Abstract
Dietary restraint theoretically increases risk for binge eating, but prospective and experimental studies have produced contradictory findings, apparently because dietary restraint scales do not identify individuals who are reducing caloric intake. Yet, experimentally manipulated caloric deprivation increases responsivity of brain regions implicated in attention and reward to food images, which may contribute to binge eating. We tested whether self-imposed acute and longer-term caloric restriction increases responsivity of attention and reward regions to images, anticipated receipt, and receipt of palatable food using functional magnetic resonance imaging among female and male adolescents (Study 1 N = 34; Study 2 N = 51/81). Duration of acute caloric deprivation correlated positively with activation in regions implicated in attention, reward, and motivation in response to images, anticipated receipt, and receipt of palatable food (e.g., anterior cingulate cortex, orbitofrontal cortex, putamen, and precentral gyrus respectively). Youth in a longer-term negative energy balance likewise showed greater activation in attention (anterior cingulate cortex, ventral medial prefrontal cortex), visual processing (superior visual cortex), reward (caudate) and memory (hippocampus) regions in response to receipt and anticipated receipt of palatable food relative to those in neutral or positive energy balance. Results confirm that self-imposed caloric deprivation increases responsivity of attention, reward, and motivation regions to food, which may explain why caloric deprivation weight loss diets typically do not produce lasting weight loss.
Keywords: dietary restraint, caloric deprivation, negative energy balance, reward, attention
1. Introduction
Theorists posit that dietary restraint increases risk for onset and maintenance of binge eating and bulimia nervosa (Fairburn, 1997; Polivy & Herman, 1985). Dieting refers to intentional and sustained restriction of caloric intake for the purposes of weight loss or maintenance. Dietary restriction must result in a negative energy balance for weight loss or a balance between intake and output for weight maintenance. Polivy and Herman (1985) argue that dieters’ chronic hunger increases the risk for binge eating and that a reliance on cognitive control over eating leaves dieters vulnerable to uncontrolled eating when these cognitive processes are disrupted. It is vital to investigate the potential adverse effects of dieting because approximately 50% of young adults report engaging in dieting behaviors (Field, Haines, Rosner, & Willett, 2009).
Consistent with dietary restraint theory, females with high versus low scores on dietary restraint scales are at greater risk for future onset of binge eating, bulimic symptoms, and bulimia nervosa (Neumark-Sztainer et al., 2006; Killen et al., 1996; Stice, Davis, Miller, & Marti, 2008a) and increases in bulimic symptoms (Johnson & Wardle, 2005; Stice, 2001; Wertheim, Koerner, & Paxton, 2001). However, randomized trials find that assignment to energy-deficit weight loss interventions versus waitlist control conditions results in significant decreases in binge eating for healthy young adults (Groesz & Stice, 2007; Presnell & Stice, 2003), overweight adults (Goodrick, Poston, Kimball, Reeves & Foreyt, 1998; Reeves et al., 2001), and young women with bulimia nervosa (Burton & Stice, 2006). It appears that these inconsistent findings emerged because the prospective studies used dietary restraint scales that do not identify individuals who are reducing caloric intake, whereas the experiments confirmed that participants entered an energy-deficit diet that produced weight loss. Individuals with high versus low scores on various dietary restraint scales do not consume fewer calories according to objective measures of intake during single eating episodes (Hetherington et al., 2000; Ouwens, van Strien, & van der Staak, 2003; Stice, Fisher, & Lowe 2004; Sysko, Walsh, & Wilson, 2007), multiple eating episodes (Jansen et al., 2003; Martin et al., 2005; Rolls et al., 1997; Sysko, Walsh, Schebendach, & Wilson, 2005), and over 2-12 week observation periods (Bathalon et al., 2000; Stice, Cooper, Schoeller, Tappe, & Lowe, 2007; Stice, Sysko, Roberto, & Allison, 2010).
Although these findings raise questions about the veracity of the dietary restraint theory, some individuals may engage in true caloric restriction, which does increase risk for binge eating (Stice et al., 2010). Several findings appear consistent with this possibility. First, rats randomized to extreme caloric deprivation conditions (in which they lost 7% - 20% of their body mass) eat significantly more calories during ad lib feeding and show a preference for high-fat foods immediately after the deprivation period relative to non-deprived rats (Hagan, Chandler, Wauford, Rybak, & Oswald, 2003; Lucas & Sclafani, 1992; Ogawa et al., 2005). Second, humans randomized to periods of caloric deprivation work longer on operant tasks to earn high-calorie snack foods and eat more snack foods than non-deprived controls (Cameron, Goldfield, Cyr, & Doucet, 2008; Epstein et al., 2003; Raynor & Epstein, 2003). Third, functional magnetic resonance imaging (fMRI) experiments indicate that activation in regions that have been implicated in attention (anterior cingulate cortex), reward valuation (amygdala), memory (hippocampus), and homeostatic feeding (hypothalamus) was significantly greater in response to pictures of palatable foods versus non-food control images after caloric deprivation versus a no deprivation condition (Fuhrer et al., 2008; LaBar et al., 2001; Leidy et al., 2011), though null effects also emerged (Siep et al., 2009; Uher et al., 2006), potentially because of the small samples used in these studies (Ns = 10 to 20). One experiment found a greater response in attention (posterior cingulate cortex) and reward (amygdala, orbitofrontal cortex [OFC], insula, and striatum) regions to pictures of high-calorie versus low-calorie foods after a period of caloric deprivation versus a non-deprivation condition (Goldstone et al., 2009), suggesting that caloric deprivation increases the reward value of high-calorie foods more than low-calorie foods. Another experiment found that there was greater response in the primary gustatory cortex (anterior insula, frontal operculum) and regions associated with reward (dorsolateral prefrontal cortex, medial prefrontal cortex) in response to intake of food (chocolate milk, chicken soup) after caloric deprivation versus a non-deprivation condition (Uher et al., 2006).
Collectively, data suggest that true caloric restriction may increase the reward value of food, such that the longer the caloric deprivation, the greater the activation of attention and reward regions in response to food, which putatively increases the likelihood of food intake. The fMRI experiments in which participants were randomly assigned to a period of caloric deprivation or a non-deprivation condition have strong internal validity, but limited external validity because participants did not voluntarily elect to engage in caloric restriction, as occurs in real-world dieters. Further, the duration of caloric deprivation ranged up to 24 hours, which is presumably much longer than most dieters go without eating. Research in which participants decide how long they would calorically restrict themselves before the fMRI scans would have more external validity and would serve as a useful complement to the experimental caloric deprivation studies. Thus, in Study 1 we investigated neural activation in response to pictures of palatable foods, unpalatable foods, and glasses of water among adolescents who varied in degree of caloric restriction before the scan (which ranged from 1 to 16 hours of caloric deprivation). By including both pictures of palatable and unpalatable foods, we were expressly able to determine whether degree of “self-inflicted” caloric deprivation correlates with hyper-responsivity of attention and reward region for palatable versus unpalatable foods. In Study 2 we tested whether the number of hours since last caloric intake (which varied from 3 to 22 hours) correlated with neural activation in response to receipt and anticipated receipt of a palatable food because only one previous study evaluated response to receipt of food (chocolate milk and chicken soup; Uher et al., 2006). Investigating neural responsivity to images of palatable food, anticipated receipt of palatable food, and actual intake of palatable food should provide a more comprehensive description of the impact of caloric restriction. We hypothesized that individuals who had deprived themselves of caloric intake for a longer period of time would show greater activation in regions implicated in attention and reward in response to images, anticipated receipt, and receipt of palatable food.
