Abstract
Parathyroid hormone (PTH) agonists and antagonists have been shown to improve hair growth after chemotherapy; however, rapid clearance and systemic side-effects complicate their usage. To facilitate delivery and retention to skin, we fused PTH agonists and antagonists to the collagen binding domain (CBD) of Clostridium histolyticum collagenase. in-vitro studies showed that the agonist fusion protein, PTH-CBD, bound collagen and activated the PTH/parathyroid hormone-related peptide receptor in SaOS-2 cells. The antagonist fusion proteins, PTH(7–33)-CBD and PTH([−1]–33)-CBD, also bound collagen and antagonized PTH(1–34) effect in SaOS-2 cells; however, PTH(7–33)-CBD had lower intrinsic activity. Distribution studies confirmed uptake of PTH-CBD to the skin at 1 and 12 hr after subcutaneous injection. We assessed in vivo efficacy of PTH-CBD and PTH(7–33)-CBD in C57BL/6J mice. Animals were depilated to synchronize the hair follicles; treated on Day 7 with agonist, antagonist, or vehicle; treated on Day 9 with cyclophosphamide (150 mg/kg i.p.) or vehicle; and sacrificed on Day 39. Normal mice (no chemo and no treatment) showed rapid regrowth of hair and normal histology. Chemo + Vehicle mice showed reduced hair regrowth and decreased pigmentation; histology revealed reduced number and dystrophic anagen/catagen follicles. Chemo + Antagonist mice were grossly and histologically indistinguishable from Chemo + Vehicle mice. Chemo + Agonist mice showed more rapid regrowth and repigmentation of hair; histologically, there was a normal number of hair follicles, most of which were in the anagen phase. Overall, the agonist PTH-CBD had prominent effects in reducing chemotherapy-induced damage of hair follicles and may show promise as a therapy for chemotherapy-induced alopecia.
Keywords: chemotherapy-induced alopecia, cyclophosphamide, PTH-CBD agonist, PTH-CBD antagonist, bone mineral density, Parathyroid hormone
Chemotherapy is a common treatment for many cancers, but it is associated with adverse side effects such as nausea, vomiting, fatigue and chemotherapy-induced alopecia (CIA), which affect quality of life.1 CIA is characterized by hair loss and impaired regrowth of hair, resulting in shorter, thinner and more fragile hair. Though this alopecia is usually transient, the effects on the patient can be psychologically devastating and may lead to discontinuation or refusal of treatment.2–4 Many preventive measures have been tried to reduce CIA, of these, the most favorable results have been reported with a scalp cooling device.5 However, scalp cooling is not recommended in patients with extensive hematological malignancies, as cooling might reduce the effect of chemotherapy on tumor cells in the scalp skin.6 Thus far, no satisfactory preventative strategy is available for CIA, and no treatment modality has been shown to be clearly effective.7–9
CIA is thought to be caused by several different mechanisms, based on the type of chemotherapeutic agents. Because many chemotherapeutic agents are potent inducers of apoptosis, alopecia may result from apoptosis-related damage to the hair follicle.10 Many chemotherapeutics also act by decreasing proliferation. Accordingly, alopecia is also attributed to the suppression of epithelial proliferation by anticancer drugs. This antiproliferative effect blocks the maturation of precursor epithelial cells to the final hair strand product.11 CIA may also result from induction of follicle dystrophy and premature induction of follicle regression (catagen) in growing (anagen) hair follicles.12
Parathyroid hormone-related peptide (PTHrP) and its receptor are prominently expressed in the skin, regulating keratinocyte proliferation and differentiation.13–15 In addition, this signaling system likely exerts important paracrine and autocrine effects on control of hair growth.12,16,17 In models of CIA, the parathyroid hormone (PTH)/PTHrP receptor agonist PTH(1–34) appears to activate the dystrophic catagen pathway by upregulating follicle keratinocyte differentiation and apoptosis. Conversely, the PTH/PTHrP receptor antagonist PTH(7–34) is thought to activate the dystrophic anagen pathway by stimulating hair follicle keratinocyte proliferation.18 Both agonist and antagonist compounds improve regrowth of hair,18 and these effects have been observed with either topical or parenteral administration.18,19 However, despite these encouraging findings in animal models, the results of Phase II clinical trials of topically administered PTH(7–34) on CIA were apparently disappointing, such that the parent company (IGI Laboratories) discontinued the clinical testing.
PTH-based compounds are quickly metabolized or redistributed, even when administered topically, which would tend to limit their efficacy, particularly for antagonist compounds. To target delivery and prolong retention of PTH agonists and antagonists to the skin, we linked them to a collagen binding domain (CBD). We synthesized fusion proteins in which PTH agonists (PTH(1–33) or antagonists (PTH(7–33), PTH([−1]–33) were linked to the amino acid chain of a CBD derived from ColH collagenase of Clostridium histolyticum. We tested the resulting peptides in-vitro for collagen binding, activity at the PTH/PTHrP receptor, and in vivo for distribution after subcutaneous injection (s.q.) and effects on CIA in mice after intraperitoneal (i.p.) administration of cyclophosphamide (CYP). For this purpose, we utilized the same mouse model as that described for the initial testing of PTH agonists and antagonists in CIA, except rather than giving multiple doses of the tested compounds to each animal, we gave only a single dose of our CBD-based compounds at the start of the study. We hypothesize that our CBD-based compounds would be retained in the skin and have more pronounced, more prolonged effects on hair growth in this model.
