Abstract
An important mechanism by which pancreatic cancer avoids anti-tumor immunity is by recruiting regulatory T cells (Treg) to the tumor microenvironment. Recent studies suggest that suppressor Treg and effector Th17 cells share a common lineage and differentiate based on the presence of certain cytokines in the microenvironment. Since IL-6 in the presence of TGF-β has been shown to inhibit Treg development and induce Th17 cells, we hypothesized that altering the tumor cytokine environment could induce Th17 and reverse tumor-associated immune suppression. Pan02 murine pancreatic tumor cells that secrete TGF-β were transduced with the gene encoding IL-6. C57BL/6 mice were injected subcutaneously with wildtype (WT Pan02), empty vector (EV Pan02), or IL-6 transduced Pan02 cells (IL-6 Pan02) to investigate the impact of IL-6 secretion in the tumor microenvironment. Mice bearing IL-6 Pan02 tumors demonstrated significant delay in tumor growth and better overall median survival compared with mice bearing WT or EV Pan02 tumors. Immunohistochemical analysis demonstrated an increase in Th17 cells (CD4+IL-23R+ cells and CD4+IL-17+ cells) in tumors of the IL-6 Pan02 group compared with WT or EV Pan02 tumors. The upregulation of IL-17-secreting CD4+ tumor-infiltrating lymphocytes was substantiated at the cellular level by flow cytometry and ELISPOT assay, and mRNA level for RORγt and IL-23 receptor by RT-PCR. Thus, the addition of IL-6 to the tumor microenvironment skews the balance toward Th17 cells in a murine model of pancreatic cancer. The delayed tumor growth and improved survival suggests that induction of Th17 in the tumor microenvironment produces an anti-tumor effect.
Keywords: T cells, Cell Differentiation, Tumor immunity
INTRODUCTION
The role of CD4+ T cells in tumor immunity is poorly understood. Naïve CD4+ T cells differentiate into mature Th1, Th2, Th17, or T regulatory cells (Treg). We and others have shown that Tregs suppress immune responses and induce tolerance at tumor sites (1–9). Treg and Th17 cells share a common lineage, and terminal differentiation of suppressor versus effector cells at the tumor site may tip the balance between tolerance and tumor rejection. Moreover, recent evidence suggests that mature Tregs can be reprogrammed into competent Th17 effector cells and the plasticity of T cell lineage may be an important mechanism by which immune homeostasis is maintained (10,11). Efforts to manipulate Treg function in order to release immune effector cells from immunosuppressive regulatory controls have become of increasing interest to tumor immunologists.
Th17 cell lineage commitment is initiated by TGF-β and IL-6, and the lineage is maintained by IL-23 and amplified by the autocrine release of IL-21 (12–18). The transcription factors RORγt and STAT3 are essential for the differentiation of Th17 cells and IL-17 cytokine expression (19–21). IL-17 is a potent inflammatory cytokine that works on a variety of cell types including fibroblasts, endothelial cells, and epithelial cells to induce the expression of other inflammatory cytokines such as IL-6, TNF, G-CSF, chemokines, and matrix metalloproteases, all which coordinate to produce a robust inflammatory response (22–27).
Th17 cells have been implicated in many autoimmune diseases, but their role in cancer has not been fully elucidated and remains controversial (22, 28–33). Increased levels of Th17 cells have been detected in many human cancers, including ovarian, pancreatic, renal cell, and gastric cancer (34, 35). Some studies report that the presence of higher levels of Th17 cells in tumor tissues or peripheral blood correlate with advanced cancer (36). Other studies describe the opposite and suggest that Th17 cells may have a potent anti-tumor effect, being found in patients with limited disease or long-term survivors (37, 38). In murine models, tumor-specific Th17-polarized cells that recognized an antigen expressed by both normal melanocytes and B16 melanoma eradicated the established melanoma tumors and improved survival (39). Also, inflammatory killing induced in the normal prostate through an IL-6/IL-17-mediated autoimmune response was shown to be effective at rejecting established metastatic murine prostate tumors (40). These studies suggest Th17 cells may play a vital role in tumor rejection based on the intimate relationship between autoimmunity and anti-tumor immunity for tissue specific antigens.
Treg cells, on the other hand, maintain immune homeostasis by inhibiting effector T cell proliferation and autoimmune responses (41, 42). Interestingly, Treg cells are upregulated in the peripheral blood and tumor microenvironment in many cancers including pancreatic cancer (1–9). High serum levels of TGF-β have also been found in many types of cancer, especially pancreatic cancer, where elevation of TGF-β in the serum may drive Treg differentiation and is correlated to tumor cell dissemination and poor survival (1, 3, 43). Likewise, an increased prevalence of Tregs correlates with more advanced cancer and is a marker of poor prognosis (5– 7). When Tregs are blocked or depleted, a more effective anti-tumor effect is seen in mouse models of cancer (9, 44–46).
Recent evidence has shown that there exists an intricate and reciprocal regulation between Th17 cells and Treg cells (10, 11, 14, 47). IL-6 plays a pivotal role in the CD4+ T cell lineage differentiation. Although TGF-β induces the differentiation of Treg cells via Foxp3 transcription, IL-6 inhibits the differentiation of Treg cells and, along with TGF-β, drives the differentiation of naïve CD4+ T cells into Th17 cells (12–14, 48–52). Thus, we hypothesized that the addition of IL-6 to the pancreatic tumor microenvironment rich in TGF-β may promote Th17 cell differentiation, which may lead to an improved anti-tumor response. In order to test this hypothesis, we genetically engineered the murine pancreatic cancer cell line Pan02, which naturally secretes TGF-β, to also produce IL-6. By changing the cytokine profile in the tumor microenvironment we aimed to alter the balance of CD4+ T cells in favor of Th17 cells in order to produce a more effective anti-tumor response.
MATERIALS AND METHODS
Cloning mIL6 into lentivirus vector; lentivirus generation and transduction
HTLV-mIL6 encoding DNA sequence form pORF9-mIL6 (InvivoGen, San Diego, CA) was subcloned into lentiviral vector pSicoR-puro with added XhoI-NheI restriction sites. For lentivirus generation, HEK-293T cells were transfected with pSicoR-mIL6 vector and VSVG in Opti-MEM media using Fugene-6 (Roche Diagnostics, Indianapolis, IN). After overnight culture, serum containing media was added and the incubation was allowed to continue for an additional 48 hours. The first and second viral harvests, performed at 48 and 72 hours after transfection, respectively, were used to transduce the murine pancreatic adenocarcinoma Pan02 cell line (aka Panc-02) (53). Viral supernatants were filtered through a 0.45 µm Millipore filter and used directly to infect cells with protamine sulfate (Sigma, Saint Louis, MO) at a concentration of 6 µg/mL. Puromycin-resistant cells were selected. Pan02 cells transduced with empty vector were used as a control. IL-6 transcript was confirmed by qRT-PCR with primers: IL-6-sense-CCGGAGAGGAGACTTCACAG and IL-6-antisense-TCCACGATTTCCCAGAGAAC. Protein expression of IL-6 was confirmed by ELISA.
Tumor cell lines
Pan02 is a murine pancreatic adenocarcinoma cell line syngeneic to C57BL/6 and was obtained from the Division of Cancer Treatment Tumor Repository (National Cancer Institute-Frederick Cancer Research and Development Center). All tumor cell lines, including wildtype (non-tranduced) Pan02 cells (WT Pan02), empty control vector Pan02 cells (EV Pan02), and IL-6 tranduced Pan02 cells (IL-6 Pan02), were maintained in culture at 37°C in humidified air (5% CO2) in complete media consisting of 1x high glucose Dulbecco’s Modified Eagle Medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 1% penicillin/streptomycin (Cellgro, Manassas, VA), and 10mM HEPES buffer (Cellgro). Puromycin was added to the media at a concentration of 2µg/mL bimonthly to ensure that transduced cell lines were stable.
Analysis of in vitro growth kinetics by carboxyfluorescein succinimidyl ester (CFSE) staining
Single cell suspensions of each of the tumor cell lines were stained with 5µM CFSE (1µL/1×107cells) in phosphate buffered saline (PBS) for 10 minutes at 37°C. After washing, cells were plated in 6-well plates at 2×105 cells/well in complete media. Cells were incubated at 37°C for 40 hours before being washed and fixed. Proliferation was detected by reduction of CSFE fluorescence. Cells stained, washed, and fixed at time 0 were included in the experiment as control. The samples were analyzed with FlowJo 7.2.4 software (Tree Star, Inc., Ashland, OR). Triplicate samples were analyzed for each of the independent experiments.