Another gap in the literature is that few studies have investigated the effects of elective longer-term caloric deprivation; the fMRI experiments described above only investigated the effects of acute caloric deprivation. It is important to investigate responsivity of attention and reward regions to food among individuals who are in a documented negative energy balance state for a prolonged period versus a balanced energy state or a positive energy balance state because dieting efforts are typically several weeks in duration (Williamson, Serdula, Anda, Levy, & Byers, 1992). In response to pictures of palatable foods, individuals who had successfully recovered from obesity showed greater activation in a region associated with inhibitory control (the superior frontal region) than obese and normal weight individuals, greater activation in a region involved in visual attention (middle temporal region) than normal weight individuals, and less activation in a motor readiness region (precentral gyrus) than obese individuals (McCaffery et al., 2009). Thus, in Study 2 we tested the hypothesis that participants in a negative energy balanced state over a 2-week period would show greater activation of attention and reward regions to receipt and anticipated receipt of a palatable food relative to participants who were in an energy balanced state or a positive energy balance state over this period. Participants in Study 2 determined whether they would be in a negative, neutral, or positive energy balance over the 2-week period during which the fMRI scans took place, thereby capturing elective dietary restriction. Another novel feature of these studies is that because we used larger samples (N = 34, 51, & 81) than employed in past studies (N = 10 to 20), we were able to conduct whole-brain analyses, rather than region of interest analyses, which should provide a more comprehensive and reliable index of the effect of elective dietary restriction in neural response to food.
2. Methods
2.1 Participants
In Study 1, participants were 34 healthy female adolescents (M age=15.5 ± 0.9, BMI=24.6 ± 5.6); 2% Asian/Pacific Islanders, 2% African Americans, 86% European Americans, 5% Native Americans, and 5% mixed racial heritage. In Study 2, participants were 162 adolescent males and females (M age=15.3 ± 1.07, BMI=20.8 ± 1.90); 4.1% Hispanic, 0.6% Native American, 0.6% Asian/Pacific Islanders, 76.5 European Americans, and 17.9% mixed racial heritage, however, for the present investigation analyses were performed on the 51 participants for whom we collected data on the degree of caloric deprivation before the scan and the 81 participants that met the weight change criteria described below. Those who reported binge eating or compensatory behaviors in the past 3 months, any use of psychotropic medications or illicit drugs, head injury with a loss of consciousness, or current Axis I psychiatric disorder per Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria (American Psychiatric Assn, 1994) were excluded. Informed consent was obtained from parents and assent from adolescents. The Oregon Research Institute Institutional Review Board approved these studies.
2.2 Anthropometrics and behavioral measures
Height was measured to the nearest millimeter using a direct reading stadiometer with the body positioned such that the heels and buttocks are against the vertical support of the stadiometer. Weight was assessed to the nearest 0.1 kg using digital scales with participants wearing light clothing without shoes or coats. At each anthropomorphic assessment, two measures of height and weight were obtained and averaged. BMI was then calculated as kg/m2. For Study 2, weight was collected twice, 14 days apart. For each anthropometric assessment, participants fasted for 5-15 hours and abstained from exercising for 24 hours prior to weighing. To assess the impact of longer-term energy balance we used weight change over the 2-week period during which the scan occurred to group participants into three a priori groups; those that lost ≥ 1kg (negative energy balance), those that remained within 0.1kg of baseline weight (energy balance), and those that gained ≥ 1kg (positive energy balance) over this period. An additional benefit of these a priori cutoffs is that they resulted in groups of similar size, which increased sensitivity to detecting between group effects. Further, requiring at least +/− 1kg of weight change is well beyond the average change observed in this sample (M = .02 kg. SD = 1.06 kg) and the +/− 1kg cutoff corresponds closely to the SD for weight change in this sample, implying that 68% of the sample showed less than a 1kg change in weight over the 2-week period. This allowed us to contrast the neural response of individuals who were in an objectively measured negative energy balance to those who were in an energy stable or positive energy balance state when the scan took place. The anthropometric assessments took place on separate days than the fMRI scan, although the fMRI scans were within approximately one week of the anthropometric assessments.
In Study 1, immediately prior to the fMRI session, participants reported the number of hours since their last dietary intake (to the nearest quarter hour). Although participants were asked to refrain from eating for 4-6 hours immediately preceding the scan, participants varied in the number of hours since last caloric intake (from 1 to 16 hours), affording an opportunity to test whether a greater number of hours since last caloric intake correlated with neural activation in response to images of palatable foods. In Study 2 we asked participants to consume their regular meals and then complete the scan approximately 5 hours after they last ate (e.g., eat lunch at 12:00 and then complete the scan at 5 PM) in an effort to better standardize caloric deprivation. However, we began to suspect that participants were varying the duration of caloric deprivation despite our efforts and therefore began collecting data on this factor; for the 51 participants for whom we recorded the degree of caloric deprivation, there was still significant variation in the number of hours since last caloric intake before the scan (M hours = 6.9 hours; ± 3.7; range 3 to 22.5).
2.3 Dutch Restrained Eating Scale
The Dutch Restrained Eating Scale (van Strien, Frijters, van Staveren, Defares, & Deurenberg, 1986), which inquires about the frequency of various dietary behaviors designed to produce weight loss and weight maintenance (sample item: Do you deliberately eat less in order not to become too heavy?), was administered in Studies 1 and 2. This scale has shown internal consistency (α’s range from .93 to .95) and temporal reliability (2-week test-retest r = .82; Stice, et al., 2004; van Strien et al., 1986). Although this scale correlates with self-reported caloric intake, like all dietary restraint or dieting scales, it does not correlate with objectively measured caloric intake (e.g., Ouwens et al., 2003; Stice, et al., 2004).
2.4 Study 1: Food Picture fMRI Paradigm
This paradigm was designed to examine blood-oxygen-level-dependent (BOLD) contrast responses to exposure to and imagined intake of palatable foods, unpalatable foods, and glasses of water shown in pictures. Before the scan, participants rated how appetizing they found various foods shown in 103 pictures. Pictures included processed foods varied by type, energy density, and macronutrient content (e.g., cheeseburger, cupcakes, crackers, cereal, grapes, peaches, cauliflower, and eggplant). Each participant was exposed to the 40 pictures of food they rated as the most appetizing and the 40 pictures of food they rated as the least appetizing, as well as 40 pictures of glasses of water. Pictures were presented via a digital projector/reverse screen display system in 2 separate runs. There were 3 events of interest in the paradigm (presented in random order): (1) viewing appetizing food, (2) viewing unappetizing food, and (3) viewing glasses of water. Pictures were presented for 5 seconds using Presentation (Version 9.81, www.neuro-bs.com). A fixation cross was presented for 2 s between each stimulus picture. The contrasts of interest included BOLD responses while viewing pictures of appetizing food versus unappetizing food (appetizing food > unappetizing food) and while viewing pictures appetizing food versus images of glasses of water (appetizing food > water).