Material and Methods
Peptides
Three fusion proteins of PTH and the CBD of C. histolyticum ColH collagenase (one agonist and two antagonists) were synthesized utilizing recombinant DNA in Escherichia coli as described previously.20 For the PTH agonist compound, PTH(1–33), which includes all of the PTH residues with known contacts or functional interaction sites with the PTH/PTHrP receptor,21,22 was linked to the CBD. Two different CBD-linked PTH antagonists were synthesized. PTH([−1]–33)-CBD has a 2-amino acid N-terminal extension; amino-terminal extended compounds have been previously shown to have antagonist activity at the PTH/PTHrP receptor.23 PTH(7–33)-CBD is an amino-terminal truncated peptide; its structure is similar to the known PTH antagonist [Leu,11 D-TRP12]PTH(7–34),24 but it includes only naturally occurring amino acids and can thus be more easily synthesized using recombinant DNA techniques. The full peptide sequences are listed below, PTH sequence is underlined:
PTH-CBD: Agonist
SVSEIQLMHN LGKHLNSMER VEWLRKKLQD VHNGINSPVY PIGTEKEPNN SKETASGPIV PGIPVSGTIE NTSDQDYFYF DVITPGEVKI DINKLGYGGA TWVVYDENNN AVSYATDDGQ NLSGKFKADK PGRYYIHLYM FNGSYMPYRI NIEGSVGR;
PTH(7–33)-CBD: Antagonist
LMHN LGKHLNSMER VEWLRKKLQD VHNGINSPVY PIGTEKEPNN SKETASGPIV PGIPVSGTIE NTSDQDYFYF DVITPGEVKI DINKLGYGGA TWVVYDENNN AVSYATDDGQ NLSGKFKADK PGRYYIHLYM FNGSYMPYRI NIEGSVGR;
PTH([−1]-33)-CBD: Antagonist
GSSVSEIQLMHN LGKHLNSMER VEWLRKKLQD VHNGINSPVY PIGTEKEPNN SKETASGPIV PGIPVSGTIE NTSDQDYFYF DVITPGEVKI DINKLGYGGA TWVVYDENNN AVSYATDDGQ NLSGKFKADK PGRYYIHLYM FNGSYMPYRI NIEGSVGR.
Biochemical assays
Collagen binding assays were preformed as previously described.20 Intracellular cAMP accumulation assays were performed in SaOS-2 cells (ATCC, Manassas, VA) as previously described.25 cAMP was measured by immunoassay using the cyclic AMP EIA Kit (Biomedical Technologies, Stoughton, MA). Calcium was measured using the Quanti-Chrom™ Calcium Kit (Bioassay Systems, Hayward, CA).
Antagonist activity of these compounds to the standard PTH/PTHrP receptor agonist, PTH(1–34), was tested in-vitro. SaOS-2 cells were grown in McCoy’s medium to 80% confluence and trypsinized. Cells (1 × 106) per well were seeded in six-well plates and were grown to 70–80% confluence. Cells were then treated with the indicated peptides (1 × 10−7 M) to assess intrinsic activity. The antagonist peptides were also tested in combination with PTH(1–34) (1 × 10−8 M) at a 10× concentration (1 × 10−7 M) to evaluate their antagonistic properties. Cyclic AMP accumulation after 1 hr of treatment was measured by immunoassay (Biomedical Technologies, Stoughton, MA).
Tissue distribution of PTH-CBD
The tissue distribution of the PTH-CBD compound was assessed by administering S35-labeled PTH-CBD via subcutaneous injection, followed by whole mount frozen and whole-body autoradiography.26 Recombinant PTH-CBD with a phosphorylation site engineered between PTH(1–33) and the CBD was purified, activated and labeled with [γ-35]ATP as described previously.27 Approximately 10.8 mcg of 35S-PTH-CBD (122 kcm/mcg) was injected subcutaneously in two mice (32–35 g, 7 weeks old). The mice were sacrificed at 1- or 12-hr postinjection and then frozen in dry ice-acetone. Frozen sections (50-μm thickness) were prepared with an autocryotome, dried at −20°C and then exposed to an image plate for 4 weeks.
Efficacy in Chemotherapy-Induced Alopecia
Animals
Twenty-eight female C57BL/6J mice aged 3–5 weeks old were obtained from the Jackson Laboratory, Bar Harbor, ME. Approval for these studies was obtained from the Institutional Animal Care and Use Committee (Ochsner Clinic Foundation, New Orleans, LA). Mice were acclimatized for 2 weeks and maintained under standard conditions, including a diet consisting of 18% protein purchased from Harlan Company (Barton, IL and Madison, WI).
Chemicals
CYP was obtained from the Ochsner Clinic Foundation Cancer Infusion Center in solution (20 mg/mL) and used within 24 hr. PTH-CBD was diluted for injection in pH 7.5, collagen binding buffer (CBD).
Study design
Mice were divided into four groups as follows:
No Chemotherapy + Vehicle (four mice)
Chemotherapy + Vehicle (eight mice)
Chemotherapy + Antagonist (PTH(7–33)-CBD) (eight mice)
Chemotherapy + Agonist (PTH-CBD) (eight mice)
On Day 0, mice were depilated on the back to synchronize the stage of the hair follicles according to the following procedure. Mice were anesthetized with pentobarbital (50 mg/kg) and then shaved to remove most of the coat. Waxing strips (Sally Hansen™ Hair Remover Wax Strip Kit – Face) with rosin were purchased from Wal-Mart (Luling, LA) and used for depilation according to the manufacturers packing instructions. On Day 7, the No Chemotherapy + Vehicle and the Chemotherapy + Vehicle group received a single subcutaneous injection of 320 μg/kg buffer solution. The Chemotherapy + Antagonist group received a single subcutaneous injection of 320 μg/kg of PTH(7–33)-CBD, and Chemotherapy + Agonist received 320 μg/kg (s.q.) of PTH-CBD. On Day 9, chemotherapy (CYP, 150 mg/kg, i.p.) was administered to all animals except those in the No Chemotherapy + Vehicle group; this was timed to maximize the damage to the hair follicles.18
Data collection (photodocumentation, histology and quantitative assessment)
Mice were observed daily for signs of alopecia as well as change in hair color. Photodocumentation was obtained every 3–4 days to monitor changes in hair growth. All mice were sacrificed at the end of the study (Day 39). Skin samples from the dorsal (nape of the neck to the middle of the back), ventral and tail regions were obtained. The skin was fixed in 10% buffered formalin, and longitudinal slices were processed for routine histology, using hematoxylin and eosin (H&E) staining. As a quantitative assessment, the number of anagen VI hair follicles per high power field (HPF) was determined by two independent observers in a blinded fashion.12,28–30 The HPF with maximum follicular density was counted on longitudinal sections, where hair follicles cut on cross-section were present, only those in subcutaneous fat layer were counted.
Bone mineral density measurements
Bone mineral density (BMD) was measured for the animals at Day 0 and at the time of sacrifice (Day 39) using a Hologic QDR 1000 plus DXA machine as described previously.31
Statistical analysis
Measurements were analyzed by a two-way Analysis of Variance (ANOVA) at each time point followed by post hoc tests, either Bonferroni or Dunnett’s as indicated for each comparison. The statistical software used is graph pad prism 5.0.
Results
in-vitro studies
Collagen binding
We have shown previously that the PTH-CBD fusion protein binds Type I collagen in-vitro.32 We assessed the collagen binding activity of the antagonist compounds, PTH(7–33)-CBD and PTH([−1]–33)-CBD, using the same collagen binding assays. Both fusion proteins were shown to bind to Type I collagen (Fig. 1).