Tumor growth and survival experiments
Young (6–8 weeks) female C57BL/6 mice were purchased from either the National Cancer Institute (Frederick, MD) or Jackson Laboratories (Bar Harbor, ME) and maintained in our animal care facility with ad libitum access to water and mouse chow. Per experiment, thirty C57BL/6 mice were divided into 3 groups of 10 mice each and were given subcutaneous injections into the right hind leg of either 1×104 or 5×104 WT Pan02 cells, EV Pan02 cells, or IL6 Pan02 cells in a total volume of 0.1 mL. Tumor growth was measured every five days and palpable tumors were measured in two perpendicular axes with a Vernier caliper. Mean tumor size was calculated by multiplying the two size measurements together. Mice were allowed to die spontaneously or sacrificed when tumor size was greater than 2 cm in one direction (in accordance with institutional guidelines) or when there was severe ulceration of the leg from the tumor that affected ambulation. All measurements were performed in a blinded fashion. Tumor growth and survival experiments were repeated independently three times with similar results. All experimental protocols were approved by the institutional Animal Studies Committee and all murine experiments were conducted in compliance with institutional guidelines for the use of research animals.
ELISA
Cytokine amounts in tissue culture supernatants were assayed with ELISA antibody pairs for IL-6 (eBioscience, San Diego, CA) and TGF-β (BD Pharmingen, San Jose, CA) in accordance with the manufacturer’s recommendations. For IL-6 and TGF-β cytokine measurements, 2×105 tumor cells were plated in 6-well plates in 2 mL complete media. After 2 hours, supernatants were assayed for cytokine amounts. For detecting TGF-β, culture supernatants were activated by acid treatment for measurement of total TGF-β. For serum IL-6 cytokine measurement, blood from mice was obtained by retro-orbital bleed. All experiments were run in triplicate.
Lymphocyte isolation from tumors
For functional studies, thirty C57BL/6 mice were divided into 3 groups of 10 mice each and were given subcutaneous injections into the right hind leg of 5×104 of WT Pan02 cells, EV Pan02 cells, or IL6 Pan02 cells in a total volume of 0.1 mL. Tumors that were approximately 1cm in diameter were harvested after mice had been sacrificed. Tumors were mechanically dissociated into 1mm3 pieces and then placed into the gentleMACS Dissociator (Miltenyi Biotec, Auburn, CA) with 10mL of enzyme digest consisting of collagenase (0.1g/100mL), DNase (0.01g/100mL), and hyaluronidase (2.5 units/mL) (all from Sigma) in RPMI-1640 (Sigma) and dissociated on a pre-set protocol. Tumor digest was then placed on a neutator and incubated at 37°C for 30 minutes before dissociating in the gentleMACS again. The cells were then washed with complete media and passed through 40µm nylon mesh to obtain single cell suspensions. CD4+ cells were isolated using BD IMag anti-CD4 magnetic particles according to the manufacturer’s instructions (BD Biosciences) and used for the functional assays.
Histopathology and Immunohistochemistry
A section of each murine pancreatic adenocarcinoma was embedded in disposable base molds containing Tissue-Tek O.C.T. Compound (Sakura) then snap frozen over liquid nitrogen and stored at −80°C. Embedded tumor tissue was sectioned on a Leica CM1900 cryostat at 10 microns, placed on SuperFrost Plus slides, and then stored at −80°C until use.
For CD4+IL-17+ and CD4+Foxp3+ double staining, frozen tissue sections were fixed for ten minutes in 1% paraformaldehyde in PBS. Sections were then permeabilized with 0.05% saponin in dH2O for 15 minutes. For CD4+IL-23R+ double staining, frozen sections were fixed in cold acetone for 10 minutes at −20°C and then dried at room temperature. All sections were blocked in Serum-Free Protein Block for 30 minutes. Primary antibodies for IL-17 (Santacruz Biotech), Foxp3 (Abcam), IL-23R (Abcam), or CD4 (eBioscience) were diluted at 1:100, 10ug/ml, 1:250, or 1:30 respectively in Antibody Diluent and added for 1 hour at room temperature.
For CD4+IL-17+ and CD4+Foxp3+ staining, Alexa Fluor 647 donkey anti-rabbit IgG and Alexa Fluor 555 goat anti-rat IgG secondary antibodies (Invitrogen) were diluted at 1:200 in Antibody Diluent and added for 30 minutes at room temperature in the dark. For CD4+IL-23R+ staining, Alexa Fluor 555 donkey anti-goat IgG (Invitrogen) and biotinylated rabbit anti-rat IgG (Vector Labs) secondary antibodies were diluted 1:200 in Antibody Diluent and added for 30 minutes at room temperature in the dark. Alexa Fluor 647 strepavidin conjugate (Invitrogen) was diluted at 1:200 in Antibody Diluent and added for 30 minutes at room temperature in the dark.
Confocal images were taken at 400X magnification on an Axiovert 100M microscope equipped with an LSM 510 confocal microscopy system (Zeiss).
Real-time PCR
RNA was isolated from CD4+ tumor-infiltrating lymphocytes using the RNeasy Protect Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. 500ng of total RNA was reverse transcribed into cDNA in a 20µl reaction containing the following reagents (Invitrogen): 200 units of Superscript II, 1X First Strand Buffer, 10mM DTT, 250ng of random primers, 0.5mM dNTPs, and 40 units of RNaseOUT. Reactions were performed according to the manufacturer’s instructions.
For real-time quantitative PCR, the following pre-designed mouse TaqMan Gene Expression Assays from Applied Biosystems were utilized: Foxp3, IL-17, IL-23R, and the endogenous control GAPDH. In addition, the following primers and probe for mouse RORγt were synthesized by Applied Biosystems: [forward: 5’-CCGCTGAGAGGGCTTCAC-3’, reverse: 5’-TGCAGGAGTAGGCCACATTACA-3’, probe: 5’-FAM-AAGGGCTTCTTCCGCCGCAGCCAGCAG-MGBNFQ-3’(20). For RORγt, a ready-to-use 20X stock primer/probe mixture was made (primers: 18µM each; probe: 5µM). Each sample was run in triplicate. Reactions were initially denatured at 95°C for 20 seconds, then a two-step amplification was performed at 95°C for 3 seconds and 60°C for 30 seconds for 40 cycles. For quantitative analysis, relative expression levels were determined with 7500 Fast Real-Time System Sequence Detection Software (Applied Biosystems, v1.3.1). Target gene expression levels were normalized to GAPDH. Relative RNA expression levels were determined using the 2−ΔΔCT method.
IL-17 Enzyme-Linked ImmunoSpot (ELISPOT) Assay
CD4+ lymphocytes were harvested from WT Pan02, EV Pan02, or IL-6 Pan02 tumors when the tumor was approximately 1cm in diameter (as described above). The CD4+ lymphocytes were assessed for their ability to produce IL-17 using a commercially available murine IL-17A ELISPOT (eBioscience, San Diego, CA). In precoated wells, 0.75×105or 1.5×105 CD4+ tumor-derived lymphocytes were plated in duplicate wells with medium containing 50ng/ml PMA plus 500ng/ml ionomycin. After approximately 20 hours, the plate was developed according to the manufacturer’s instructions except for the substitution of avidin-alkaline phosphatase for avidin-HRP followed by the alkaline phosphatase substrate BCIP/NBT, and the spots were counted with an ImmunoSpot Series I analyzer (Cellular Technology, Cleveland, OH). Results are presented as the average number of spot-forming cells (SFC) per 5×105 cells plated and corrected for the background medium.
In vitro stimulation
Tumors were harvested from both WT and IL-6 tumor bearing mice when tumors were ~1cm in greatest diameter. Tumors were weighed before creating single cell suspensions using a combination of mechanical and enzymatic digestion per the mPAC tumor protocol provided by Miltenyi Biotec (as described above). Tumor cell suspensions were passed over 70µm and 40µm filters in succession in sterile RPMI before being resuspended in complete RPMI and counted for absolute number of cells. Cells were then incubated for 6 hours at 37°C in complete RPMI in the presence of 5ng/mL PMA and 10uM Ionomycin (Invitrogen, Carlsbad, CA) with Brefeldin-A (Biolegend, San Diego, CA). After stimulation, cells were washed and used for flow cytometry.