2.5 Study 2: Milkshake receipt and anticipated fMRI paradigm
The paradigm used in Study 2 examined BOLD response to receipt and anticipated receipt of a chocolate milkshake and a calorie-free tasteless solution. The milkshake (270 kcals, 13.5g fat, 28g sugar per 150mL) was prepared with 60g of vanilla Häagen-Dazs® ice cream, 80mL of 2% milk, and 15mL of Hershey’s® chocolate syrup. The tasteless solution was designed to mimic the natural taste of saliva to avoid activation of the taste cortex. Stimuli were presented in 5 separate randomized scanning runs. Participants were presented with cartoon pictures of either milkshake or a water glass followed by a jittered time span (1-7 secs) and then administration of the corresponding tastant, another jitter, and subsequently a swallow cue. The jitter allows sampling at multiple points of the hemodynamic response function, thereby increasing design efficiency and decreasing the possible impact of conditioning. Milkshake receipt was followed by a tasteless rinse to cleanse the palate. On 40% of the milkshake and tasteless solution trials, the taste was not delivered following the cue to allow the investigation of the neural response to anticipation of a taste that was not confounded with actual receipt of the taste (unpaired trials). A digital projector/reverse screen display mirror system presented visual stimuli. Tastants were delivered using programmable syringe pumps (insuring consistent timing, delivery and volume) and tubing leading to a manifold, which fit into the participants’ mouths, delivering the taste to a consistent segment of the tongue. To identify brain regions activated in response to palatable food intake, BOLD response was contrasted during receipt of milkshake versus tasteless solution (milkshake receipt > tasteless receipt). The arrival of a taste in the mouth was considered to be receipt. To identify brain regions activated in response to anticipated receipt, BOLD response during presentation of the unpaired cue signaling impending delivery of the milkshake was contrasted with response during presentation of the unpaired cue signaling impending delivery of the tasteless solution (milkshake cue > tasteless cue).
2.6 Imaging acquisition, preprocessing and analysis
A Siemens Allegra 3 Tesla head-only MRI was used for scanning. A birdcage coil acquired data from the entire brain. Functional scans used a T2* weighted gradient single-shot echo planar imaging (EPI) sequence (TE = 30ms, TR = 2000ms, flip angle=80°) with an in plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). Thirty-two 4mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse oblique plane, as determined by the midsagittal section. Prospective acquisition correction (PACE) was applied to adjust slice position and orientation, as well as to re-grid residual volume-to-volume motion in real-time during data acquisition for the purpose of reducing motion-induced effects in Study 2 (Thesen et al., 2000). Participants who moved greater 2mm or 2° in any direction were excluded from analyses. A high-resolution inversion recovery T1 weighted sequence (MP-RAGE; FOV = 256 × 256 mm2, 256 × 256 matrix, thickness = 1.0 mm, slice number ≈ 160) was acquired.
Data were pre-processed and analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, London, England) in MATLAB (Mathworks, Inc., Sherborn, MA). Images were manually reoriented to the AC-PC line and skull stripped. Functional images were then realigned to the mean and both the anatomical and functional images were normalized to the standard Montreal Neurological Institute (MNI) T1 template brain (ICBM152). Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for high-resolution anatomical images. Functional images were smoothed with a 6 mm FWHM isotropic Gaussian kernel. Vectors of the onset time and durations for stimuli were compiled and entered into the design matrix so that responses could be modeled by the canonical hemodynamic response function, as implemented in SPM8. A 128 second high-pass filter was used to remove low-frequency noise and signal drift.
Random effects analyses were used to account for both within-group and within-subject variance. For Study 1, individual’s statistical parametric maps of the (appetizing food > unappetizing food) and (appetizing food > water) contrasts were entered into second-level regression models including the duration since last food intake as a covariate. We conducted parallel regression models for Study 2, in which statistical parametric maps of the milkshake receipt > tasteless receipt and milkshake cue > tasteless cue contrasts were regressed on the hours since last caloric intake. Also in Study 2, statistical parametric maps of the milkshake receipt > tasteless receipt and milkshake cue > tasteless cue contrasts were entered into a mixed between and within-subjects ANOVA. To assess the impact of short-term energy balance, we compared the negative energy balance, energy balance, and positive energy balance groups in an ANOVA model. Based on the evidence that reward-related neural function in women is heightened during mid-follicular phase (Dreher et al., 2007), we created a dichotomous variable that reflected whether participants completed the fMRI scans during the mid-follicular phase (4-8 days after menses onset) or not. Because findings did not change when this covariate was included, results from analyses that did not include this covariate are reported.
A cluster level threshold of p < 0.05 corrected for multiple comparisons across the whole brain for each study were derived using Monte Carlo simulations (10,000 iterations) of random noise distribution in the whole brain mask (3×3×3mm) using the 3dClustSim in AFNI (Cox, 1996; Forman et al., 1995). This approach combines individual voxel probability threshold and minimum cluster size to estimate the probability of a false positive. The resulting threshold of p < 0.001 with a cluster (k) ≥ 11 was considered corrected for multiple comparisions across the whole brain. Data presented in the figures represent parameter estimates of the indicated peak voxel ± SEM. Stereotactic coordinates are presented in MNI space (http://bic.mni.mcgill.ca/) and images are presented on the mean anatomical brain image for the sample. Effect sizes (r) were derived from the Z-values (Z/√N). Non-fMRI statistical analysis, descriptive statistics (means ± SDs), and analyses of self-report data were performed using SPSS (version 19).
3.1 Results
3.2 Study 1: Acute calorie deprivation & BOLD responsivity to pictures of appetizing foods
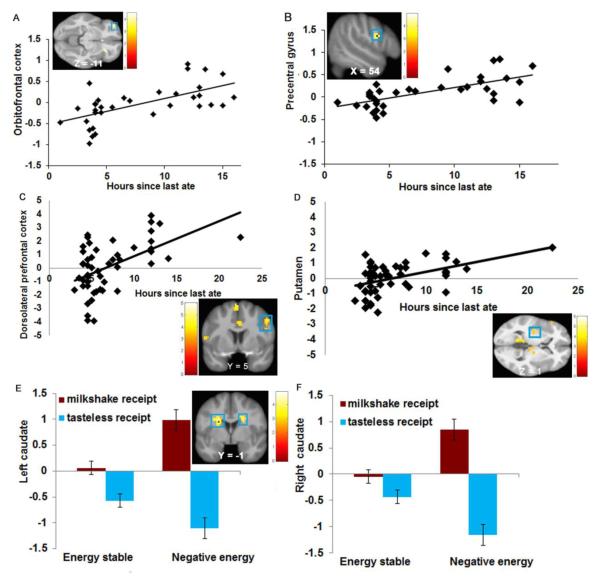
Participants (N = 34) reported a mean deprivation of 7.8 ± 4.6 hours (range 1 to 16). Participant’s Dutch Restrained Eating Scale scores were not significantly related to hours of food deprivation (P = 0.98; r = 0.004), providing further evidence that this scale does not identify people who restrict caloric intake. In the appetizing food > unappetizing food contrast, duration of caloric deprivation was positively related to activity in the left OFC (Figure 1A). In the appetizing food > water contrast, duration of caloric deprivation was positively related to activity in the right precentral gyrus (Figure 1B). There were no significant effects when examining the unappetizing > appetizing food or water > appetizing food contrasts. There was no evidence of significant quadratic relations between hours of caloric deprivation and neural activation in response to images of appetizing foods. The effects remained when potential outliers were excluded for these and subsequently reported effects.