Figure 1.
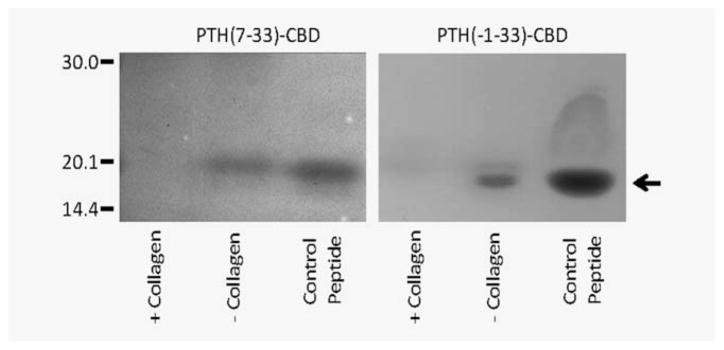
In-vitro biochemical properties of fusion proteins. Collagen binding in-vitro. The indicated proteins were incubated in collagen binding buffer in the presence (+) or absence (−) of insoluble Type I collagen at 25°C for 30 min in 0.22-μm Ultrafree-MC microcentrifuge tubes, followed by centrifugation. The filtrate was analyzed by SDS-PAGE (7.5% acrylamide).
PTH/PTHrP receptor activation
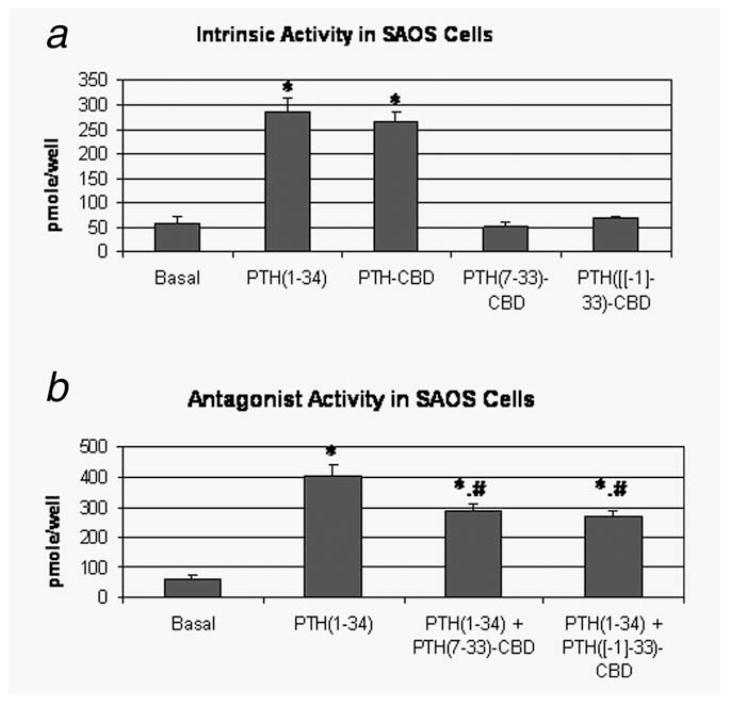
PTH-CBD has been previously shown to stimulate cAMP accumulation with similar potency and efficacy to human PTH(1–34) in LL-CPK cells stably transfected with the PTH/PTHrP receptor.32 We first confirmed the efficacy of PTH-CBD in SaOS-2 cells, which have a lower density of PTH/PTHrP receptor than do LL-CPK cells. Similar efficacy of PTH(1–34) and PTH-CBD was again observed (PTH(1–34): 286 ± 26 pmol/well, PTH-CBD: 268 ± 18 pmol/well, Fig. 2a). We next tested agonist and antagonist properties of two compounds considered for use as antagonists in in vivo experiments, PTH(7–33)-CBD and PTH([−1]–33)-CBD. While PTH(7–33)-CBD showed no change in cAMP accumulation in SaOS-2 cells from baseline levels, administration of PTH([−1]–33)-CBD did show an indication of increased cAMP accumulation, although this did not achieve statistical significance (Fig. 2b). Both peptides functioned as antagonists, as evidenced by reduced cAMP accumulation seen when PTH(1–34) was added in combination with 10× excess of either PTH (7–33)-CBD (27% inhibition) or PTH([−1]–33)-CBD (27% inhibition) (Fig. 2b). The antagonistic effects of the two experimental compounds were thus similar to each other and were similar to those observed with other known PTH antagonists.33 However, the intrinsic activity of PTH(7–33)-CBD at the PTH/PTHrP receptor may be lower. Therefore, we selected PTH(7–33)-CBD for use in the in vivo studies as a compound which at least partially blocks the PTH/PTHrP receptor without activating it.
Figure 2.
In-vitro biochemical properties of fusion proteins. Antagonist activity of these compounds to the standard PTHrP agonist, PTH(1–34) was tested in-vitro. SaOS-2 cells (osteosarcoma cells) were grown in McCoy’s medium to 80% confluence and trypsinized. Cells (1 × 106) were seeded in each well of a six-well plate and were grown to 70–80% confluence. The cells were then treated with different concentrations of the peptide after making appropriate dilutions with binding buffer. Cyclic AMP accumulation after 1 hr of treatment was measured. (a) Intrinsic activity. cAMP was measured after treatment with the indicated peptides at a concentration of 1 × 10−7 M. *p < 0.01 versus basal (ANOVA followed by Dunnett’s). (b) Antagonist activity. cAMP was measured after treatment with the indicated peptides in the presence of PTH(1–34) (1 × 10−8 M) at a 10× concentration (1 × 10−7 M). *p < 0.01 versus basal, #p < 0.05 versus PTH(1–34) (ANOVA followed by Bonferroni).