Flow cytometry
In order to determine the frequency of Treg cells and Th17 cells within the tumor-infiltrating lymphocyte (TIL) population, flow cytometry was used to analyze TILs after brief stimulation (as described above). After washing, cells were stained with CD45, CD4, CD8, and NK1.1 (all from BioLegend, San Diego, CA) for cell surface analysis. After cell surface staining, cells were stained for intracellular IFN-γ, Foxp3, and IL-17A (all from eBioscience, San Diego, CA) using the Foxp3 staining kit from eBioscience per manufacturer protocol. After gating on CD45+ cells, cells were gated for either CD4+ or CD8+ cells. CD4+ cells were subsequently analyzed for Foxp3 or IL-17. Treg cells were identified as being CD45+CD4+Foxp3+ and Th17 cells were identified as CD45+CD4+IL-17+ cells. CD8+ cells were further analyzed for IFN-γ secretion.
To correct for variation in tumor size, cells of interest (i.e. Th17 cells and Treg cells) were quantified per gram of tumor tissue (cells/gram). The ratio of Th17 and Treg cells could then be calculated for both tumor types. All flow cytometry was analyzed using the LSRII (BD Biosciences, San Jose, CA) and FlowJo analysis software ( FlowJo, Ashland, OR). Pooled samples were used for the analysis.
Statistical analysis
Kaplan-Meier survival curves were generated to compare differences in survival probabilities with the log-rank test. Tumor growth over a period of time was analyzed by using linear trend analysis. Data from ELISA and ELISPOTS were analyzed by the Student’s t-test. All tests were two sided and p<0.05 was considered statistically significant.
RESULTS
Transduction and characteristics of murine pancreatic cancer cell lines
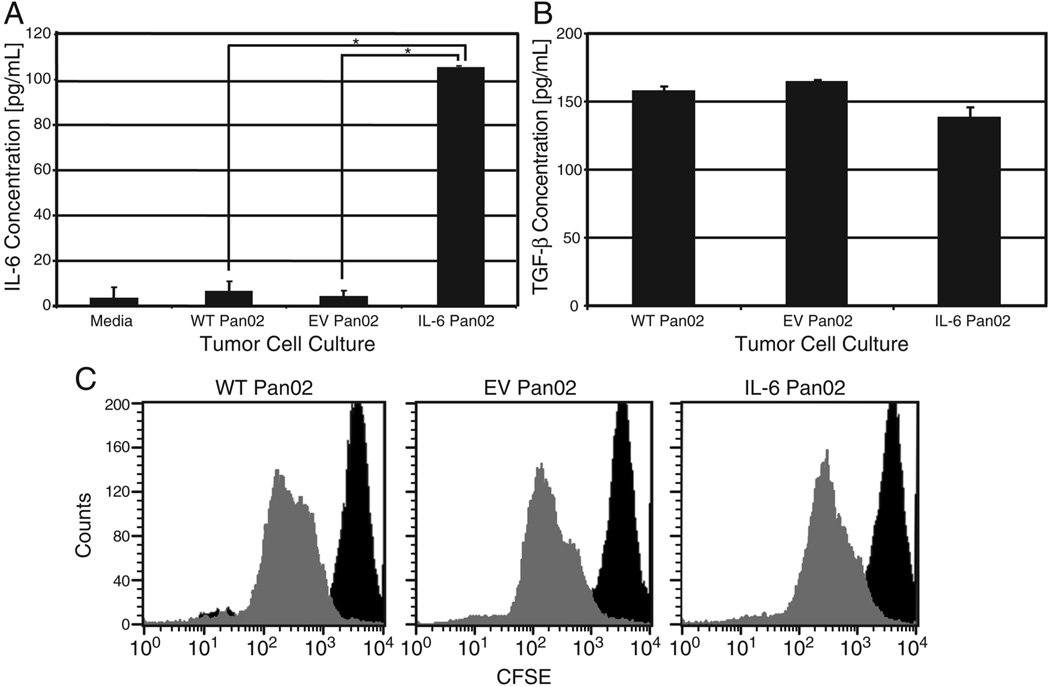
Wild-type Pan02 cells (WT Pan02) were genetically modified to express the murine IL-6 gene (IL-6 Pan02) or empty vector (EV Pan02). IL-6 secretion was confirmed by ELISA for the IL-6 Pan02 whereas no IL-6 was produced by WT Pan02 or EV Pan02 (Figure 1A). The transduced IL-6 Pan02 cells retained their ability to produce and secrete IL-6 in vivo as determined by detection of IL-6 in the serum of IL-6 Pan02 tumor-bearing mice (supplemental Figure 1), and by in vitro analysis of IL-6 Pan02 tumors grown ex vivo (data not shown). ELISA confirmed that all three cell lines produced similar amounts of TGF-β, ensuring that the gene for TGF-β had not been disrupted by the transduction with the lentiviral vector (Figure 1B). All three cell lines had nearly identical in vitro growth characteristics (proliferative rate, doubling time) as determined by CFSE fluorescence (Figure 1C).
Figure 1. Characterization of the IL-6 transduced murine pancreatic cancer cell line Pan02.
(A) Wild-type (WT), empty vector (EV), or IL-6 transduced Pan02 tumor cells were plated in 6-well plates at a density of 2×105 cells/well in 2mL media. The cells were cultured for 2 hours, and IL-6 concentrations in the supernatants were measured by ELISA. *p<0.01. (B) Cells were cultured as in (A) for 2 hours, and TGF-β concentrations in the supernatants were measured by ELISA. Results were normalized to media. In (B), p>0.1 for all Student’s t-tests, indicating no significant difference in TGF-β concentrations for all cell lines. (C) CFSE extinction kinetics, as measured by flow cytometry, showed similar proliferation rates in the WT, EV, and IL-6 Pan02 tumor cell lines. The negative control, at time 0, is indicated by the dark curve on the right. The dilution of CFSE from proliferation at 40 hours is represented by the lighter gray curve on the left. Results represent mean ± SD. Experiments run in triplicate.
In vivo tumor growth and survival
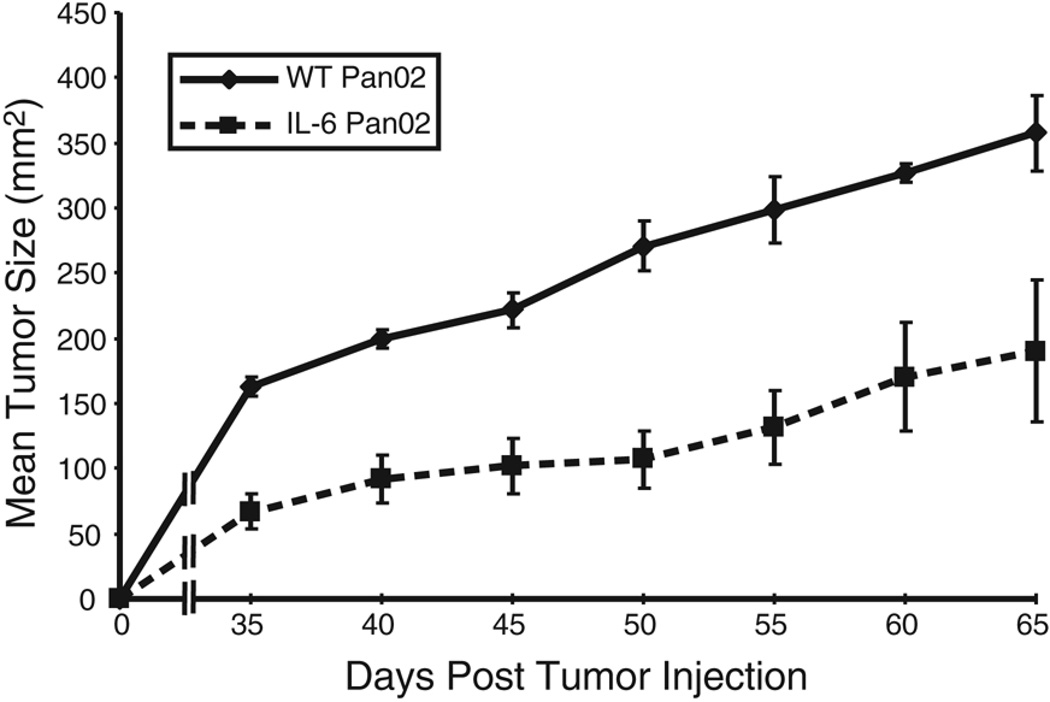
To investigate the impact of IL-6 in the TGF-β-rich pancreatic tumor microenvironment, 1×104 IL-6 Pan02 cells or WT Pan02 cells were injected subcutaneously into the right hind leg of C57BL/6 mice. Tumors were measured every 5 days. Mice injected with IL-6 Pan02 developed significantly smaller tumors compared to mice injected with WT Pan02 tumors (p<0.05, Figure 2). Of the mice injected with IL-6 Pan02 tumors, three of the ten mice never developed palpable tumor. This experiment was repeated three times with similar results. In contrast, all mice injected with WT Pan02 tumor grew tumors and eventually died of disease. Therefore, the production of IL-6 and TGF-β in the Pan02 tumor microenvironment caused a significant reduction in tumor growth.