Figure 1.
Activation in A) the left orbitofrontal cortex (MNI coordinates: −36, 47, −11, Z = 3.94, k = 12) and B) the right precentral gyrus (MNI: 54, 5, 22, Z = 4.32, k = 17) in response to images of food as a function of acute caloric deprivation in Study 1. Activation in C) the right dorsolateral prefrontal cortex (MNI coordinates: 51, 5, 40, Z = 3.99, k = 28) and D) the left putamen (MNI coordinates: −36, −13, 1, Z = 3.62, k = 43) in response to milkshake receipt and anticipated milkshake receipt as a function of acute caloric deprivation in Study 2. Greater activation in E) the left caudate (MNI coordinates: −21, −1, 22, Z = 4.71, k = 12) and F) the right caudate (MNI coordinates: 21, 8, 22, Z = 4.22, k = 11) in response to milkshake receipt and anticipated milkshake receipt in the negative energy balance group compared to the energy stable group.
3.3 Study 2: Relation between acute calorie deprivation and BOLD responsivity to receipt and anticipated receipt of milkshake
Participants (n = 51) reported a mean deprivation of 6.9 ± 3.7 hours (range 3 to 22.5). Again, participant’s Dutch Restrained Eating Scale scores were not significantly related to hours of food deprivation (P = 0.46, r = 0.10). On 1-20 point scales, participants rated the chocolate milkshake as highly pleasant (M = 14.2) and edible (M = 15.8) immediately before the scan. In the milkshake cue > tasteless solution cue contrast, hours of caloric deprivation correlated positively with activation in the right hemisphere in the middle temporal gyrus (MTG), precentral gyrus, medial superior frontal gyrus (medial SFG), lingual gyrus, dorsolateral prefrontal cortex (dlPFC, Figure 1C), dorsal anterior cingulate cortex (dorsal ACC), and middle frontal gyrus (MFG), in the left hemisphere in the inferior occipital gyrus (IOG), ventrolateral prefrontal cortex (vlPFC), precentral gyrus, parahippocampal gyrus, and putamen (Figure 1D), and bilaterally in the cerebellum, thalamus (including the mediodorsal thalamus), and middle occipital gyrus (MOG) (Table 2). In the milkshake receipt > tasteless receipt contrast, hours of caloric deprivation correlated positively with activation in the left mid insula (Table 2). No significant effects emerged for the reverse contrasts. There was no evidence of significant quadratic relations between caloric deprivation and neural activation in response to receipt and anticipated receipt of palatable food.
Table 2.
BOLD activity in response to receipt and anticipated receipt of palatable food as a function of acute caloric deprivation1 in Study 2.
| x, y, z | k | Z value | r | |
|---|---|---|---|---|
| Milkshake cue > Tasteless solution cue | ||||
|
Positive correlation with fasting hours Cerebellum |
−33, −64, −26 | 144 | 5.17 | 0.72 |
| 30, −58, −23 | 17 | 3.71 | 0.52 | |
| Middle temporal gyrus | 39, −58, 7 | 18 | 5.09 | 0.71 |
| Precentral gyrus | 6, −22, 64 | 45 | 4.43 | 0.62 |
| Inferior occipital gyrus | −39, −70, −11 | 33 | 4.40 | 0.62 |
| Thalamus | 18, −13, 16 | 35 | 4.22 | 0.59 |
| −9, −10, 10 | 29 | 4.19 | 0.59 | |
| Ventrolateral prefrontal cortex | −51, 32, 7 | 63 | 4.20 | 0.59 |
| Lingual gyrus | 18, −46, −5 | 21 | 4.18 | 0.58 |
| Precentral gyrus | −51, 11, 10 | 27 | 4.18 | 0.58 |
| Parahippocampal gyrus | −12, −40, −2 | 42 | 4.12 | 0.58 |
| Dorsolateral prefrontal cortex | 51, 5, 40 | 28 | 3.99 | 0.56 |
| Dorsal anterior cingulate cortex | 3, 5, 34 | 45 | 3.87 | 0.54 |
| Middle frontal gyrus | 36, −1, 52 | 19 | 3.75 | 0.53 |
| Putamen | −36, −13, 1 | 43 | 3.62 | 0.51 |
| Middle occipital gyrus | −27, −76, 31 | 16 | 3.61 | 0.50 |
| 27, −76, 34 | 13 | 3.45 | 0.48 | |
| Mediodorsal thalamus | 6, −19, 4 | 15 | 3.37 | 0.47 |
| Milkshake receipt > Tasteless solution receipt | ||||
| Positive correlation with fasting hours | ||||
| Mid insula | −45, 11, −2 | 16 | 3.78 | 0.53 |
Significant at P < 0.05 whole brain corrected for multiple comparisons, BOLD = Blood oxygen level dependent, x,y,z = MNI coordinates, k = cluster size, r = effect size.
3.4 Study 2: Two-week energy balance and BOLD responsivity to receipt and anticipated receipt of milkshake
Participants in the negative energy balance group (≥ 1kg in weight loss; n = 27) showed a mean weight change of −1.5 ± 0.5kg (range −3.0- −1.0kg) over the 2-week period. Participants in the energy stable group (remained within 0.1kg, n = 31) showed a trivial increase in mean weight (−0.01 ± 0.08kg; range −0.1 - 0.1kg). Those in the positive energy balance group (≥ 1kg in weight gain; n = 23) showed a mean weight gain of 1.7 ± 0.9kg (range 1.1 - 5.6kg) over the 2-week period. There were no significant differences in Dutch Restrained Eating Scale scores between the energy balance groups (p = 0.92; negative energy group M = 15.6 ± 7.2, energy stable group M = 15.9 ± 6.8, positive energy group M = 15.1 ± 6.5), providing further evidence that this scale does not identify people in a negative energy balance.
In the milkshake cue > tasteless solution cue contrast, participants in the negative energy balance group showed greater activation in the left precuneus, left medial prefrontal cortex (mPFC), and right ventral ACC relative to the positive energy balance group and greater activation in the left cuneus, left dlPFC, and left posterior cingulate cortex (PCC) relative to the energy stable group (Table 3). The energy stable group did not show significantly greater activation in response to the milkshake cue > tasteless solution cue compared to either positive or negative energy balance groups. The positive energy balance group did not show significantly greater activation in response to the milkshake cue > tasteless solution cue contrast compared to either energy stable or negative energy balance groups. No significant effects emerged for the reverse contrasts.
Table 3.
