are environmental, familial or sporadic in origin. Both apoptotic and necrotic cell death may be observed in neurons [8], but here we will focus on apoptotic mechanisms. Perhaps the most obvious difference between neuronal and non-neuronal cell death is the time that takes to complete apoptosis. The final stages of apoptotic cell death are rapid and similar in both neuronal and nonneuronal cells. However, in neurons the early stages begin in the distal axon and presynaptic terminals and progress slowly. Months or even years may elapse between the first decrements in neuronal function and the initiation of a final apoptotic sequence.
The ways in which cell death pathways are managed in neurons differ from nonneuronal cells. The enormous size and complex functional architecture of neurons requires additional layers of complexity, which are seen both in development and in pathological states. Cell death plays a critical role in sculpting the functional architecture of the nervous system during development [9, 15]. During development, programmed cell death via apoptosis assures that appropriate matches exist between neurons and their target cells. Nuclear changes are late events and the first steps leading toward developmental neuronal death typically occur in synaptic and axonal compartments. If these steps are limited in scope and extent, synaptic and axonal changes might lead to pruning of nonproductive synaptic contacts or axonal branches[9]. Such pruning is essential for establishing sensory maps in the cortex such as those corresponding to visual space [16]. The atrophy of an axonal branch follows shutdown of synaptic function and degeneration of the presynaptic terminal, with subsequent degeneration of the distal axon. The distal axonopathy seen in early stages of neurodegenerative disease follows a similar sequence but involves a most or all of the presynaptic terminals in an affected neuron. The result is a classic dying-back neuropathy[17–19].
The sequence of events associated with loss of presynaptic function and degeneration of distal axons may be prolonged for years after the first decrement in synaptic function take place [10, 20]. This is in large part due to the size and complex morphology of neurons, which create an abundance of heterogenous microdomains and provide new arenas for cell death-related molecular components to perform diverse functions. In some neurons, activation of kinases or caspases in a presynaptic compartment may occur a meter or more away from the nucleus. This topographic separation prevents the interaction with key downstream targets required for the progression and execution of apoptosis. Under these conditions, the critical apoptosis executioner caspase-3[11, 12, 21] and pro-apoptotic kinases like JNK [22–25] may be activated in a pre- or postsynaptic compartment, but apoptosis does not result. A growing body of evidence indicates that some components of cell death pathways may play important roles in normal neuronal functions like the synaptic plasticity that underlies learning and memory[11–13].
However, a failure in the trophic relationships of neurons with target cells does trigger apoptosis in neurons[26–28]. This is due to the uniquely symbiotic relationship between a given neuron and the hundreds or thousands of other cells that interact with that neuron. Important interactions are associated with presynaptic and postsynaptic specializations as well as with diverse glial cells. Target cells (i.e., muscle cells, other neurons, etc.) act as a source of neurotrophins, which in turn promote survival of neurons innervating these targets. The neuron integrates these various relationships in ways that are incompletely understood. This integration is essential not only for neuronal survival, but also for determining size, morphology and various functional aspects of the neuron. Moreover, these trophic relationships are tightly tied to neuronal activity, so only productive relationships are preserved[29, 30].