In vivo studies
Distribution of PTH-CBD
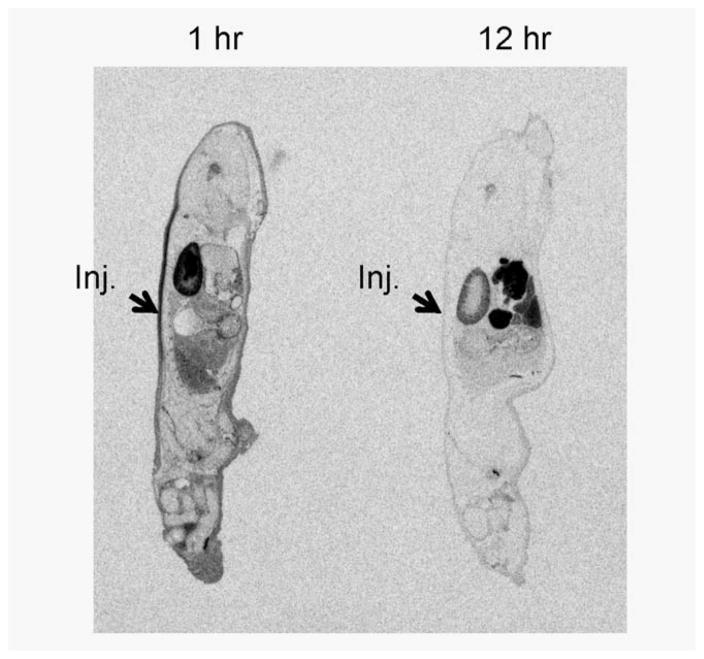
We next determined the biodistribution of S35-labeled PTH-CBD in mice. Specifically, we sought to determine how far from the site of injection PTH-CBD could be detected, to determine where the hair growth observations and histological evaluation should be focused during the in vivo testing. One hour after a single subcutaneous injection of S35-labeled PTH-CBD, the site of injection had increased levels of S35-labeled PTH-CBD (Fig. 3). However, to our surprise, the radioactivity was not localized around the site of injection, but rather had spread across the back of the mouse, and had already started to redistribute to other tissues. By 12 hr, the S35-labeled PTH-CBD had been nearly completely absorbed from the site of injection and redistributed throughout the body. Radioactivity was distributed primarily to skin and skeletal regions throughout the animal; radioactivity was also detected in the intestinal lumen, kidney and bladder. Thus, although S35-labeled PTH-CBD was absorbed systemically after subcutaneous injection, it was ultimately distributed at least in part to the intended target, the skin. Therefore, we proceeded with in vivo efficacy studies using subcutaneous administration of PTH-CBD but included investigations for systemic side-effects, particularly in bone.
Figure 3.
Tissue distribution of S35 labeled PTH-CBD. Whole-body autoradiogram obtained 1 hr after s.q. injection of S35-labeled PTH-CBD. Whole-body autoradiogram obtained 12 hr after s.q. injection of S35-labeled PTH-CBD.
Efficacy in Chemotherapy-Induced Alopecia
After determining the pharmacodynamics and in vivo distribution of the fusion peptides, we next assessed the in vivo activity of these compounds on hair growth in a mouse model of CIA.
Body weights
Animals were observed daily for their health status and weighed every 2 weeks. There was no apparent (or statistically significant) difference in the weights of the animals between different groups at any time point (not shown).
Photodocumentation
Hair growth proceeds cranial to caudal and the length of the regrowth at any given time point can be used to compare between groups. The No Chemotherapy + Vehicle mice showed rapid regrowth of hair after depilation on gross observation, as expected (Fig. 4). The Chemotherapy + Vehicle mice showed maximal chemotherapy-induced hair loss on Day 17, only partial regrowth of hair afterward, and that hair showed decreased pigmentation. There was also hair thinning and color change in the nondepilated regions. The Chemotherapy + Antagonist (PTH(7–33)-CBD) mice showed no appreciable difference from those in the Chemotherapy + Vehicle group. The Chemotherapy + Agonist (PTH-CBD) mice again showed maximal chemotherapy-induced hair loss on Day 17 but showed more rapid regrowth and repigmentation of hair than Chemotherapy + Vehicle mice, approaching that seen in the No Chemotherapy + Vehicle mice. Interestingly, Chemotherapy + Agonist mice also showed greater regrowth of hair prior to the chemotherapy-induced hair loss on Day 17. As would be predicted from the distribution studies, these effects were not restricted to the site of injection, and reduced thinning and color change in the nondepilated regions was also noticeable. In all groups, there was no observable change in hair growth in regions where the hair coat is normally thin, that is, ears and tail. Overall, it appeared that the agonist compound, PTH-CBD, had a more noticeable effect on hair growth than did the antagonist compound.
Figure 4.
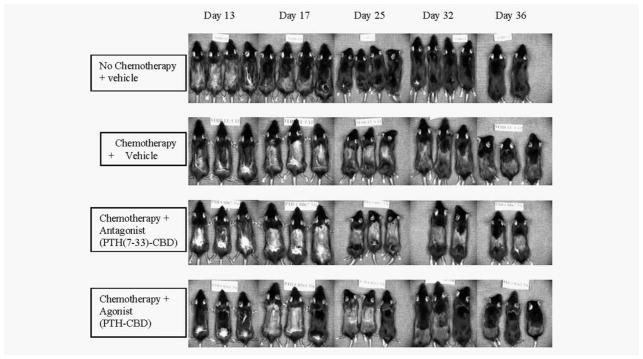
Photodocumentation. Seven-week-old female C57BL/6J mice received single subcutaneous injections of the indicated peptides at Day 7 followed by treatment with CYP at Day 9. Serial photographs of one representative set of animals from each group, obtained at the indicated time points, are shown.
Histology
Skin samples form the no Chemotherapy + Vehicle group showed normal anagen and telogen hair follicles (Fig. 5). Histological examination revealed morphological changes in the hair follicles after CYP therapy, which were more superficially located and exhibited clumped melanocytes around the bulb, characteristics of the dystrophic anagen and catagen phase. While PTH(7–33)-CBD appeared to have little effect on these changes, PTH-CBD pretreatment led to deeper rooting and reduced melanocyte clumping, thus reversing the dystrophic changes. Furthermore, there appeared to be a larger number of hair follicles in the subcutaneous layer after PTH-CBD therapy; hair follicles in this anatomical location can be presumed to be in the anagen VI phase. Histological sections of regions of the tail, where the hair coat is normally thin, were indistinguishable between groups (not shown).
Figure 5.