Difference in BOLD activity in response to receipt and anticipated receipt of palatable food by energy balance group1in Study 2.
| x, y, z | k | Z value | r | |
|---|---|---|---|---|
| Milkshake cue > Tasteless solution cue | ||||
| Negative energy balance > positive energy balance group | ||||
| Precuneus | −6, −64, 34 | 14 | 3.73 | 0.41 |
| Medial prefrontal cortex | −12, 41, 25 | 26 | 3.70 | 0.41 |
| Ventral anterior cingulate cortex | 9, 26, 16 | 11 | 3.38 | 0.43 |
| Negative energy balance > energy stable group | ||||
| Cuneus | −18, −79, 31 | 33 | 4.07 | 0.45 |
| Dorsolateral prefrontal cortex | −33, 41, 31 | 46 | 3.95 | 0.44 |
| Posterior cingulate cortex | −6, −31, 49 | 12 | 3.45 | 0.38 |
| Milkshake receipt > Tasteless solution receipt | ||||
| Negative energy balance > positive energy balance group | ||||
| Caudate | −21, −1, 19 | 13 | 4.06 | 0.45 |
| Precentral gyrus | 9, −13, 61 | 31 | 3.90 | 0.43 |
| Negative energy balance > energy stable group | ||||
| Caudate | −21, −1, 22 | 12 | 4.71 | 0.52 |
| 21, 8, 22 | 11 | 4.22 | 0.47 | |
| Hippocampus | 39, −25, −14 | 21 | 3.97 | 0.44 |
| Middle occipital gyrus | −15, −91, −5 | 15 | 3.60 | 0.40 |
| Fusiform gyrus | −27, −76, −8 | 28 | 3.58 | 0.40 |
Significant at P < 0.05 whole brain corrected for multiple comparisons
BOLD = Blood oxygen level dependent, x,y,z = MNI coordinates, k = cluster size, r = effect size.
In the milkshake receipt > tasteless receipt contrast, the negative energy balance group showed greater activation in left caudate and right precentral gyrus relative to the positive energy balance group (Table 3). The negative energy balance group also showed greater activation in the bilateral caudate, right hippocampus, left MOG, and left fusiform gyrus relative to the energy stable group (Table 3). The energy stable group did not show significantly greater activation in response to milkshake receipt > tasteless solution receipt contrast compared to either the positive or negative energy balance groups. The positive energy balance group did not show significantly greater activation in response to milkshake receipt > tasteless solution receipt contrast relative to the energy stable and negative energy balance groups1. No significant effects emerged for the reverse contrasts.
4. Discussion
In Study 1, the duration of elective acute caloric deprivation correlated positively with activation in the OFC in response to pictures of appetizing food. Activation in the OFC has been associated with the subjective evaluation of (food) reward (Kringelbach, 2004) and craving (Wang et al., 1999). This finding extends previous evidence that activation in the medial and lateral OFC in response to pictures of high-calorie over low-calorie foods is greater in a fasted state compared to a sated state (Goldstone et al., 2009). Duration of acute caloric deprivation also correlated with elevated activation in the precentral gyrus. Activation in this region is associated with motor responses and is thought to be related to planning to acquire or consume food (Geliebter et al. 2006). Activation in this region in response to palatable food pictures extends findings from previous fMRI studies in healthy people who report a craving for food after experimentally manipulated caloric deprivation (Goldstone et al., 2009, Siep et al., 2009). Increased motor responses to food images may therefore reflect an anticipated desire for food consumption.
In Study 2, participants reporting a longer duration of elective acute caloric deprivation exhibited a pattern of neural activation in regions implicated in (food) reward, motivation, and attention in response to receipt and anticipated receipt of palatable food, such as the thalamus, parahippocampal gyrus, dlPFC, dorsal ACC, MFG, putamen, and mid insula (Haase et al., 2009; Koob & Volkow, 2010; Pessoa et al., 2002; Stice, Spoor, Bohon, Veldhuizen, & Small, 2008b; Uher et al., 2006). The dlPFC has also found to be associated with planning and goal-directed behavior (Heller, 2004). The positive association between duration of acute caloric deprivation and activation in the dlPFC extends the finding of Uher et al. (2006) who found that dlPFC activation during food pictures was stronger in the fasted compared to sated state. In response to anticipated food receipt, duration of acute caloric deprivation was also positively correlated with activation in regions associated with visual processing, such as the IOG, lingual gyrus, MTG (Pessoa et al., 2002, Hahn et al., 2006) and motor responses, such as the precentral gyrus (Geliebter et al., 2006). Thus, a longer duration of acute caloric deprivation may prompt greater attention to food cues and increased motivation to obtain the food. Duration of caloric deprivation was also positively related to activation in the cerebellum in response to anticipated food receipt. Cerebellum activation has been associated with gustatory and olfactory stimulation (Sobel et al., 1998), images of high-calorie versus low-calorie foods (Killgore et al., 2003) and oral glucose intake (Liu et al., 2000). The cerebellum finding dovetails the findings of past positron emission tomography (PET) studies that have shown that increased regional cerebral blood flow in the cerebellum is associated with hunger and appetite (Tataranni et al., 1999), whereas decreased blood flow in this region is linked with satiation (Gautier et al., 2001). Overall, the findings from Study 1 and Study 2 suggest that duration of acute calorie deprivation is related to greater reward valuation of and attention to palatable food pictures and food cues and potentially with greater motivation to obtain or consume the food.
Participants in a negative energy balance state during the scan in Study 2 compared to those in a positive or stable energy balance showed greater activation in the precuneus, mPFC, ventral ACC, cuneus, dlPFC, and PCC in response to anticipated receipt of milkshake. Activations in the precuneus and cuneus are related to visual attention and memory retrieval (Cavanna & Trimble, 2006) and have found to be associated with experimental caloric deprivation (Uher et al., 2006) and cue-induced craving (Due et al., 2002). The mPFC and ventral ACC have been linked with motivation, emotional decision-making, and reward processing (Dolcos, LaBar, & Caneza, 2004; Koob & Volkow, 2010; Haase et al., 2009). The PCC has found to be associated with processing of emotional salient stimuli (Maddock, 1999). For example, the PCC showed activation in states of both high motivation and aversion of eating, while activation was decreased in a neutral condition (Small et al., 2001). This region has also been linked to spatial attention (e.g., Small et al., 2003) and visual imagery (Hassabis et al., 2007). Overall, results suggest that a longer-term negative energy balance state is associated with elevated activation in regions implicated in reward, attention, and motivation during anticipated palatable food receipt.
Participants in a negative energy balance relative to those in a stable or positive energy balance state also showed greater activation in the caudate, precentral gyrus, hippocampus, MOG, and fusiform gyrus in response to actual intake of palatable food. The caudate is involved in incentive motivation and is thought to encode consummatory food reward (Small et al., 2001; Stice et al., 2008b). The hippocampus modulates saliency of stimuli through regulation of ventral striatum dopamine (DA) release (Berridge & Robinson, 1998) and has been implicated in the development of memories of food (Van Vugt, 2010), food craving (Pelchat et al., 2004), physiological hunger (Haase et al., 2009), and processing of food tastes (Gautier et al., 1999). Both the MOG and fusiform gyrus have found to be involved in visual processing particularly in response to food images (Hahn et al., 2006). The elevated activation in the fusiform gyrus dovetails with evidence that experimentally manipulated caloric deprivation results in elevated activation in this region in response to food stimuli (LaBar et al., 2001; Uher et al., 2006). Results may suggest that individuals in a longer-term negative energy balance state are more sensitive to the hedonic sensations associated with the palatable food intake.