Synaptic function and neurotrophin-dependent neuronal survival
How then does a neuron progress to apoptosis either in development or in slowly progressing adult-onset neurodegenerative diseases like Alzheimer’s, Parkinson’s and Amyotrophic Lateral Sclerosis? In both, the apoptotic process occurs as a dying-back neuropathy or distal axonopathy[31], following a characteristic sequence of events (Figure 1). Lack of effective synaptic activity coupling neurotransmitter release to neurotrophin uptake will initiate degeneration of the presynaptic terminal[9, 17]. Loss of synaptic activity may result from reductions in critical components for neurotransmitter release in the presynaptic compartment or by failure of appropriate responses (i.e., trophic support) from target cells. In disease states, deficiencies in presynaptic components may be more common, while during development inadequate neurotrophin supplies from nonresponsive targets is more likely. For example, blockade of neurotrophin uptake during development interferes with the establishment of ocular dominance columns[32]. Similarly, both uptake and retrograde transport of neurotrophins by sensory neurons in dorsal root ganglia is needed to prevent cell death of these sensory neurons[33]. Survival of motor neurons in the spinal cord is also dependent on neuronal activity[34] and activity dependent release of neurotrophins is critical for their biological actions[35]. Although a full review of the actions of neurotrophins on synaptic plasticity[36, 37] and neuronal survival is beyond the scope of this perspective, a number of reviews have considered this issue[35, 38–41]. Regardless, both formation and maintenance of neuronal connections are closely linked to establishment of trophic relationships with target cells.
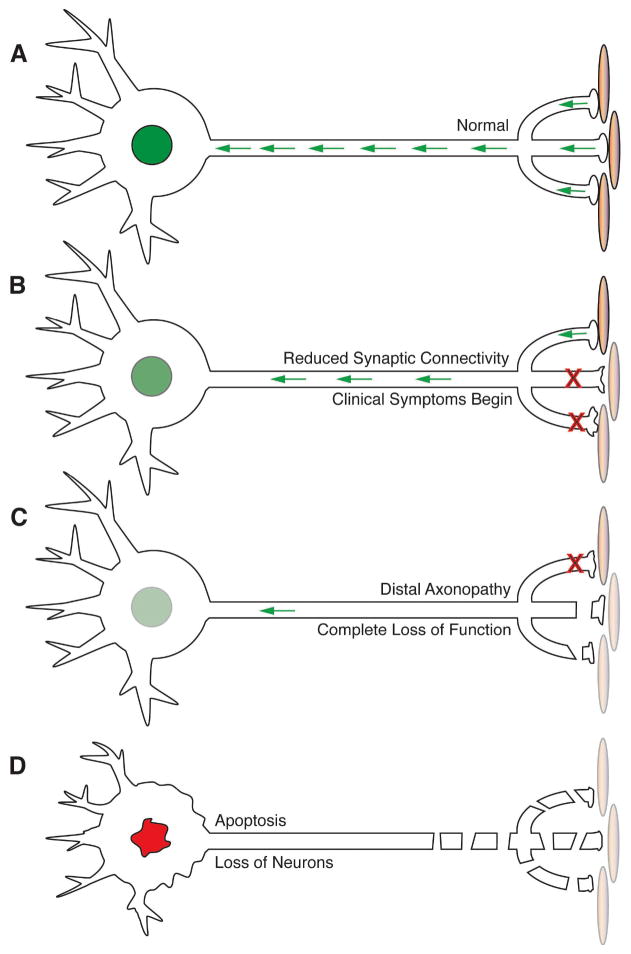
Figure 1. Stages in neuronal death as a dying back neuropathy.
A) In the intact nervous system, neurons and targets are well matched, so activity and neurotrophic support (green arrows) are well-coordinated. B) When the activity at a given synapse is compromised, then presynaptic terminals are retracted and neurotrophin return is reduced. Changes in gene expression may occur, but the perikaryon is still intact at this stage. C) When the number of functional synapses falls below a critical threshold, the remaining presynaptic terminals are typically shut down and retracted. Consequently, target-derived neurotrophin supplies are no longer sufficient to maintain the distal axon or to sustain neuronal viability. D) As the distal axon atrophies, the neuronal perikaryon begins to exhibit the characteristics of classical apoptotic cell death, including pycnotic nuclei, shrinkage of cell body, TUNEL staining and blebbing of the plasma membrane. The time from the earliest changes in synaptic function seen in B to the clear activation of apoptotic pathways in D may be months or years.