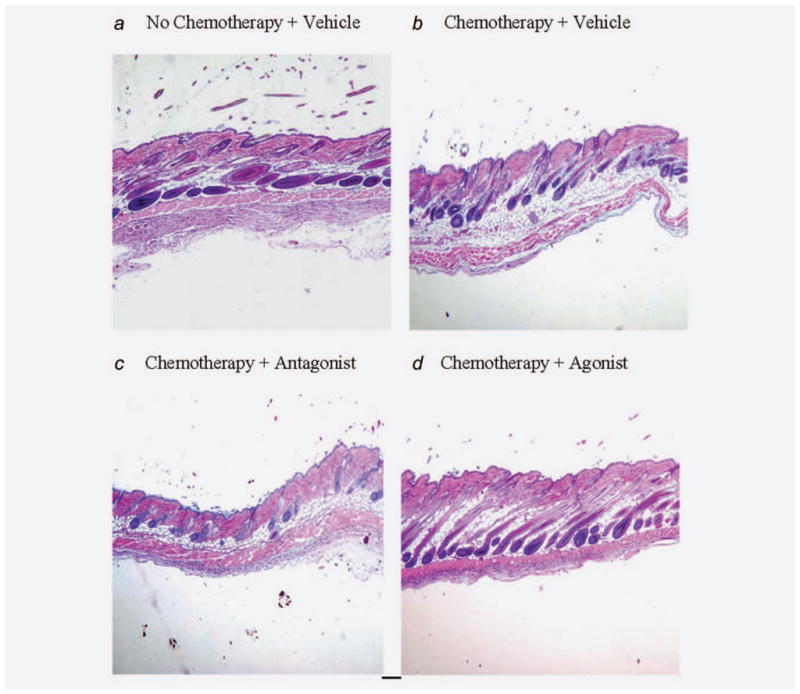
(a–d) Histology (Day 39). Seven-week-old female C57BL/6J mice received single subcutaneous injections of the indicated peptides at Day 7 followed by treatment with CYP at Day 9. All mice were sacrificed at the end of the study. Skin samples from the dorsal (nape of the neck to the middle of the back), ventral and tail regions were obtained. The skin was fixed in 10% buffered formalin, and longitudinal slices were processed for routine histology, using hematoxylin and eosin (H&E) staining.
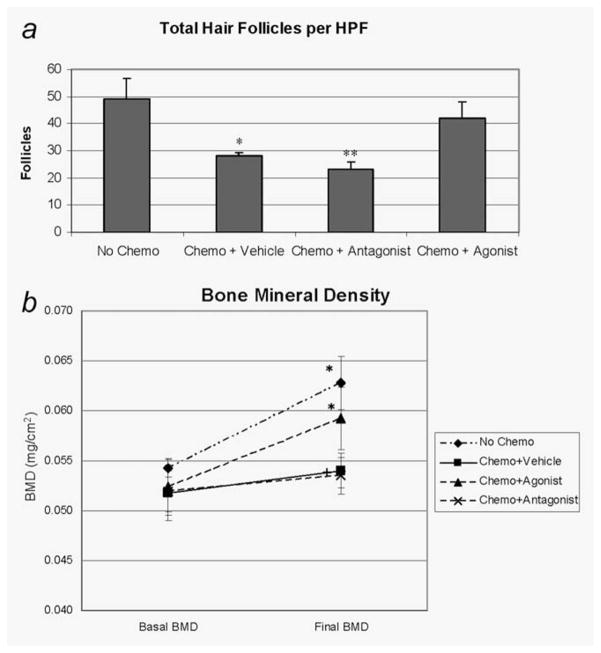
Hair follicles were assessed quantitatively by counting the number of anagen VI hair follicles per HPF on longitudinal sections at the region of maximum follicle density. When compared with the No Chemotherapy + Vehicle group (49 ± 7), the number of anagen VI hair follicles was significantly reduced in the Chemotherapy + Vehicle group (28 ± 1, p = 0.012) and the Chemotherapy + Antagonist group (23 ± 3, p = 0.004) (Fig. 6a). However, the Chemotherapy + Agonist group (42 ± 6, p > 0.5, not significant (NS)) showed no significant reduction in the hair follicles compared to animals not receiving chemotherapy. The greater number of presumed anagen VI follicles, together with the reversal of the dystrophic changes in those follicles, likely accounts for the observed changes in hair growth.
Figure 6.
(a) Hair follicle counts. Quantitative assessment of the hair follicles was done to determine the number per HPF by two independent observers in a blinded fashion. Results are expressed as mean ± standard deviation. *p < 0.05 versus vehicle, **p < 0.01 versus no chemo ANOVA followed by Dunnett’s test. (b) Lumbar spine BMD after single dosing of PTH-CBD. Seven-week-old female C57BL/6J mice received single subcutaneous injections of the indicated peptides at Day 7 followed by chemotherapy with CYP at Day 9. Animals were anesthetized, and the lumbar spine BMD was measured by DXA using a Hologic QDR-1000plus at the start and the end of the experiment period (Day 39). The analysis was performed by the same investigator in a blinded fashion. Lumbar spine BMD in excised spine: Results are expressed as mean ± standard deviation. *p < 0.05 versus baseline, +p < 0.05 versus normal vehicle, ANOVA followed by Dunnett’s test.
Bone Mineral Density
The distribution studies showed evidence of systemic redistribution of the CBD peptides to bone, and PTH is a major regulator of bone metabolism. Therefore, we measured the bone mineral densities in each animal at the start and end of the experimental period (Day 39). We found significant increases in BMD from baseline values in the No Chemotherapy + Vehicle group (0.054 ± 0.002 vs. 0.061 ± 0.006, p < 0.05), as expected in normal young mice (Fig. 6b). There was no significant change in BMD in the Chemotherapy + Vehicle group (0.052 ± 0.007 vs. 0.054 ± 0.004, NS), consistent with the known effect of CYP to reduce bone formation.34,35 The Chemotherapy + Agonist group showed a similar increase in BMD as that seen in the No Chemotherapy + Vehicle group (0.052 ± 0.007 vs. 0.059 ± 0.007, p < 0.05), whereas the Chemotherapy + Antagonist group again showed no change (0.052 ± 0.005 vs. 0.054 ± 0.004, NS). Thus, it appears that the agonist compound, PTH-CBD, restored the normal increase in BMD after CYP treatment, whereas the antagonist compound had no effect on BMD. Orthogonal analysis of the BMD values at 6 weeks by ANOVA followed by Dunnett’s test (comparing all groups to the No Chemotherapy + Vehicle group) indicated that BMD values were significantly reduced in the Chemotherapy + Vehicle and Chemotherapy + Antagonist groups, but not in the Chemotherapy + Agonist group. Thus, PTH-CBD corrected the CYP-induced bone loss to levels which were statistically indistinguishable from those of normal mice.
Serum calcium
PTH is also a major regulator of calcium metabolism. As the distribution studies showed evidence of systemic absorption of the CBD peptides, we measured serum calcium levels at the time of sacrifice. Serum calcium levels showed no significant changes between the groups (p = 0.22, NS, data not shown).