Figure 2. Tumor growth is reduced in mice injected with IL-6 Pan02 compared to WT Pan02 tumor cells.
Mice (n=10 per group) were injected subcutaneously with 1×104 IL-6 Pan02 or WT Pan02 tumor cells. Tumors were measured every five days. The mice bearing the IL-6 transduced Pan02 cells demonstrated a statistically significant reduction in tumor growth compared to WT Pan02 controls. Three mice receiving the IL-6 Pan02 tumor cells did not grow palpable tumors and are not included in the tumor growth curve. This experiment was repeated independently three times with similar results. p<0.05 for the separation of the tumor growth curves.
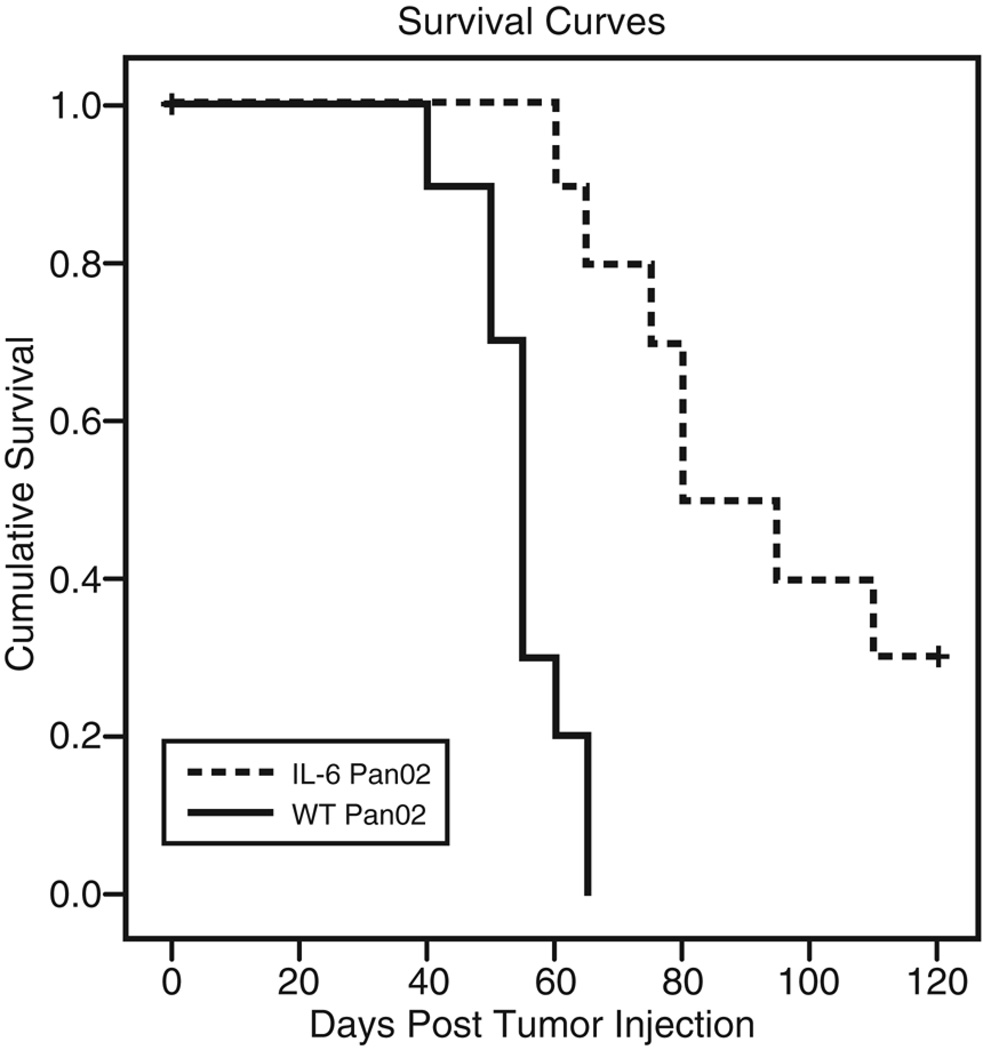
To determine if the reduction in tumor growth also translated into a survival advantage, mice were followed until death. Mice bearing IL-6 Pan02 tumors showed a significant survival advantage compared with mice bearing WT Pan02 tumors (Figure 3). The median survival of mice bearing IL-6 Pan02 tumors was 80 days (range: 60–110 days) compared with 55 days (range: 40–65 days) for WT Pan02 tumors (p<0.001). Thus, the secretion of IL-6 in the tumor microenvironment significantly reduced tumor growth and prolonged survival.
Figure 3. Mice injected with IL-6 Pan02 have a survival advantage over mice receiving WT Pan02 tumor cells.
Mice (n=10 per group) were injected subcutaneously with 1×104 IL-6 Pan02 or WT Pan02 tumor cells and were allowed to die spontaneously or were sacrificed when tumor size was greater than 2 cm or there was severe ulceration of the leg from the tumor that affected ambulation. Either death event was used to generate Kaplan-Meier survival estimates. Mice bearing IL-6 Pan02 tumors showed a significant survival advantage compared with mice bearing WT Pan02 tumors (p<0.001). This experiment was repeated independently three times with similar results.
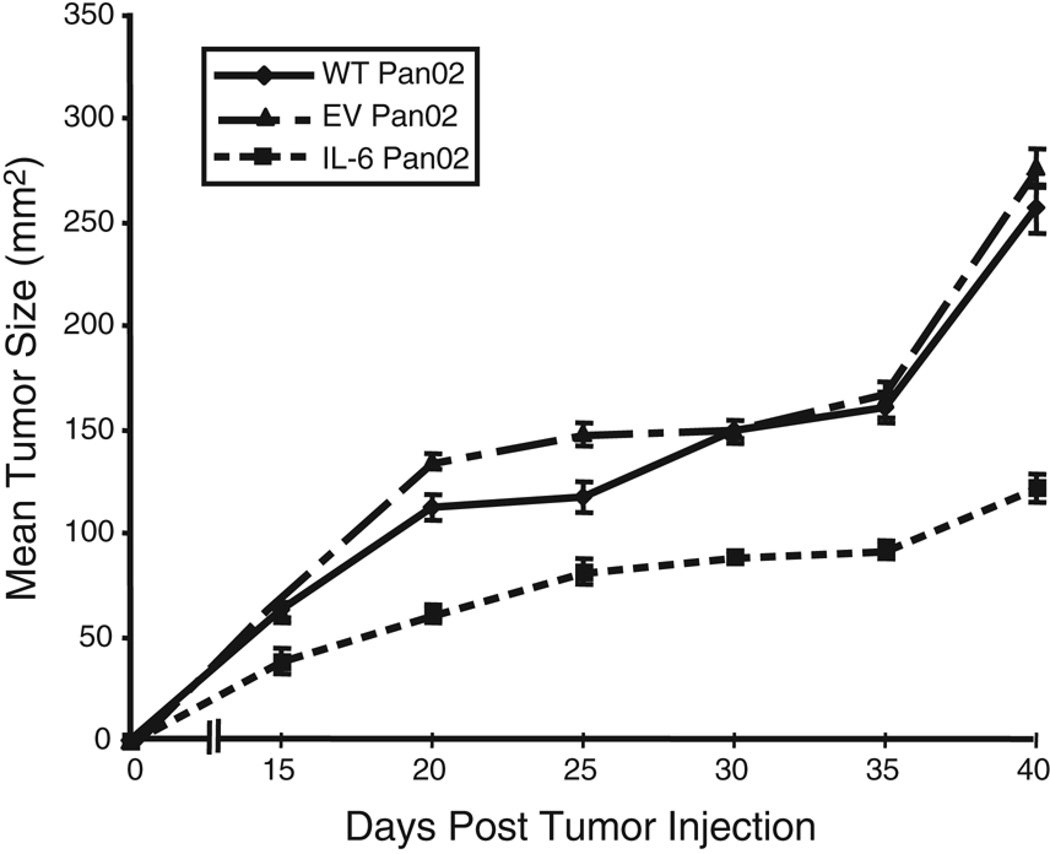
To perform mechanistic studies, mice were inoculated with a greater number of tumor cells so that sufficient numbers of CD4+ tumor-infiltrating lymphocytes (TIL) could be obtained from both IL-6 Pan02 tumors and control tumors. Tumor growth analysis in mice injected with 5×104 tumor cells showed that there was a significant reduction in growth of IL-6 Pan02 tumors compared with either EV Pan02 tumors or WT Pan02 tumors (p<0.05, Figure 4). Analysis of tumor weights at 5 weeks also confirmed that IL-6 Pan02 tumors were smaller than either WT or EV Pan02 tumors. IL-6 Pan02 tumors had an average weight of 0.94g compared with WT Pan02 tumors with an average weight of 1.74g or EV Pan02 tumors with an average weight of 2.12g (p<0.05 for IL-6 tumor weights compare with WT or EV tumor weights).