It was noteworthy that caloric deprivation was related to altered neural response in a much wider array of reward, attention, and motivation regions in response to receipt and anticipated receipt of palatable foods versus images of palatable foods herein. Past fMRI studies have not investigated the effects of caloric deprivation on neural response to all three of these events. Yet, Uher et al. (2006) did find that experimentally manipulated caloric deprivation increased responsivity of reward and sensory/hedonic regions to receipt of chocolate milk and chicken soup, but not images of palatable foods versus non-deprived participants. Collectively, results imply that caloric deprivation increases responsivity to real food much more than it does to images of food, probably because pictures of food hold no caloric value for hungry people. However, it is also important to consider alternative explanations for this pattern of findings. First, participant may have shown habituation to the palatable and unpalatable food images because they viewed and rated palatability two days before completing the scan on average. However, an earlier paper that examined the relation of BMI to BOLD response to the images of palatable foods versus unpalatable foods and water glasses using data from Study 1 (Stice et al., 2010) found 16 significant peaks, which is within the range of peaks identified in the other studies that have examined this question with subjects viewing the food images for the first time (range 3 – 26 peaks; Bruce et al., 2010; Martin et al., 2010; Rothemund et al., 2007; Stoeckel et al., 2008). Second, it is possible that the fact that Study 2 included both sexes, whereas Study 1 included only females, contributed to the differential findings. However, post hoc analyses confirmed that all effects remained significant when we controlled for sex in the Study 2 analyses. Third, it is also possible that the fact that Study 2 involved a much narrower range of BMI values contributed to the differential effects. Again, post hoc analyses indicated that only 3 of the 34 peaks became non-significant when we controlled for BMI in analyses.
The evidence that elective caloric restriction in the present studies was associated with greater responsivity of attention and reward regions to palatable food images, anticipated receipt of palatable food, and receipt of palatable food more broadly extend findings from caloric deprivation experiments. In those experiments, manipulated caloric restriction was similarly associated with elevated response in attention (ACC), reward valuation (OFC, amygdala), reward (ventral striatum, insula), and memory (hippocampus) regions in response to images of palatable foods (e.g., Fuhrer et al., 2008; Goldstone et al., 2009; Leidy et al, 2011). The similarity in the findings from studies that investigated elective caloric deprivation and experimentally manipulated caloric deprivation suggest that these effects are very robust. Experimental research with rats has revealed that acute food deprivation and chronic caloric deprivation results in increased DA release at feeding and increased D2 receptor binding (Thanos et al., 2008), which suggests that caloric deprivation results in greater signaling capacity of DA-based reward circuitry in response to food. Interesting, animal experiments show that caloric deprivation selectively increases preferences for high-fat foods (Lucas & Sclafani, 1992).
One potential mechanism for the effects observed herein comes from an experiment with rats involving discontinuation of cocaine use (Cameron & Carelli, 2012). Abstinence from cocaine after a period of regular use resulted in a 17% increase in cells that selectively fire in response to cocaine directed behavior, suggesting that caloric deprivation may likewise result in an increase in DA-neurons that fire in response to food cues. Another potential mechanism for the effects observed herein comes from animal experiment involving caloric deprivation. In vivo dialysis experiments show higher food-induced DA release in fasted versus satiated rats (Wilson et al., 1995). Intracerebroventricular injection of the DR2 agonist quinpirole produced a more pronounced striatal neuronal activation in caloric restricted rats versus ad lib fed controls (Carr et al., 2003). Administration of the D1R agonist SFK-82958 produced enhanced striatal activation and elevated D2Rs showed enhanced effector striatal coupling (Carr et al., 2003). Accumbens DA levels have been shown to increase in response to caloric intake more following longer versus shorter caloric deprivation periods (Yoshida et al., 1992) and in response to caloric deprivation weight loss diets versus baseline (Avena et al., 2008). These data collectively suggest that caloric deprivation increases D2R receptor sensitization, which could explain the greater reward value of food after caloric deprivation.
Thus, results collectively suggest that caloric deprivation increases the reward value of food, particularly high-calorie palatable food and cues predicting food receipt. One implication of these data is that weight loss diets characterized by caloric deprivation may be bound to fail because they increase the reward value of food with every passing hour of deprivation. Ironically, our findings imply that the more successful people are at caloric-restriction dieting, the greater likelihood that it will not last. Indeed, this phenomenon may explain why most weight loss diets are ineffective in producing lasting weight loss. Future studies should investigate whether absolute caloric deprivation increases the reward value of food more than reducing overall caloric intake by replacing high-fat/high-sugar foods with low-fat/low-sugar foods. However, trials have not found that weight loss interventions involving fewer meals produce significantly greater weight loss than those that involve more frequent meals (Groesz & Stice, 2007; Schlundt, Hill, Sbrocco, Pope-Cordle, & Sharp, 1992). Future fMRI studies should also investigate whether negative energy balance induced by reducing caloric intake has similar effects on neural responsivity as an energy deficit induced by increasing physical activity.
The present findings may also explain why people who attempt to diet do not typically succeed per objective measures of caloric intake (e.g., Hetherington et al., 2000; Stice et al., 2004; Sysko et al., 2007). Our results imply that if people attempt to lose weight by abstaining from food intake for long durations of time, it will have the effect of increasing the reward value of food, which may lead to poor food choices when the individual eventually does eat. Thus, results imply that dieting that is characterized by meal skipping and fasting would be less successful than weight loss efforts characterized by intake of low energy dense foods. Hours since last caloric intake and negative energy balance status showed no correlation with scores on the Dutch Restrained Eating Scale, implying that restraint scale scores identify individuals who are attempting to reduce caloric intake, rather than those who typically in a negative energy balanced state. These findings imply that future studies should measure duration of fasting efforts or whether participants are actually in a negative energy balance state, rather than continue to use dietary restraint scales. Interestingly, hours of caloric deprivation and negative energy balance status showed markedly stronger relations to intake, anticipated intake, and images of palatable foods than to the Dutch Restrained Eating Scale in the present data sets: dietary restraint scores correlated positively with activation in the OFC and dlPFC in response to milkshake receipt, but did not correlate with activation in response to anticipated receipt of milkshake or pictures of palatable food (Burger & Stice, 2011).
Another implication of the present results is that it will be important for future fMRI studies that investigate neural response to palatable food images, receipt, and anticipated receipt to more carefully standardize caloric intake before the scans. It may be optimal to invite participants to the lab several hours before the scan so that they can be fed a standardized meal.