Discussion
PTH-CBD and PTH(7–33)-CBD are fusion proteins of a bacterial CBD and a PTH agonist or antagonist, respectively. These compounds were designed to promote distribution and retention of the active component to collagen-containing tissues, such as skin. The compounds retained their activity at the PTH/PTHrP receptor and bound to Type I Collagen in-vitro. Whole-animal biodistribution studies indicated that the compound was absorbed after subcutaneous injection, but distributed back to sites where collagen is most abundant, including skin and bone. Subcutaneous administration of PTH-CBD prior to chemotherapy with CYP resulted in faster regrowth of hair and partial correction of chemotherapy-induced hair changes, such as thinning and color change, when compared to animals receiving CYP alone. These changes were grossly apparent in the photodocumentation record (Fig. 4). The histological examination of the skin at the time of sacrifice showed a greater number of hair follicles in the subcutaneous fat layer in mice pretreated with PTH-CBD and resolution of the chemotherapy-induced dystrophy. The mice treated with CYP alone showed hair follicles predominantly in the dystrophic anagen and catagen phases, where as those pretreated with PTH-CBD led to deeper rooting and reduced melanocyte clumping thus reversing the dystrophic changes. These follicles appear to be predominant in the subcutaneous fat layer indicating that these are anagen VI hair follicles. The presence of continued effects of a single dose of PTH-CBD throughout the duration of the study suggests a prolonged effect of the compound, as might have been predicted based on its collagen binding activity.
Although the observed beneficial effects of PTH-CBD on hair growth in animals treated with CYP is certainly encouraging, it is also important to compare the overall results with those seen in animals which did not receive chemotherapy, as a measure of the clinical significance of the results. Although PTH-CBD pre-treatment did increase hair growth on the photodocumentation record, there was only a partial correction towards the degree of regrowth seen in animals not receiving chemotherapy. We plan to conduct additional studies, including a dose–response study and topical administration of PTH-CBD, to attempt to achieve full correction of the CIA. Interestingly, PTH-CBD resulted in more rapid regrowth of hair after depilation prior to the administration of CYP, suggesting a more global effect on hair growth which may be effective in treating other forms of alopecia as well. Importantly, there was no apparent change in hair growth, nor there were any evident histological changes, in regions of skin (tail, ears) which do not normally have a full coat.
The distribution studies of PTH-CBD showed evidence of systemic redistribution to other collagen-containing tissues, including bone, following subcutaneous injection. We have previously reported that PTH-CBD has an anabolic effect in bone.32 Conversely, CYP has direct effect to reduce bone formation and also induces hypogonadism, both of which reduce BMD.34,35 Therefore, we measured the bone mineral densities of the mice in this study. As expected, CYP-treated mice had lower BMD than those not receiving chemotherapy. Animals pretreated with PTH-CBD showed higher BMD levels than those receiving CYP alone, approaching those of the normal mice. In some circumstances, this anabolic bone effect may be desirable as a means of reducing fracture risk. On the other hand, it could be concerning in some patients, particularly those at increased risk for bone tumors. A modified route of delivery, that is, topical administration, may result in reduced systemic absorption and is currently being pursued. Topical administration may also help reduce concerns of unanticipated toxicity and immunogenicity relating to the fact that PTH-CBD is a fusion protein.
Hypercalcemia is a common side effect of PTH(1–34) therapy.36 Serum samples obtained at the end of the study (Day 39) showed no significant changes in the calcium levels among the groups. Given the fragility of the animals after CYP chemotherapy, we could not obtain blood samples during the study without risking excess mortality. Additional studies are underway with PTH-CBD in rats to assess acute and chronic effects of therapy on serum calcium after subcutaneous injection.
In designing our antagonist compound, we took great care to choose a compound with minimal intrinsic activity, as effects seen with a weak partial agonist could be the result of either agonist or antagonist activity. CBD-linked peptides are concentrated in the skin, and even weak partial agonist activity could result in a measurable biological effect. We considered two models, amino-terminal truncation of PTH(1–33) and amino-terminal extension of PTH(1–33). Amino-terminal truncation has been well-described to result in conversion of PTH from an agonist to an antagonist.37,38 Amino-terminal extension is a newer concept in design of PTH antagonists, based on the relative structure of PTH(1–34) versus tuberoinfundibular peptide.23 We found that the antagonist activity of PTH(7–33)-CBD and PTH([−1]–33)-CBD was quite similar, but the intrinsic activity of PTH(7–33)-CBD was apparently lower. Therefore, we selected the purer antagonist compound for use in these studies. PTH(7–33)-CBD is also more structurally similar to the previously tested PTH antagonist, PTH(7–34), studied extensively in preclincal19 and in Phase II clinical trials for the treatment of CIA by IGI Laboratories.
As PTH antagonists have also been described as stimulating hair growth in mice, including those receiving CYP chemotherapy,18 we expected PTH(7–33)-CBD to show prominent effects in our study as well. Surprisingly, PTH(7–33)-CBD had no effect on hair growth in CYP-induced alopecia. There were no evident changes at any time point in the photodocumentation record between mice pretreated with PTH(7–33)-CBD and those receiving CYP chemotherapy alone. The histological appearance of the hair follicles was also indistinguishable, exhibiting clumped melanocytes around the bulb, both groups showed smaller follicles which lacked hair shafts, and the follicles were predominantly in the dystrophic anagen and catagen phase. Furthermore, there were no differences observed in the number of anagen VI hair follicles per HPF between theses two groups.
There are several possible explanations as to why PTH(7–33)-CBD did not result in hair growth in our studies. Although the CBD domain concentrates the peptide in the skin, it may also have resulted in partial sequestration of the compound from the PTH/PTHrP receptor-containing fibro-blasts. Such sequestration might be expected to have greater effects on the function of an antagonist than that of an agonist, as antagonists need to be present in higher concentrations to achieve their receptor blocking effects. Given the relatively high dose of topically applied PTH(7–34) in previous studies (500 mcg/kg/day),19 it is also possible that some of the observed effects of PTH antagonists on hair growth may be the result of weak partial agonist activity (PTH(7–33)-CBD had no measurable agonist activity at the PTH/PTHrP receptor). Most of the studies of hair growth effects of PTH antagonists have been conducted in the Hairless mouse17,19; it is possible that PTH(7–33)-CBD does have positive effects on hair growth in this model.