Figure 4. Tumor growth is reduced in mice injected with 5×104 IL-6 Pan02 compared to EV Pan02 or WT Pan02 tumor cells.
Ten mice were included in each group, and tumors were measured every five days. The mice bearing IL-6 Pan02 tumors demonstrated a statistically significant reduction in tumor growth (p<0.05). All mice grew palpable tumors. This experiment was repeated and the data confirmed three independent times.
Immunohistochemical analysis of the CD4+ tumor lymphocyte infiltrate
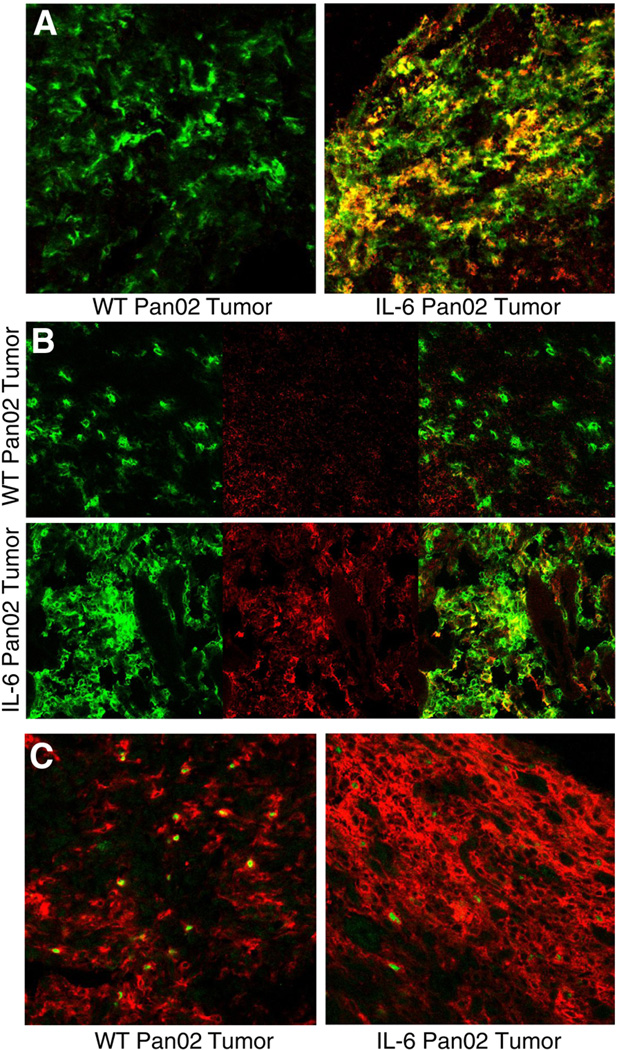
By modifying the tumor cytokine environment with the addition of IL-6 and showing an antitumor effect, we hypothesized that there was either a downregulation of Treg cells or an induction of Th17 cells. Immunohistochemistry was initially performed to analyze the tumor immune infiltrate when tumor sizes were, on average, 1 cm in diameter. IL-6 Pan02 tumors qualitatively showed increased staining for the cell surface IL-23 receptor (IL-23R) and intracellular IL-17 (both markers for Th17 cells) when compared to WT Pan02 tumors (Figures 5A and 5B). Of note, there also appeared to be a greater CD4+ infiltrate in the IL-6 Pan02 tumors. Intracellular staining for Foxp3 was observed in both the IL-6 Pan02 tumors and WT Pan02 tumors (Figure 5C). Thus, it appeared that there was an induction of Th17 cells in the tumors secreting IL-6 and TGF-β.
Figure 5. Immunohistochemical analysis of CD4+ lymphocytic infiltrate from IL-6 Pan02 or WT Pan02 tumor sections for Th17 cells and Treg cells.
(A) Cell surface staining of IL-23 receptor (IL-23R) suggested that IL-6 Pan02 tumors demonstrated more CD4+IL-23R+ cells, indicative of Th17 cells, in the lymphocytic infiltrate compared to the WT Pan02 tumors. (Green: CD4+, Red: IL-23R+, Yellow: CD4+IL-23R+). (B) Intracellular staining of IL-17 cytokine suggested that IL-6 Pan02 tumors again demonstrated more CD4+IL-17+ cells, indicative of Th17 cells, in the lymphocytic infiltrate compared to the WT Pan02 tumors in IHC. (Green: CD4+, Red: IL-17+, Yellow: CD4+IL-17+). (C) Intracellular staining of Foxp3 transcription factor in the WT and IL-6 Pan02 tumors. (Red: CD4+, Green: Foxp3+). More CD4+ lymphocytes were noted in the IL-6 Pan02 tumor sections compared with WT Pan02 tumors.
Quantification of mRNA for Th17 and Treg markers from CD4+ tumor lymphocytes
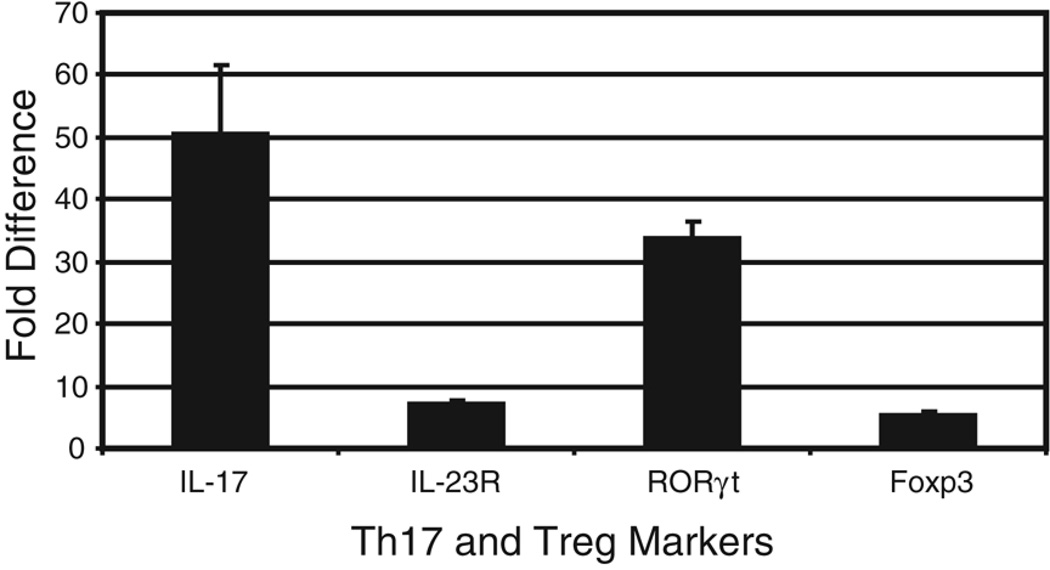
Freshly isolated CD4+ TIL from tumors approximately 1 cm in diameter were lysed and mRNA was purified. Complementary DNA was synthesized from this mRNA and used in a real-time PCR assay as described in the Methods section. RT-PCR for all of the markers of interest were first normalized to the internal control glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Messenger RNA from CD4+ TIL of WT Pan02 tumor cells was used as a reference to compare EV and IL-6 Pan02 CD4+ TIL marker profiles, expressed as a fold difference. Markers of interest included IL-17, RORγt, IL-23R (for Th17 cells) and Foxp3 (for Treg cells). Expression levels of all markers were similar between the WT Pan02 tumors and EV Pan02 tumors (i.e., no fold difference), as expected. However, expression of IL-17, RORγt, and IL-23R in CD4+ TIL from IL-6 Pan02 tumors was significantly higher than in CD4+ TIL from either WT Pan02 tumors or EV Pan02 tumors, further demonstrating that Th17 cells are upregulated in the tumor microenvironment of tumors secreting IL-6 and TGF-β (Figure 6). A small increase in expression of Foxp3 was also noted in the CD4+ TIL from the IL-6 Pan02 tumors.