In a healthy connection, the release of neurotrophins from target cells leads to binding of neurotrophins to neuronal receptors, uptake of receptor-neurotrophin complexes into the neuron, and eventually transport of these complexes to the distant neuronal cell body. There is a close relationship between synaptic function and neurotrophin support, so neurotrophin uptake correlates with the release of neurotransmitter from the synapse [42, 43]. In turn, elevated neurotransmitter levels at the synapse stimulates release of neurotrophins from the target cell and helps maintain postsynaptic structures as well as enhancing endocytosis in the presynaptic terminal[43]. For neurotrophic influences to be effective, receptors with bound ligand must be endocytosed and processed into a signaling endosome for return to the cell body[44–47]. Both local and perikaryal signaling by neurotrophin receptors are important in the maintenance of presynaptic function [29, 30]. An appropriate supply of synaptic components and neurotrophin receptors from the neuronal perikaryon to synapses and the corresponding return of signaling endosomes to the neuronal cell body are both essential processes for neuronal survival. From these observations, it is clear that the intracellular trafficking of synaptic and trophic factor components along axons plays a critical role in maintaining neuronal viability.
Axonal Transport: A vital supply line for synapses
As discussed above, synaptic activity and neurotrophic factor support represent critical cellular processes underlying the neuronal survival. An understanding of apoptotic cell death in neurons thus requires an analysis of molecular mechanisms underlying maintenance and functionality of synapses. Neurons are uniquely dependent on intracellular transport of proteins and organelles[18, 48]. Human neurons may be a meter or more in length and even small mammalian neurons are significantly larger than nonneuronal cells. Their large size imposes certain demands on a neuron, but the polarization and complexity of neuronal morphologies creates further challenges. A typical neuron has thousands of pre- and post-synaptic specializations. Some of these may be maintained for decades and others must be rapidly adjusted in response to local signals. The ability of synapses to respond to localized signals requires alterations in their biochemical composition. Despite periodic reports that small amounts of protein synthesis may occur in axons (particularly growing axons), >99.9% of the proteins in axonal domains and >80% of the proteins in dendrites of mature neurons are synthesized in cell bodies and transported to sites of utilization [48]. As a result, neuronal cells face daunting logistical challenges in the coordinate synthesis, packaging, transport and targeting of proteins to pre and postsynaptic compartments. To fullfill this need, neurons must continually synthesize, transport and deliver remarkable amounts of membrane proteins, mitochondria, cytoskeletal elements, and cytoplasmic enzymes via axonal transport mechanisms [48]. This dependence of axons and synapses on delivery of material from the cell body was recognized as early as Ramon y Cajal[49], although the responsible molecular motors were not discovered until the 1980’s[50]. At that time, advances in digital video microscopy methods permitted real time visualization of individual membrane bounded organelles (MBOs) moving along single microtubules (MTs) in living cells for the first time. Application of video microscopic methods to axoplasm isolated from squid giant axons[51] produced real time images of MBOs translocating on MTs and allowed biochemical and pharmacological characterization of fast axonal transport (FAT)[51–56]. Studies of FAT in axoplasm led to the discovery of conventional kinesin, a new class of molecular motor that defined a superfamily of proteins [55–58]. Subsequently, a cytoplasmic form of dynein [59] was identified. Although there are exceptions, most kinesins move toward the plus end of MTs and dyneins are minus end motors. The polarized distribution of MTs in the axon allows for kinesins to move MBOs from the cell body towards the cell periphery in the anterograde direction and for dyneins to move MBOs from the cell periphery towards the cell body in the retrograde direction [60, 61].