The limitations of the present studies should be considered. First, although the repeated measures of weight at the beginning and end of the 2-week period during which the fMRI scans occurred allowed us to classify participants into those who were in an energy deficit on average versus energy balance or a positive energy state in Study 2, there was no way to confirm that on the day of the actual scan that they consumed fewer caloric than needed to maintain their weight. Yet, the fact that they showed increased responsivity in attention and reward regions in response to food that were similar to regions that showed elevated responsivity in response to food in those who reported a longer period of caloric deprivation and participants in previous studies who were assigned to caloric deprivation conditions increases confidence in the negative energy balance findings. Second, we investigated neural response to receipt and anticipated receipt of only one palatable food and it was a beverage. Results should be generalized with caution to other palatable foods, particularly solid foods. Third, because participants in these two studies were adolescents, results may not generalize to adults. Fourth, although the samples used in these studies are larger than those typically used in brain imaging studies, they were still only moderate in magnitude, which may have limited our statistical power and the generalizability of our findings.
4.1 Conclusions
The results from the current elective caloric deprivation studies taken in conjunction with findings from the earlier caloric deprivation experiments suggest that acute and prolonged caloric deprivation increases the reward value of food, particularly energy dense palatable foods. These data may explain for why weight loss diets typically do not produce lasting weight loss. Most critically, findings imply that weight loss diets that involve the replacement of unhealthy energy dense foods with healthy low energy density foods should be more effective than diets that involve long periods of caloric deprivation.
Highlights.
Experimentally manipulated caloric deprivation increases brain responsivity
Increases activation in attention & reward regions to food may contribute to overeating
We tested whether self-imposed kcal restriction related to neural response to food in 2 studies
Self-imposed kcal deprivation increased brain responsivity to food
This may explain why kcal deprivation diets typically do not produce lasting weight loss
Table 1.
BOLD activity in response to palatable food images as a function of acute caloric deprivation1in Study 1.
| x, y, z | k | Z value | r | |
|---|---|---|---|---|
|
Appetizing food > Unappetizing food Orbitofrontal cortex |
−36, 47, −11 | 12 | 3.94 | 0.68 |
|
Appetizing food > Water Precentral gyrus |
54, 5, 22 | 17 | 4.32 | 0.74 |
Significant at P < 0.05 whole brain corrected for multiple comparisons
BOLD = Blood oxygen level dependent, x,y,z = MNI coordinates, k = cluster size, r = effect size.
Acknowledgements
This study was supported research grants R1MH64560A, DK080760, and DK092468 from the National Institutes of Health. The authors would like thank the Lewis Center for Neuroimaging at the University of Oregon for their assistance in data collection for these projects. The authors declare no conflict of interest.
Footnotes
We also conducted regression models for Study 2, in which statistical parametric maps of the (milkshake receipt > tasteless receipt) and (milkshake cue > tasteless cue) contrasts were regressed on the continuous weight change over the 2-week period. The results were similar to the mixed between and within-subjects ANOVA findings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed Author; Washington, DC: 1994. [Google Scholar]
- Avena N, Rada P, Hoebel B. Underweight rates have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156:865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathalon G, Tucker K, Hays N, Vinken A, Greenberg A, McCrory M, et al. Psychological measures of eating behavior and the accuracy of 3 common dietary assessment methods in healthy postmenopausal women. American Journal of Clinical Nutrition. 2000;71:739–745. doi: 10.1093/ajcn/71.3.739. [DOI] [PubMed] [Google Scholar]
- Berridge K, Robinson T. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Burger K, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipate intake, and food pictures. NeuroImage. 2011;55:233–239. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton E, Stice E. Evaluation of a healthy-weight treatment program for bulimia nervosa: A preliminary randomized trial. Behaviour Research & Therapy. 2006;44:1727–1738. doi: 10.1016/j.brat.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron C, Carelli R. Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. European Journal of Neuroscience. 2012;35:940–951. doi: 10.1111/j.1460-9568.2012.08024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J, Goldfield G, Cyr M, Doucet E. The effects of prolonged caloric restriction leading to weight-loss on food hedonics and reinforcement. Physiology and Behavior. 2008;94:474–480. doi: 10.1016/j.physbeh.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Carr K, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Cavanna A, Trimble M. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LBar KS, Caneza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Due D, Huettel S, Hall W, Rubin D. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Epstein L, Truesdale R, Wojcik A, Paluch R, Raynor H. Effects of deprivation on hedonics and reinforcing value of food. Physiology and Behavior. 2003;78:221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- Fairburn C. Eating disorders. In: Clark D, Fairburn C, editors. Science and practice of cognitive behaviour therapy. Oxford University Press; Oxford: 1997. pp. 209–241. [Google Scholar]
- Field A, Haines J, Rosner B, Willett W. Weight-control Behaviors and Subsequent Weight Change among Adolescents and Young Adult Females. American Journal of Clinical Nutrition. 2009;91:147–53. doi: 10.3945/ajcn.2009.28321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: An exploratory visually stimulated fMRI study. Obesity. 2008;16:945–950. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gautier J, Chen K, Bandy D, Salbe A, Lawson M, Ravussin E, et al. Increased cortical representation of hunger and satiation in obese men. Diabetes. 1999;48:A312. [Google Scholar]
- Gautier J, Del Parigi A, Chen K, Salbe A, Bandy D, et al. Effect of satiation on brain activity in obese and lean women. Obesity research. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Ladell T, Logan M, Schweider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Goldstone A, Hernandez C, Beaver J, Muhammed K, Croese C, Durighel G, et al. Fasting biases brain reward systems towards high-calorie foods. European Journal of Neuroscience. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- Goodrick G, Poston W, Kimball K, Reeves R, Foreyt J. Nondieting versus dieting treatments for overweight binge-eating women. Journal of Consulting and Clinical Psychology. 1998;66:363–368. doi: 10.1037//0022-006x.66.2.363. [DOI] [PubMed] [Google Scholar]
- Groesz L, Stice E. An experimental test of the effects of dieting on bulimic symptoms: Impact of eating episode frequency. Behaviour Research and Therapy. 2007;45:49–62. doi: 10.1016/j.brat.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage. 2009;44:1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan M, Chandler P, Wauford P, Rybak R, Oswald K. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. International Journal of Eating Disorders. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross T, Stein E. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. NeuroImage. 2006;32:842–853. doi: 10.1016/j.neuroimage.2006.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire E. Using imagination to understand the neural basis of episodic memory. Journal of Neuroscience. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W. Emotion. In: Banich M, editor. Cognitive neuroscience and neuropsychology. Houghton Mifflin Company; Boston, MA: 2004. pp. 393–427. [Google Scholar]
- Hetherington M, Bell A, Rolls B. Pleasure and monotony: Effects of repeat exposure on pleasantness, preference and intake. British Food Journal. 2000;102:507–521. [Google Scholar]
- Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, Roefs A. Overweight children overeat after exposure to food cues. Eating Behaviors. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Johnson F, Wardle J. Dietary restraint, body dissatisfaction, and psychological distress: A prospective analysis. Journal of Abnormal Psychology. 2005;114:119–124. doi: 10.1037/0021-843X.114.1.119. [DOI] [PubMed] [Google Scholar]