Figure 6. Expression of IL-17, RORγt, IL-23R, and Foxp3 in CD4+ TIL from mice bearing IL-6 Pan02, EV Pan02, or WT Pan02 tumors.
Real-time PCR quantification of IL-17, RORγt, IL-23R, and Foxp3 in CD4+ TIL from tumors approximately 1cm in diameter. Messenger RNA from CD4+ TIL of WT Pan02 tumor cells was used as a reference to compare EV and IL-6 Pan02 CD4+ TIL marker profiles, expressed as a fold difference. Expression levels of all markers of interest were similar between the WT Pan02 tumors and EV Pan02 tumors. IL-6 Pan02 tumors expressed significantly higher levels of Th17 cell markers (IL-17, RORγt, and IL-23R) and higher levels of the Treg marker Foxp3 compared with CD4+ TIL from the WT Pan02 tumor. Error bars indicate standard error of mean.
Enhanced secretion of IL-17 by CD4+ TIL from IL-6 Pan02 tumors
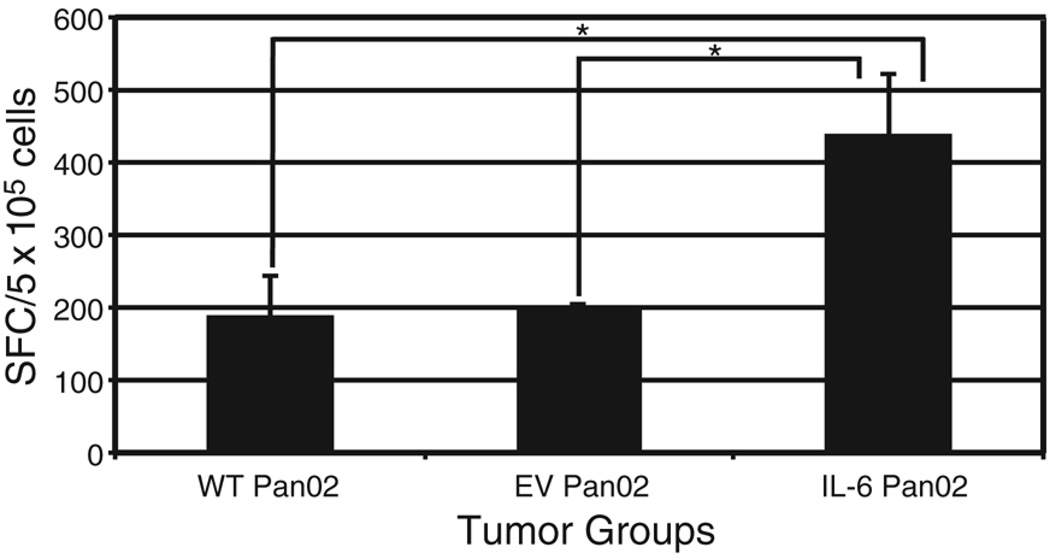
To obtain further confirmation that there was an upregulation of Th17 cells in the tumor microenvironment of mice bearing IL-6 Pan02 tumors, ELISPOT assay was used to detect IL-17 secreting, CD4+ TIL. CD4+ TIL were isolated from IL-6 Pan02, WT Pan02, and EV Pan02 tumors, and stimulated with PMA and ionomycin in IL-17 antibody-coated ELISPOT plates for approximately 20 hours. The ELISPOT assay depicted a significantly higher number of IL-17 secreting CD4+ TIL from the IL-6 Pan02 tumors compared with the EV Pan02 or WT Pan02 CD4+ TIL (Figure 7). This result again supports the hypothesis that Th17 cells are being induced in the tumor microenvironment rich in IL-6 and TGF-β.
Figure 7. A greater number of Th17 cells is present in IL-6 Pan02 tumors compared to WT and EV Pan02 tumors.
ELISPOT quantification of IL-17, a marker for Th17 cells, depicted significant differences between the mean number of IL-17 secreting CD4+ TIL from the IL-6 Pan02 tumors compared with the EV Pan02 or WT Pan02 CD4+ TIL. Shown is the average number of spot-forming cells (SFCs) per 5×105 cells. *p<0.05. Error bars indicate standard error of mean. Experiment run in duplicates.
IL-6 in the tumor microenvironment induces Th17
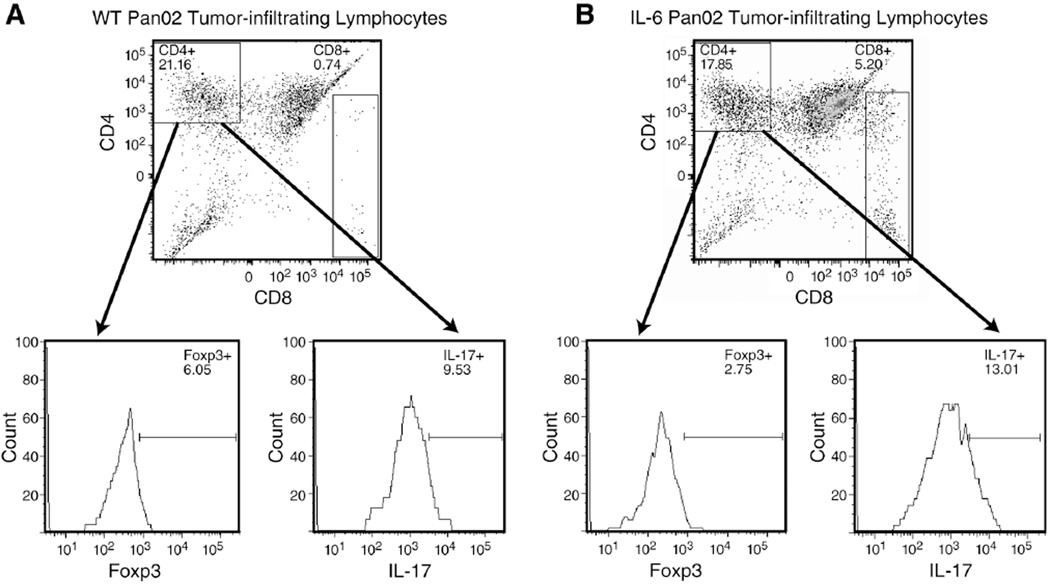
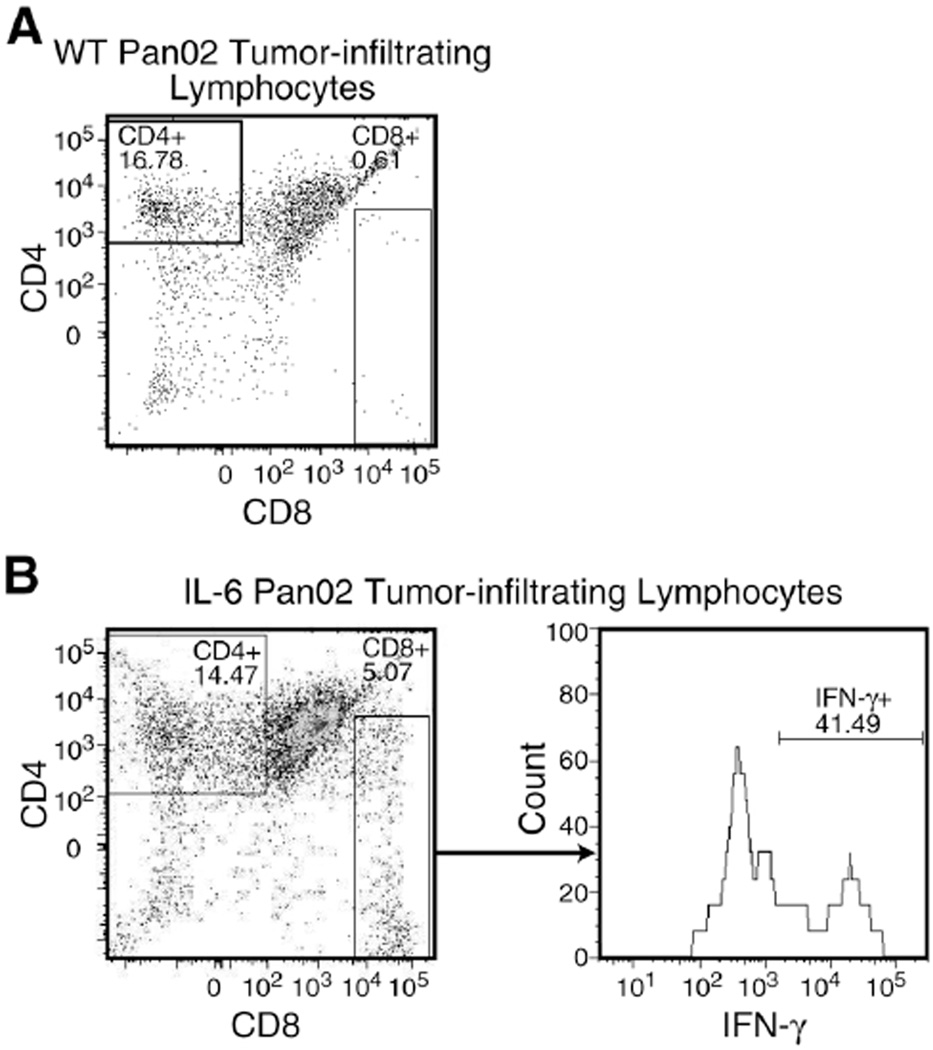
In order to assess if the Treg/Th17 ratio was altered by the addition of IL-6 in the tumor microenvironment, flow cytometry was used to quantify the Th17 and Treg cell infiltration in both tumors (as described in the Methods). Both the WT and IL-6 Pan02 tumors contained similar absolute number of cells per gram of tumor tissue (3.1×107 cells/gram vs. 3.0×107 cells/gram, respectively), but the ratio of Th17 cells to Treg cells were different between the two tumors. The WT Pan02 tumor contained 5.0×104 Treg cells/gram and 8.5×104 Th17 cells/gram. The ratio of Th17 cells to Treg cells in the TIL population of the WT tumor was 1.7. In contrast, the IL-6 Pan02 tumor contained 3.0×104 Treg cells/gram and 2.5×105 Th17 cells/gram. So for the IL-6 Pan02 tumor, the ratio of Th17 cells to Treg cells was 8.3. In addition, the absolute number of Tregs in IL-6 Pan02 tumors was 1.7 times lower than in the WT Pan02 tumors, whereas the number of Th17 cells was 2.9 times higher in IL-6 tumors compared to WT tumors, suggesting that Th17 cells are most likely induced from naïve CD4+ T cells. Gated CD45+CD4+ cells and percentage of Foxp3+ and IL-17+ cells for the WT and IL-6 Pan02 tumor are depicted in Figures 8A and 8B. Thus, the addition of IL-6 to the tumor microenvironment induced Th17 cells and altered the balance of Th17 and Treg cells in the tumor.
Figure 8. Ratio of Th17 to Treg cells is altered in the tumor microenvironment of IL-6 Pan02 tumors compared to WT Pan02 tumors.
Gated CD45+CD4+ cells from stimulated single tumor cell suspensions were analyzed for Foxp3 (Treg cells) and IL-17 (Th17 cells). Calculations were performed using the absolute number of cells per gram of tumor tissue in order to increase accuracy. (A) Tumor suspensions from the WT Pan02 showed a similar ratio of Th17 and Treg cells. The ratio of Th17 cells to Treg cells was 1.7. (B) Tumor suspensions from the IL-6 Pan02 tumor showed a predominance of Th17 cells compared with Treg cells with a ratio of 8.3. Pooled samples were used for analysis.
Greater amount of IFN-γ+ CD8+ cells are in the TIL from IL-6 Pan02 tumors
Since both IL-6 and TGF-β may affect other effector cells, flow cytometry was used to analyze the tumor suspensions for IFN-γ+CD8+ T cells and natural killer cells. Single tumor cell suspensions were again stimulated briefly and flow cytometry was used to analyze the effector cells. In the WT tumor, there were too few CD8+ cells in the TIL population to analyze for IFN-γ secretion (Figure 9A). However, there were considerably more CD8+ cells in the IL-6 Pan02 TIL population with 41.5% secreting IFN-γ (Figure 9B). Cells were also stained for NK1.1, the cell surface marker for natural killer cells, and analyzed by flow cytometry. In both the WT and IL-6 Pan02 TIL population, there were <2% natural killer cells (data not shown). Thus, there was a greater population of IFN-γ+CD8+ T cells in the TIL of the IL-6 Pan02 tumor, and natural killer cells were not predominant effector cells in either the WT or IL-6 Pan02 TIL.
Figure 9. Greater amount of IFN-γ+CD8+ T cells demonstrated in the TIL from IL-6 Pan02 tumors compared with WT Pan02 tumors.