Cumulative evidence indicates that conventional kinesin is the main molecular motor involved in the anterograde FAT of various MBOs, including mitochondria, synaptic vesicles, and plasma membrane components. Signaling complexes (i.e., activated neurotrophin receptors) and MBOs carrying degradation products (i.e., lysosomes and multivesicular bodies) are transported retrogradely to the neuronal cell body by the multi-subunit molecular motor cytoplasmic dynein [44, 50]. However, the complexity of neuronal cell biology has also raised the issue of differential regulation of kinesins and dyneins [18, 62]. Coordination of kinesin and dynein-based FAT represents a critical component underlying synaptic function and plasticity [63]. As discussed below, an establishment of functional relationships between synaptic activity, trophic support and axonal transport has shed important insights on the mechanisms underlying neuronal dysfunction and death in various human neurodegenerative diseases.
Axonal transport and Dysferopathies
Adult-onset neurodegenerative diseases are among the most difficult and puzzling disorders of the nervous system. Genes associated with these diseases have been identified and characterization of pathogenic mutations constituted major breakthroughs. However, identification of mutant genes often failed to illuminate specific pathogenic mechanisms. Biological roles for the associated gene product were often not apparent. Some diseases were associated with multiple mutations in a given gene or with mutations in different unrelated genes that all resulted in comparable pathologies. Many diseases existed in both familial and sporadic forms with indistinguishable clinical presentation. Moreover, a number of these mutant proteins were expressed in a variety of neuronal and nonneuronal cells, but only specific neuronal populations would be affected. Few of the identified mutations explained either the unique vulnerability of neurons in these diseases, or why affected neurons functioned normally for decades before appearance of pathology. However, one class of neurodegeneration-associated genes were illuminating
Recently, evidence that alteration in motor function may underlie some neuropathologies has accumulated [18, 62, 64–67], and these typically manifest as neurodegenerative diseases with the features of a dying back neuropathy[19]. For example, loss of function mutations leading to a 50% reduction in the kinesin-1 isoform kinesin-1A (representing approximately 10% of total kinesin-1 in motor neurons) leads to a form of spastic paraplegia[68, 69], a disease involving gradual degeneration of upper motor neurons. Similarly, mutations in the cytoplasmic dynein heavy chain subunit [67, 70] or dynactin[71] result in late onset neurodegeneration of specific neuronal populations. Curiously, some mutations in dynein heavy chain lead to motor neuron degeneration[67] while other mutations produce degeneration of sensory neurons[70]. Although mutations in motor proteins are rare and can account for only a small fraction of neurodegenerative diseases, recent evidence indicates that FAT may be affected in a much larger fraction of neurodegenerative diseases. These alterations occurred through changes in the activity of protein kinases involved in regulation of FAT [19]. Diseases that involve compromises in FAT as an intrinsic element in their pathogenesis may be categorized as dysferopathies (from the Greek “fero” meaning to transport or carry)[19, 72].
The complexity of neuronal cell biology raised the issue of differential regulation of kinesins and dyneins [18, 62]. Altered protein phosphorylation is a common feature of neurodegenerative diseases, and several kinase and phosphatase activities have been implicated in the regulation of FAT through phosphorylation of motor protein subunits (reviewed by Morfini et al[18, 62]). These kinase pathways affect a variety of cellular activities, including pro-apoptotic pathways. However, in cases where adult-onset, slowly progressive neurodegeneration is observed, compromised FAT is likely to be a primary lesion leading to loss of neuronal connectivity and eventually to neuronal cell death. Significantly, kinases activated in some of these dysferopathies include ones with pro-apoptotic activity, including JNK, P38, PKC, and GSK3β[73–79].
For example, Alzheimer’s disease neurons with familial Alzheimer’s disease mutations in presenilin-1 (PS1) increased activity of GSK-3β, a regulator of kinesin-based motility in neurons[80] and a facilitator of apoptosis[73]. Analysis of FAT in PS-1 mutant neurons showed a 20%–30% reduction in kinesin-based motility[81]. The tau filaments present in the neurofibrillary tangles characteristic of both familial and sporadic Alzheimer’s brains[82] also activate GSK3β in neurons and affect FAT[83]. Subsequently, oligomeric forms of the Aβ peptide associated with the amyloid characteristic of Alzheimer’s was found to activate casein kinase 2, which also inhibits FAT[84] and leads to failure of synaptic transmission[85]. In some cases, CK2 may be antiapoptotic in some cellular contexts[79], but in Alzheimer’s elevated CK2 activity has the paradoxical effect in shutting down FAT and hastening loss of synaptic connectivity.