- Killgore W, Young A, Femia L, Bogorodzki P, Rogowska J, et al. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Killen J, Taylor C, Hayward C, Haydel K, Wilson D, Hammer L, et al. Weight concerns influence the development of eating disorders: A 4-year prospective study. Journal of Consulting and Clinical Psychology. 1996;64:936–940. doi: 10.1037//0022-006x.64.5.936. [DOI] [PubMed] [Google Scholar]
- Koob G, Volkow N. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. Food for thought: hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126:807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- LaBar K, Gitelman D, Parrish T, Kim Y, Nobre A, Mesulam M. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Leidy H, Lepping R, Savage C, Harris C. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: A pilot fMRI study. Obesity. 2011;19:2019–2025. doi: 10.1038/oby.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gao J, Liu H, Fox P. The temporal response of the brain after eating revealed by funcfional MRI. Nature. 2000;405:1058–1061. doi: 10.1038/35016590. [DOI] [PubMed] [Google Scholar]
- Lucas F, Sclafani A. Food deprivation increases the rats preference for a fatty flavor over sweet taste. Chemical Senses. 1992;21:169–179. doi: 10.1093/chemse/21.2.169. [DOI] [PubMed] [Google Scholar]
- Maddock R. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends in Neurosciences. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Martin C, Williamson D, Geiselman P, Walden H, Smeets M, Morales S, Redman S. Consistency of food intake over four eating sessions in the laboratory. Eating Behaviors. 2005;6:365–372. doi: 10.1016/j.eatbeh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- McCaffery J, Haley A, Sweet L, Phelan S, Raynor H, Del Parigi A, et al. Differential functional magnetic responance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. American Journal of Clinical Nutrition. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Wall M, Guo J, Story M, Haines J, Eisenberg M. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: How do dieters fare 5 years later? Journal of the American Dietetic Association. 2006;106:559–568. doi: 10.1016/j.jada.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Ogawa R, Strader A, Clegg D, Sakai R, Seeley R, Woods S. Chronic food restriction and reduced dietary fat: Risk factors for bouts of overeating. Physiology and Behavior. 2005;86:578–585. doi: 10.1016/j.physbeh.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Ouwens M, van Strien T, van der Staak C. Tendency toward overeating and restraint as predictors of food consumption. Appetite. 2003;40:291–298. doi: 10.1016/s0195-6663(03)00006-0. [DOI] [PubMed] [Google Scholar]
- Pelchat M, Johnson A, Chan R, Valdez J, Ragland J. Images of desire: food-craving activation during fMRI. NeuroImage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider L. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman C. Dieting and binge eating: A causal analysis. American Psychologist. 1985;40:193–204. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Presnell K, Stice E. An experimental test of the effect of weight-loss dieting on bulimic pathology: Tipping the scales in a different direction. Journal of Abnormal Psychology. 2003;112:166–170. [PubMed] [Google Scholar]
- Raynor H, Epstein L. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- Reeves R, McPherson R, Nichaman M, Harrist R, Foreyt J, Goodrick G. Nutrient intake of obese female binge eaters. Journal of the American Dietetic Association. 2001;101:209–215. doi: 10.1016/S0002-8223(01)00055-4. [DOI] [PubMed] [Google Scholar]
- Rolls B, Castellanos V, Shide D, Miller D, Pelkman C, Thorwart M, Peters J. Sensory properties of a nonabsorbable fat substitute did not affect regulation of energy intake. American Journal of Clinical Nutrition. 1997;65:1375–1383. doi: 10.1093/ajcn/65.5.1375. [DOI] [PubMed] [Google Scholar]
- Schlundt D, Hill J, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: A randomized clinical trial. American Journal of Clinical Nutrition. 1992;55:645–651. doi: 10.1093/ajcn/55.3.645. [DOI] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermanns R, Bonte M, Jansen A. Hunger is the best spice: An fMRI study of the effecgts of attention, hunger, and caloric content on food reward processing in the amygdala and orbitofrontal cortex. Behavior Brain Research. 2009;198:149–158. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Small D, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Small D, Zatorre R, Dagher A, Evans A, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley C, Desmond J, Zhao Z, et al. Odorant-induced and sniff-induced activation in the cerebellum of the human. Journal of Neuroscience. 1998;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E. A prospective test of the dual pathway model of bulimic pathology: Mediating effects of dieting and negative affect. Journal of Abnormal Psychology. 2001;110:124–135. doi: 10.1037//0021-843x.110.1.124. [DOI] [PubMed] [Google Scholar]
- Stice E, Cooper J, Schoeller D, Tappe K, Lowe M. Are dietary restraint scales valid measures of moderate- to long-term dietary restriction? Objective biological and behavioral data suggest not. Psychological Assessment. 2007;19:449–458. doi: 10.1037/1040-3590.19.4.449. [DOI] [PubMed] [Google Scholar]
- Stice E, Davis K, Miller N, Marti C. Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. Journal of Abnormal Psychology. 2008a;117:941–946. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Fisher M, Lowe M. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychological Assessment. 2004;16:51–59. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of reward from food intake and anticipated intake to obesity: A functional magnetic resonance imaging study. Journal of Abnormal Psychology. 2008b;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Sysko R, Roberto C, Allison S. Are dietary restraint scales valid measures of dietary restriction? Additional objective behavioral and biological data suggest not. Appetite. 2010;54:331–339. doi: 10.1016/j.appet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysko R, Walsh T, Schebendach J, Wilson G. Eating behaviors among women with anorexia nervosa. American Journal of Clinical Nutrition. 2005;82:296–301. doi: 10.1093/ajcn.82.2.296. [DOI] [PubMed] [Google Scholar]
- Sysko R, Walsh B, Wilson G. Expectancies, dietary restraint, and test meal intake among undergraduate women. Appetite. 2007;49:30–37. doi: 10.1016/j.appet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Tataranni P, Gautier J, Chen K, Uecker A, Bandy D. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos P, Michael M, Piyis Y, Wang G, Volkow N. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo μPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer M, Campbell I. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behavioural Brain Research. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- van Strien T, Frijters J, van Staveren W, Defares P, Deurenberg P. The predictive validity of the Dutch Restrained Eating Scale. International Journal of Eating Disorders. 1986;5:747–755. [Google Scholar]
- Van Vugt D. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Human Reproduction Update. 2010;16:276–292. doi: 10.1093/humupd/dmp051. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow N, Fowler J, Cervany P, Hitzemann R, Pappas N, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sciences. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wertheim E, Koerner J, Paxton S. Longitudinal predictors of restrictive eating and bulimic tendencies in three different age groups of adolescent girls. Journal of Youth and Adolescence. 2001;30:69–81. [Google Scholar]
- Williamson D, Serdula M, Anda K, Levy A, Byers T. Weight loss attempts in adults: Goals, duration, and rate of weight loss. American Journal of Public Health. 1992;82:1251–1257. doi: 10.2105/ajph.82.9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. Dopaminergic correlates of motivated behavior: Importance of drive. Journal of Neuroscience. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Mizoguchi K, Kawahara J, Tsuda A, Nishikawa T, et al. Eating and drinking cause increased DA release in the nucleus accumbens and ventral tegmental area in the rate: Measurement by in vivo microdialysis. Neuroscience Letters. 1992;139:73–76. doi: 10.1016/0304-3940(92)90861-z. [DOI] [PubMed] [Google Scholar]