Gated CD45+CD8+ cells from stimulated single tumor cell suspensions were analyzed for IFN-γ secretion. (A) There were too few CD8+ T cells in the suspension from the WT Pan02 tumor to analyze for IFN-γ secretion. (B) IL-6 Pan02 TIL had a substantial number of IFN-γ+CD8+ T cells in the tumor microenvironment. Pooled samples were used for analysis.
DISCUSSION
In this study, we showed that induction of Th17 and altering the Treg/Th17 balance in the tumor microenvironment improves survival. Previous research has shown that Tregs are increased in the tumor microenvironment of patients and mice with pancreatic cancer (1, 6–9). By genetically engineering the murine Pan02 pancreatic adenocarcinoma cell line to express IL-6 cytokine, a Th17 cell population was induced compared with the WT Pan02 or control EV Pan02 tumors as shown by the increase in Th17 cell markers, especially IL-17 and RORγt. Mice injected with the IL-6 expressing Pan02 tumor cells demonstrated significantly delayed tumor growth and improved survival compared to mice bearing WT or EV Pan02 tumors. The different in vivo proliferation was not a direct effect of the IL-6 transduction since in vitro proliferation kinetics were the same between the WT, EV, and IL-6 transduced Pan02 tumors. Thus, the induction of Th17 cells in the tumor microenvironment appeared to mediate an anti-tumor response to the weakly immunogenic Pan02 pancreatic adenocarcinoma.
The few recently published studies on Th17 cells and cancer did not clearly define a pro-tumor or anti-tumor role for Th17 cells. One study found an increased prevalence of Th17 cells in ovarian, renal, and pancreatic cancer and another study found there was a higher percentage of Th17 cells in more advanced gastric cancer (34–36). More specifically, Zhang et al. noted an increased prevalence of Th17 cells in tumor draining lymph nodes of patients with advanced gastric cancer and reported an association between higher gastric cancer stages and increasing percentages of Th17 cells in tumor tissues and peripheral blood (36). Although this may suggest an association between advancing cancer and Th17 cells, it does not take into account the ratio of Treg cells to Th17 cells. It is not surprising that the cytokines in the tumor microenvironment would support the differentiation of both Th17 cells and Treg cells, especially since high levels of both IL-6 and TGF-β are found in late stage tumors (54). Kryczek et al. noted that most of the Th17 cell differentiation in advanced cancers occurred within the tumor and not within the tumor draining lymph nodes, suggesting the importance of cytokine expression in the tumor microenvironment for immune cell differentiation (34). Also, Kryczek et al. showed that the ratio of Treg cells to Th17 cells was significantly higher throughout murine B16 melanoma growth (34). Finally, the increased prevalence of Th17 cells in advanced cancers does not clarify Th17 cell function or how the larger percentage of Treg cells may influence Th17 cell function. Thus, it may be more important to look at Treg to Th17 cell ratio in tumors in order to better elucidate the role of Th17 cells and how their function may be compromised by Treg cells.
There are also recent studies that have shown an anti-tumor effect for Th17 cells. One study showed an inverse correlation between Th17 prostate-infiltrating lymphocytes and Gleason tumor grade in prostate cancer (37). Another study reported a larger percentage of Th17 cells in long-term survivors of small cell lung cancer (SCLC) and patients with limited disease, while patients with extended disease SCLC had more Treg cells compared with effector CD4+ cells, including Th17 cells (38). Here again, the balance between effector CD4+ cells and Treg cells appear to reflect the disease progression, suggesting that a Treg dominant, Treg/Th17 balance is associated with advanced cancer. Further support of Th17 cells in anti-tumor immunity has come from murine models looking at the impact of tumor-specific Th17 cells. Muranski et al. reported Th17-polarized cells specific for a shared self (melanocyte) and tumor antigen, tyrosinase-related protein 1 (TRP-1), could eradicate established B16 murine melanoma tumors and improve survival suggesting a potent anti-tumor effect (39). Even more convincingly, Kottke et al. used a murine model to show that induced inflammatory killing of normal prostate, enhanced through a hsp70 immune adjuvant and IL-6 expression, was mediated by a Th17 cell response. That Th17 cell autoimmune effector response was also found to reject established metastatic prostate tumors but not tumors of another histiologic type (B16 melanoma), suggesting that the resultant anti-tumor response was against tissue-specific antigens (40). The data presented in this paper showed that mice bearing Pan02 tumors that secreted IL-6 also induced the differentiation of Th17 cells in the tumor microenvironment which resulted in delayed tumor growth and improved survival. Thus, it is likely that Th17 cells play some role in immune defense against tumor cells and that there may be an intimate connection between the Th17 cell response in autoimmune disease and anti-tumor immunity.