Similarly, polyQ expansion diseases like Huntington’s disease and spinal bulbar muscular atrophy lead to activation of the stress activated protein kinase JNK3, which phosphorylates kinesin and reduces FAT[86, 87]. JNK family kinases are well known pro-apoptotic kinases, although they can also be protective in some situations[77, 78]. In neurons, this dual role may depend on different JNK isoforms, because JNK1 activity appears to be essential and constitutive, while stressors activate JNK3 (a neuron-specific JNK) and neuronal damage [88]. Significantly, JNK3 inhibits FAT, while JNK1 does not[87]. Finally, exposure to certain toxic agents like MPTP lead to a form of Parkinson’s disease[89, 90]. MPTP and its metabolites activate caspase 3[72, 91], but this occurs in primarily in axons and terminals where dopamine transporters are enriched. When caspase 3 activated in the axon or the terminal, it cleaves and activates PKCδ[72, 92], which in turn alters FAT and leads to failure of neurotransmission[72, 93]. Thus, activation of pro-apoptotic signals in neurons can lead to deficits in FAT that eventually result in loss of synaptic function and distal axonopathy.
Summary
Although neuronal cell death through apoptotic pathways represents a common feature of dysferopathies, the canonical apoptotic changes familiar from non-neuronal cells are late events. Loss of neuronal function occurs at a much early time, when synaptic-based neuronal connectivity fails. In this context, apoptotic pathways may normally serve a clean-up role, rather than a pathogenic one. Reframing the consideration of cell death in the nervous system to include the early stages of axonal degeneration provides a better understanding of the roles played by various apoptotic signaling pathways in neurodegenerative diseases. Focusing on disease-specific mechanisms that initiate the sequence that eventually leads to neuronal loss should facilitate development of therapies that preserve neuronal function as well as neuronal numbers.
Acknowledgments
This work was supported by grants from NINDS (NS23868) and MDA to S.B, and from the Huntington’s Disease Society of America to (G.M.).
Bibliography
- 1.Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Lipton SA. Cell death: protein misfolding and neurodegenerative diseases. Apoptosis. 2009;14:455–468. doi: 10.1007/s10495-008-0301-y. [DOI] [PubMed] [Google Scholar]
- 3.Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:676–687. doi: 10.1016/j.bbadis.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiesa R, Piccardo P, Dossena S, Nowoslawski L, Roth KA, Ghetti B, Harris DA. Bax deletion prevents neuronal loss but not neurological symptoms in a transgenic model of inherited prion disease. Proc Natl Acad Sci U S A. 2005;102:238–243. doi: 10.1073/pnas.0406173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waldmeier P, Bozyczko-Coyne D, Williams M, Vaught JL. Recent clinical failures in Parkinson’s disease with apoptosis inhibitors underline the need for a paradigm shift in drug discovery for neurodegenerative diseases. Biochem Pharmacol. 2006;72:1197–1206. doi: 10.1016/j.bcp.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 7.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 8.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger KA. Challenges in neuronal apoptosis. Curr Alzheimer Res. 2006;3:377–391. doi: 10.2174/156720506778249434. [DOI] [PubMed] [Google Scholar]
- 11.Bravarenko NI, Onufriev MV, Stepanichev MY, Ierusalimsky VN, Balaban PM, Gulyaeva NV. Caspase-like activity is essential for long-term synaptic plasticity in the terrestrial snail Helix. Eur J Neurosci. 2006;23:129–140. doi: 10.1111/j.1460-9568.2005.04549.x. [DOI] [PubMed] [Google Scholar]