When considering the mechanism by which PTH-CBD promotes hair growth in CIA, it is important to note that the mechanism by which chemotherapy results in hair loss is itself not fully described. Chemotherapeutics target rapidly dividing cells, and one proposed mechanism for alopecia is direct damage to the rapidly dividing cells in the hair follicle.39,40 Another proposed mechanism is an indirect activation of apoptosis in the hair follicles.10 In our studies, we found evidence that PTH-CBD resulted in a greater number of hair follicles in the subcutaneous layer, and that those hair follicles were predominantly in the anagen VI or growth phase. The increase in anagen VI follicles after PTH-CBD treatment is a critical observation, consistent with known effects of parathyroid hormone to increase production of beta-catenin, which induces hair follicle transition to the anagen phase. In this phase, the hair follicle may be paradoxically more susceptible to initial damage from chemotherapy; however, the continued regenerative stimulus provided by PTH-CBD would result in more rapid repair of this damage, with a net positive effect on hair growth after chemotherapy. Consistent with this theory, PTH-CBD treatment did not prevent the chemotherapy-induced hair loss seen in all groups on Day 13 but did result in more rapid and more complete regrowth of hair by the end of the experimental period.
These studies represent a preliminary evaluation of the effectiveness of PTH agonists and antagonists linked to a bacterial CBD in preventing CIA. The results show evidence of efficacy for the agonist compound. Additional studies, optimizing the dose and route of administration and testing in tumor-bearing models and in models where chemotherapy is administered in a cyclical fashion, are currently underway. Formal toxicological and immunogenicity assessments also need to be performed. Further test of targeted delivery of PTH agonists to the skin may ultimately yield an effective therapy for this psychologically devastating complication of chemotherapy.
Acknowledgments
Grant sponsors: Ochsner Clinic Foundation, New Orleans, LA, AR Biosciences Institute (ABI); Grant sponsor: National Institutes of Health Center for Protein Structure and Function; Grant numbers: NCRR COBRE 1 P20RR15569, INBRE P20RR16460; Grant sponsor: Japan Society for the Promotion of Science and Kagawa University Project Research Fund; 2005–2006
The authors are thankful to Dr. Alan Brushell, Section Head, Department of Endocrinology at Ochsner Clinic Foundation, New Orleans, Lousiana for his kind support and scientific advice throughout the study.
Abbreviations
- BMD
bone mineral density
- CBD
collagen binding domain
- CIA
chemotherapy-induced alopecia
- CYP
cyclophosphamide
- HPF
high power field
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone related peptide
Footnotes
Disclosure PTH-CBD is patented and exclusively licensed to Biologics MD, LLC. Robert Gensure is Chief Medical Officer of Biologics MD. Joshua Sakon has a stock ownership in Biologics MD
References
- 1.Lotfi-Jam K, Carey M, Jefford M, Schofield P, Charleson C, Aranda S. Nonpharmacologic strategies for managing common chemotherapy adverse effects: a systematic review. J Clin Oncol. 2008;26:5618–29. doi: 10.1200/JCO.2007.15.9053. [DOI] [PubMed] [Google Scholar]
- 2.Cline BW. Prevention of chemotherapy-induced alopecia: a review of the literature. Cancer Nurs. 1984;7:221–8. [PubMed] [Google Scholar]
- 3.Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562–70. [PubMed] [Google Scholar]
- 4.Botchkarev VA. Molecular mechanisms of chemotherapy-induced hair loss. J Investig Dermatol Symp Proc. 2003;8:72–5. doi: 10.1046/j.1523-1747.2003.12175.x. [DOI] [PubMed] [Google Scholar]
- 5.Grevelman EG, Breed WP. Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol. 2005;16:352–8. doi: 10.1093/annonc/mdi088. [DOI] [PubMed] [Google Scholar]
- 6.Christodoulou C, Tsakalos G, Galani E, Skarlos DV. Scalp metastases and scalp cooling for chemotherapy-induced alopecia prevention. Ann Oncol. 2006;17:350. doi: 10.1093/annonc/mdj008. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, Connelly EA, Chen Q, Zou J, Goldenberg C, Voellmy R. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones. 2008;13:31–8. doi: 10.1007/s12192-007-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesketh PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, Yardley D. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer. 2004;12:543–9. doi: 10.1007/s00520-003-0562-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Lu Z, Au JL. Protection against chemotherapy-induced alopecia. Pharm Res. 2006;23:2505–14. doi: 10.1007/s11095-006-9105-3. [DOI] [PubMed] [Google Scholar]
- 10.Schilli MB, Paus R, Menrad A. Reduction of intrafollicular apoptosis in chemotherapy-induced alopecia by topical calcitriol-analogs. J Invest Dermatol. 1998;111:598–604. doi: 10.1046/j.1523-1747.1998.00350.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis ST, Benson BG, Bramson HN, Chapman DE, Dickerson SH, Dold KM, Eberwein DJ, Edelstein M, Frye SV, Gampe RT, Jr, Griffin RJ, Harris PA, et al. Prevention of chemotherapy-induced alopecia in rats by CDK inhibitors. Science. 2001;291:134–7. doi: 10.1126/science.291.5501.134. [DOI] [PubMed] [Google Scholar]
- 12.Paus R, Handjiski B, Eichmuller S, Czarnetzki BM. Chemotherapy-induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol. 1994;144:719–34. [PMC free article] [PubMed] [Google Scholar]
- 13.Danks JA, Ebeling PR, Hayman J, Chou ST, Moseley JM, Dunlop J, Kemp BE, Martin TJ. Parathyroid hormone-related protein: immunohistochemical localization in cancers and in normal skin. J Bone Miner Res. 1989;4:273–8. doi: 10.1002/jbmr.5650040221. [DOI] [PubMed] [Google Scholar]
- 14.Lee K, Deeds JD, Segre GV. Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology. 1995;136:453–63. doi: 10.1210/endo.136.2.7835276. [DOI] [PubMed] [Google Scholar]
- 15.Shin JH, Ji C, Casinghino S, McCarthy TL, Centrella M. Parathyroid hormone-related protein enhances insulin-like growth factor-I expression by fetal rat dermal fibroblasts. J Biol Chem. 1997;272:23498–502. doi: 10.1074/jbc.272.38.23498. [DOI] [PubMed] [Google Scholar]