The skewing of tumor-infiltrating lymphocytes in the Pan02 murine model was dependent on the secretion of the IL-6 cytokine. As mentioned above, the skewing of tumorinfiltrating lymphocytes towards Treg dominance implies immune suppression and cancer progression, while T effector cell dominance (including Th17 cells) supports tumor rejection. The addition of IL-6 to the TGF-β-rich tumor microenvironment from our transduced cells skewed the tumor-infiltrating lymphocytes toward Th17 cells. We also anticipated inhibition of Treg cell differentiation since IL-6 has been shown to inhibit Tregs in vitro (14). Interestingly, there appeared to be similar, if not slightly lower, absolute numbers of CD4+Foxp3+ cells in the IL-6 Pan02 tumors compared to controls. Although the quantity of Treg cells remained almost unchanged, the skewing of tumor-infiltrating lymphocytes towards Th17 cells appeared to overcome the function of the Tregs. There has been much research demonstrating that the blockade or depletion of Treg cells slowed tumor growth and improved survival (9, 44–46). We have now discovered a novel way to suppress Treg function to promote a tumor-specific immune response.
The question that remains is how the upregulation of Th17 cells in the tumor microenvironment mediates its anti-tumor effect. Th17 cells secrete many cytokines that allows for the bridging of innate and adaptive immunity. IL-17 cytokine alone has been implicated in both impairing and promoting tumor immunity (55–58). IL-17 promotes dendritic cell maturation and may allow for better tumor antigen presentation and, consequently, a stronger T cell response. Th17 cells also secrete IL-6 which has been shown to have strong anti-tumor immunity and may activate tumor-specific cytotoxic T lymphocytes (59–61). Our results, with the greater population of IFN-γ+CD8+ T cells in the TIL of the IL-6 Pan02 tumor, support that thought. Finally, there may also be a neutrophil or natural killer cell recruitment to the tumor site that cooperates with other effector cells for a tumor-specific immune response. However, natural killer cells were not a large part of the lymphocyte infiltrate in either the WT or IL-6 Pan02 tumor. Therefore, the downstream effects of Th17 cells and the cytokines secreted need to be more fully investigated to illuminate the anti-tumor effects.
In conclusion, these findings provide evidence that altering the cytokines in the tumor microenvironment can shift the balance between Treg and Th17 cells in the tumor-infiltrating lymphocytes. In this murine model of pancreas cancer, the addition of IL-6 to a TGF-β-rich tumor microenvironment allowed for the differentiation of Th17 cells and a greater population of IFN-γ+CD8+ T cells. This skewing of the tumor-infiltrating lymphocytes towards effector Th17 cells resulted in significantly delayed tumor growth and improved survival. This suggests that Th17 cells may play an important role in anti-tumor immunity and strategies aimed at improving Th17 recruitment or differentiation in the tumor microenvironment may improve the efficacy of cancer treatments.
Acknowledgments
This research was supported by the 5T32CA009621-19 Surgical Oncology Training Grant, awarded by the National Institutes of Health and in part by the Frank Cancer Research Fund, awarded by the Barnes-Jewish Hospital Foundation. Tissue sectioning was performed through the Washington University Digestive Disease Research Core Center (DDRCC) (Grant 5P30 DK052574).
Abbreviations used in this paper
- Treg
regulatory T cell
- WT
wildtype
- EV
empty vector
- TIL
tumor-infiltrating lymphocytes
REFERENCES
- 1.Liyange UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 2.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 3.von Bernstorf W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7(3 Suppl):925s–932s. [PubMed] [Google Scholar]
- 4.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. CD4(+)CD25(+) T cells in tumors from patients with early stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 5.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 6.Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fuji M, Maekawa Y, Yasutomo K, Shimada M. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas. 2006;33(4):386–390. doi: 10.1097/01.mpa.0000240275.68279.13. [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 8.Liyanage UK, Goedegebuure PS, Moore TT, Viehl CT, Moo-Young TA, Larson JW, Frey DM, Ehlers JP, Eberlein TJ, Linehan DC. Increased prevalence of regulatory T cells (Treg) is induced by pancreas adenocarcinoma. J Immunother. 2006;29(4):416–424. doi: 10.1097/01.cji.0000205644.43735.4e. [DOI] [PubMed] [Google Scholar]
- 9.Linehan DC, Goedegebuure PS. CD25+CD4+ regulatory T cells in cancer. Immunol Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 10.Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Keulen VPVan, Marler RJ, Felts SJ, Pease LR. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008;181:3137–3147. doi: 10.4049/jimmunol.181.5.3137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Sharma MD, Hou DY, Liu Y, koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in the tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 15.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181(9):55948–55955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs Th-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 18.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and type 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 19.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park H, Zhaoxia L, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71(1):1–8. [PubMed] [Google Scholar]
- 24.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14(1):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 25.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Mahapatra BDas, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettelli E, Oukka M, Kuchroo V. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 29.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 30.Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176(12):7768–74. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- 31.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17 deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 32.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;188(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting Edge: Th17 and regulatory T cell dynamics and regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178(11):6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 35.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105(40):15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374(3):533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 37.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14(11):3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyama K, Kagamu H, Miura S, Hiura T, Miyabayashi T, Itoh R, Kuriyama H, Tanaka H, Tanaka J, Yoshizawa H, Nakata K, Gejyo F. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62highCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14(21):6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 39.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kottke T, Sanchez-Perez L, Diaz RM, Thompson J, Chong H, Harrington K, Calderwood SK, Pulido J, Georgopoulos N, Selby P, Melcher A, Vile R. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67(24):11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakauchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 42.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moo-Young TA, Larson JW, Belt BA, Tan MC, Hawkins WG, Eberlein TJ, Goedegebuure PS, Linehan DC. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J Immunother. 2009;32(1):12–21. doi: 10.1097/CJI.0b013e318189f13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 45.Sutmuller RP, Duivenvoorde LMvan, Elsas Avan, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viehl C, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol. 2006;13(9):1252–1258. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- 47.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naïve T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park HB, Paik DJ, Jang E, Hong S, Youn J. Acquisition of anergic and suppressive activities in transforming growth factor-beta costimulated CD4+CD25− T cells. Int Immunol. 2004;16(8):1203–1213. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- 50.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schramm C, Huber S, Protshcka M, Czochra P, Burg J, Schmitt E, Lohse AW, Galle PR, Blessing M. TGFbeta regulates the CD4+CD25+ T-cell pool and the expression of Foxp3 in vivo. Int Immunol. 2004;16(9):1241–1249. doi: 10.1093/intimm/dxh126. [DOI] [PubMed] [Google Scholar]
- 52.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173(11):6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 53.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Jr., Schabel FM., Jr. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–726. [PubMed] [Google Scholar]
- 54.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 55.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, Banchereau J, Fridman WH, Wijdenes J, Lebecque S, Sautes-Fridman C. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59(15):3698–3707. [PubMed] [Google Scholar]
- 56.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 57.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 58.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99(6):2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 59.Porgador A, Tzehoval E, Katz A, Vadai E, Revel M, Feldman M, Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992;52(13):3679–3686. [PubMed] [Google Scholar]
- 60.Mullen CA, Coale MM, Levy AT, Stetler-Stevenson WG, Liotta LA, Brandt S, Blaese RM. Fibrosarcoma cells transduced with the IL-6 gene exhibited reduced tumorigenicity, increased immunogenicity, and decreased metastatic potential. Cancer Res. 1992;52(21):6020–6024. [PubMed] [Google Scholar]
- 61.Mule JJ, Custer MC, Travis WD, Rosenberg SA. Cellular mechanisms of antitumor activity of recombinant IL-6 in mice. J Immunol. 1992;148(8):2622–2629. [PubMed] [Google Scholar]