- 12.Kudryashova IV, Onufriev MV, Kudryashov IE, Gulyaeva NV. Caspase-3 activity in hippocampal slices reflects changes in synaptic plasticity. Neurosci Behav Physiol. 2009;39:13–20. doi: 10.1007/s11055-008-9089-z. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP, Duan W. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- 14.Mattson MP, Bazan NG. Apoptosis and Necrosis. In: Siegel G, Albers RW, Brady ST, Price D, editors. Basic Neurochemistry. 7. Boston, MA: Elsevier Academic Press; 2006. pp. 603–615. [Google Scholar]
- 15.Burek MJ, Oppenheim RW. Programmed cell death in the developing nervous system. Brain Pathol. 1996;6:427–446. doi: 10.1111/j.1750-3639.1996.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 16.LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 17.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 18.Morfini G, Pigino G, Brady ST. Polyglutamine Expansion Diseases: Failing to Deliver. Trends Molec Med. 2005;11:64–70. doi: 10.1016/j.molmed.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH, Jr, Brown H, Tiwari A, Hayward L, et al. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–12786. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu X, Raina AK, Perry G, Smith MA. Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res. 2006;3:393–396. doi: 10.2174/156720506778249470. [DOI] [PubMed] [Google Scholar]
- 21.Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- 22.Ahn SM, Choe ES. Alterations in GluR2 AMPA receptor phosphorylation at serine 880 following group I metabotropic glutamate receptor stimulation in the rat dorsal striatum. J Neurosci Res. 2009 doi: 10.1002/jnr.22275. [DOI] [PubMed] [Google Scholar]
- 23.Jong YJ, Kumar V, O’Malley KL. Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J Biol Chem. 2009 doi: 10.1074/jbc.M109.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 25.Yue X, Dreyfus C, Kong TA, Zhou R. A subset of signal transduction pathways is required for hippocampal growth cone collapse induced by ephrin-A5. Dev Neurobiol. 2008;68:1269–1286. doi: 10.1002/dneu.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochem Soc Trans. 2007;35:424–427. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- 27.Blochl A, Blochl R. A cell-biological model of p75NTR signaling. J Neurochem. 2007;102:289–305. doi: 10.1111/j.1471-4159.2007.04496.x. [DOI] [PubMed] [Google Scholar]
- 28.Nykjaer A, Willnow TE, Petersen CM. p75NTR--live or let die. Curr Opin Neurobiol. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Barker PA, Hussain NK, McPherson PS. Retrograde signaling by the neurotrophins follows a well-worn trk. Trends Neurosci. 2002;25:379–381. doi: 10.1016/s0166-2236(02)02199-9. [DOI] [PubMed] [Google Scholar]
- 30.Weible MW, 2nd, Hendry IA. What is the importance of multivesicular bodies in retrograde axonal transport in vivo? J Neurobiol. 2004;58:230–243. doi: 10.1002/neu.10318. [DOI] [PubMed] [Google Scholar]
- 31.Conforti L, Adalbert R, Coleman MP. Neuronal death: where does the end begin? Trends Neurosci. 2007;30:159–166. doi: 10.1016/j.tins.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 33.Hamburger V, Brunso-Bechtold JK, Yip JW. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci. 1981;1:60–71. doi: 10.1523/JNEUROSCI.01-01-00060.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittman R, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979;187:425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- 35.Kuczewski N, Porcher C, Lessmann V, Medina I, Gaiarsa JL. Activity-dependent dendritic release of BDNF and biological consequences. Mol Neurobiol. 2009;39:37–49. doi: 10.1007/s12035-009-8050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 37.Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2008;153(Suppl 1):S310–324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 40.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator: rapid effects of BDNF on ion channels. Bioessays. 2004;26:1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt JT. Activity-driven sharpening of the retinotectal projection: the search for retrograde synaptic signaling pathways. J Neurobiol. 2004;59:114–133. doi: 10.1002/neu.10343. [DOI] [PubMed] [Google Scholar]