- 16.Orloff JJ, Wu TL, Stewart AF. Parathyroid hormone-like proteins: biochemical responses and receptor interactions. Endocr Rev. 1989;10:476–95. doi: 10.1210/edrv-10-4-476. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF, Ray S, Chen TC, Tian X, Persons KS. A parathyroid hormone antagonist stimulates epidermal proliferation and hair growth in mice. Proc Natl Acad Sci USA. 1994;91:8014–6. doi: 10.1073/pnas.91.17.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters EM, Foitzik K, Paus R, Ray S, Holick MF. A new strategy for modulating chemotherapy-induced alopecia, using PTH/PTHrP receptor agonist and antagonist. J Invest Dermatol. 2001;117:173–8. doi: 10.1046/j.0022-202x.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- 19.Safer JD, Ray S, Holick MF. A topical parathyroid hormone/parathyroid hormone-related peptide receptor antagonist stimulates hair growth in mice. Endocrinology. 2007;148:1167–70. doi: 10.1210/en.2006-1442. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita O, Jung CM, Minami J, Katayama S, Nishi N, Okabe A. A study of the collagen-binding domain of a 116-kDa Clostridium histolyticum collagenase. J Biol Chem. 1998;273:3643–8. doi: 10.1074/jbc.273.6.3643. [DOI] [PubMed] [Google Scholar]
- 21.Chorev M. Parathyroid hormone 1 receptor: insights into structure and function. Receptors Channels. 2002;8:219–42. [PubMed] [Google Scholar]
- 22.Gensure RC, Gardella TJ, Juppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–78. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson KB, John MR, Gensure RC, Gardella TJ, Juppner H. Tuberoinfundibular peptide 39 binds to the parathyroid hormone (PTH)/PTH-related peptide receptor, but functions as an antagonist. Endocrinology. 2001;142:704–9. doi: 10.1210/endo.142.2.7945. [DOI] [PubMed] [Google Scholar]
- 24.Maretto S, Schievano E, Mammi S, Bisello A, Nakamoto C, Rosenblatt M, Chorev M, Peggion E. Conformational studies of a potent Leu11,D-Trp12-containing lactam-bridged parathyroid hormone-related protein-derived antagonist. J Pept Res. 1998;52:241–8. doi: 10.1111/j.1399-3011.1998.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 25.Bergwitz C, Jusseaume SA, Luck MD, Juppner H, Gardella TJ. Residues in the membrane-spanning and extracellular loop regions of the parathyroid hormone (PTH)-2 receptor determine signaling selectivity for PTH and PTH-related peptide. J Biol Chem. 1997;272:28861–8. doi: 10.1074/jbc.272.46.28861. [DOI] [PubMed] [Google Scholar]
- 26.Tamai E, Ishida T, Miyata S, Matsushita O, Suda H, Kobayashi S, Sonobe H, Okabe A. Accumulation of Clostridium perfringens epsilon-toxin in the mouse kidney and its possible biological significance. Infect Immun. 2003;71:5371–5. doi: 10.1128/IAI.71.9.5371-5375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimamoto S, Tamai E, Matsushita O, Minami J, Okabe A, Miyata S. Changes in ganglioside content affect the binding of Clostridium perfringens epsilon-toxin to detergent-resistant membranes of Madin-Darby canine kidney cells. Microbiol Immunol. 2005;49:245–53. doi: 10.1111/j.1348-0421.2005.tb03726.x. [DOI] [PubMed] [Google Scholar]
- 28.Paus R, Schilli MB, Handjiski B, Menrad A, Henz BM, Plonka P. Topical calcitriol enhances normal hair regrowth but does not prevent chemotherapy-induced alopecia in mice. Cancer Res. 1996;56:4438–43. [PubMed] [Google Scholar]
- 29.Tobin DJ, Hagen E, Botchkarev VA, Paus R. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? J Invest Dermatol. 1998;111:941–7. doi: 10.1046/j.1523-1747.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 30.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 31.Katikaneni R, Ponnapakkam A, Miller E, Ponnapakkam T, Gensure RC. A new technique for precisely and accurately measuring lumbar spine bone mineral density in mice using clinical dual energy X-ray absorptiometry (DXA) Toxicol Mech Methods. 2009;19:225–31. doi: 10.1080/15376510802499030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnapakkam T, Matsushita O, Sakon J, Gensure RC. Weekly administration of a novel parathyroid hormone-collagen binding domain fusion protein increases bone mineral density by more than 15% in normal mice. American Society for Bone and Mineral Research (ASBMR); 2025 M Street, NW, Suite 800, Washington DC 20036-3309: 2007. [Google Scholar]
- 33.Carter PH, Juppner H, Gardella TJ. Studies of the N-terminal region of a parathyroid hormone-related peptide (1–36) analog: receptor subtype-selective agonists, antagonists, and photochemical cross-linking agents. Endocrinology. 1999;140:4972–81. doi: 10.1210/endo.140.11.7102. [DOI] [PubMed] [Google Scholar]
- 34.Hirbe A, Morgan EA, Uluckan O, Weilbaecher K. Skeletal complications of breast cancer therapies. Clin Cancer Res. 2006;12:6309s–14s. doi: 10.1158/1078-0432.CCR-06-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SH, Lim SK, Hahn JS. Effect of pamidronate on new vertebral fractures and bone mineral density in patients with malignant lymphoma receiving chemotherapy. Am J Med. 2004;116:524–8. doi: 10.1016/j.amjmed.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Barbehenn EK, Lurie P, Wolfe SM. Osteosarcoma risk in rats using PTH 1–34. Trends Endocrinol Metab. 2001;12:383. doi: 10.1016/s1043-2760(01)00489-1. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblatt M, Potts JT., Jr Analogues of an in-vitro parathyroid hormone inhibitor: modifications at the amino terminus. Calcif Tissue Int. 1981;33:153–7. doi: 10.1007/BF02409428. [DOI] [PubMed] [Google Scholar]
- 38.Carter PH, Petroni BD, Gensure RC, Schipani E, Potts JT, Jr, Gardella TJ. Selective and nonselective inverse agonists for constitutively active type-1 parathyroid hormone receptors: evidence for altered receptor conformations. Endocrinology. 2001;142:1534–45. doi: 10.1210/endo.142.4.8103. [DOI] [PubMed] [Google Scholar]
- 39.Tosi A, Misciali C, Piraccini BM, Peluso AM, Bardazzi F. Drug-induced hair loss and hair growth. Incidence, management and avoidance. Drug Saf. 1994;10:310–7. doi: 10.2165/00002018-199410040-00005. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima-Kamimura N, Nishimaki K, Mori T, Asoh S, Ohta S. Prevention of chemotherapy-induced alopecia by the anti-death FNK protein. Life Sci. 2008;82:218–25. doi: 10.1016/j.lfs.2007.11.011. [DOI] [PubMed] [Google Scholar]