- 43.Lim KC, Lim ST, Federoff HJ. Neurotrophin secretory pathways and synaptic plasticity. Neurobiol Aging. 2003;24:1135–1145. doi: 10.1016/j.neurobiolaging.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Cosker KE, Courchesne SL, Segal RA. Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol. 2008;18:270–275. doi: 10.1016/j.conb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009;72:46–55. doi: 10.1016/j.jprot.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68. doi: 10.1016/s0896-6273(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 48.Brady ST. Axonal Dynamics and Regeneration. In: Gorio A, editor. Neuroregeneration. New York City, NY: Raven Press; 1993. pp. 7–36. [Google Scholar]
- 49.Ramon y Cajal S. Degeneration and Regeneration in the Nervous System. 1992. Oxford, England: Oxford University Press; 1928. [Google Scholar]
- 50.Hirokawa N, Takemura R. Molecular motors in neuronal development, intracellular transport and diseases. Curr Opin Neurobiol. 2004;14:564–573. doi: 10.1016/j.conb.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Brady ST, Lasek RJ, Allen RD. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982;218:1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- 52.Brady ST, Lasek RJ, Allen RD, Yin H, Stossell T. Gelsolin inhibition of fast axonal transport indicates a requirement for microfilaments. Nature. 1984;310:56–58. doi: 10.1038/310056a0. [DOI] [PubMed] [Google Scholar]
- 53.Brady ST, Lasek RJ, Allen RD. Video microscopy of fast axonal transport in isolated axoplasm: A new model for study of molecular mechanisms. Cell Motility. 1985;5:81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- 54.Brady ST. Fast axonal transport in isolated axoplasm from the squid giant axon. In: Smith RS, Bisby M, editors. Axonal Transport. Vol. 25. New York City: Alan R. Liss; 1987. pp. 113–137. [Google Scholar]
- 55.Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- 56.Lasek RJ, Brady ST. Adenylyl imidodiphosphate (AMPPNP), a nonhydrolyzable analogue of ATP, produces a stable intermediate in the motility cycle of fast axonal transport. Biol Bull. 1984;167:503. [Google Scholar]
- 57.Lasek RJ, Brady ST. Attachment of transported vesicles to microtubules in axoplasm is facilitated by AMP-PNP. Nature. 1985;316:645–647. doi: 10.1038/316645a0. [DOI] [PubMed] [Google Scholar]
- 58.Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shpetner HS, Paschal BM, Vallee RB. Characterization of the microtubule-activated ATPase of brain cytoplasmic dynein (MAP1C) J Cell Biol. 1988;107:1001–1009. doi: 10.1083/jcb.107.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 61.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 62.Morfini G, Pigino G, Beffert U, Busciglio J, Brady ST. Fast axonal transport misregulation and Alzheimer’s disease. Neuromolecular Med. 2002;2:89–99. doi: 10.1385/NMM:2:2:089. [DOI] [PubMed] [Google Scholar]
- 63.Puthanveettil SV, Monje FJ, Miniaci MC, Choi YB, Karl KA, Khandros E, Gawinowicz MA, Sheetz MP, Kandel ER. A new component in synaptic plasticity: upregulation of kinesin in the neurons of the gill-withdrawal reflex. Cell. 2008;135:960–973. doi: 10.1016/j.cell.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirokawa N, Takemura R. Biochemical and molecular characterization of diseases linked to motor proteins. Trends Biochem Sci. 2003;28:558–565. doi: 10.1016/j.tibs.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Holzbaur EL. Motor neurons rely on motor proteins. Trends Cell Biol. 2004;14:233–240. doi: 10.1016/j.tcb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 67.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, et al. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 68.Fichera M, Lo Giudice M, Falco M, Sturnio M, Amata S, Calabrese O, Bigoni S, Calzolari E, Neri M. Evidence of kinesin heavy chain (KIF5A) involvement in pure hereditary spastic paraplegia. Neurology. 2004;63:1108–1110. doi: 10.1212/01.wnl.0000138731.60693.d2. [DOI] [PubMed] [Google Scholar]
- 69.Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, Graham FL, Gaskell PC, Dearlove A, Pericak-Vance MA, et al. A Kinesin Heavy Chain (KIF5A) Mutation in Hereditary Spastic Paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–14524. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, Kennedy WR, Wendelschafer-Crabb G, Vortmeyer A, Powers R, et al. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol. 2005;57:687–694. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morfini G, Pigino G, Opalach K, Serulle Y, Moreira JE, Sugimori M, Llinas RR, Brady ST. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci U S A. 2007;104:2442–2447. doi: 10.1073/pnas.0611231104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X, Bijur GN, Jope RS. Glycogen synthase kinase-3beta, mood stabilizers, and neuroprotection. Bipolar Disord. 2002;4:137–144. doi: 10.1034/j.1399-5618.2002.40201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- 75.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weishaupt JH, Neusch C, Bahr M. Cyclin-dependent kinase 5 (CDK5) and neuronal cell death. Cell Tissue Res. 2003;312:1–8. doi: 10.1007/s00441-003-0703-7. [DOI] [PubMed] [Google Scholar]
- 77.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 78.Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591–598. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 80.Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen Synthase Kinase 3 Phosphorylates Kinesin Light Chains and Negatively Regulates Kinesin-based Motility. EMBO Journal. 2002;23:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pigino G, Morfini G, Mattson MP, Brady ST, Busciglio J. Alzheimer’s Presenilin 1 Mutations Impair Kinesin-Based Axonal Transport. J Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer’s disease. Biochim Biophys Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 83.Lapointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, Brady ST. The amino terminus of tau inhibits kinesin-dependent axonal transport: Implications for filament toxicity. J Neurosci Res. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady ST. Disruption of Fast Axonal Transport Is a Pathogenic Mechanism for Intraneuronal Amyloid Beta. Proc Natl Acad Sci U S A. 2009;106:5907–5912. doi: 10.1073/pnas.0901229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moreno HH, Yu E, Pigino G, Hernandez I, Kim N, Moreira JE, Sugimori M, Llinas R. Synaptic transmission block by presynaptic injection of oligomeric amyloid beta. Proc Natl Acad Sci U S A. 2009;106:5901–5906. doi: 10.1073/pnas.0900944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady ST. JNK Mediates Pathogenic Effects of Polyglutamine-expanded Androgen Receptor on Fast Axonal Transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- 87.Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coffey ET, Smiciene G, Hongisto V, Cao J, Brecht S, Herdegen T, Courtney MJ. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J Neurosci. 2002;22:4335–4345. doi: 10.1523/JNEUROSCI.22-11-04335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Speciale SG. MPTP: insights into parkinsonian neurodegeneration. Neurotoxicol Teratol. 2002;24:607–620. doi: 10.1016/s0892-0362(02)00222-2. [DOI] [PubMed] [Google Scholar]
- 91.Turmel H, Hartmann A, Parain K, Douhou A, Srinivasan A, Agid Y, Hirsch EC. Caspase-3 activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Mov Disord. 2001;16:185–189. doi: 10.1002/mds.1037. [DOI] [PubMed] [Google Scholar]
- 92.Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- 93.Serulle Y, Morfini G, Pigino G, Moreira JE, Sugimori M, Brady ST, Llinas RR. 1-Methyl-4-phenylpyridinium induces synaptic dysfunction through a pathway involving caspase and PKC{delta} enzymatic activities. Proc Natl Acad Sci U S A. 2007;104:2437–2441. doi: 10.1073/pnas.0611227104. [DOI] [PMC free article] [PubMed] [Google Scholar]