Abstract
Background. Recent advances in rational adjuvant design and antigen selection have enabled a new generation of vaccines with potential to treat and prevent infectious disease. The aim of this study was to assess whether therapeutic immunization could impact the course of Mycobacterium tuberculosis infection with use of a candidate tuberculosis vaccine antigen, ID93, formulated in a synthetic nanoemulsion adjuvant, GLA-SE, administered in combination with existing first-line chemotherapeutics rifampicin and isoniazid.
Methods. We used a mouse model of fatal tuberculosis and the established cynomolgus monkey model to design an immuno-chemotherapeutic strategy to increase long-term survival and reduce bacterial burden, compared with standard antibiotic chemotherapy alone.
Results. This combined approach induced robust and durable pluripotent antigen-specific T helper–1-type immune responses, decreased bacterial burden, reduced the duration of conventional chemotherapy required for survival, and decreased M. tuberculosis–induced lung pathology, compared with chemotherapy alone.
Conclusions. These results demonstrate the ability of therapeutic immunization to significantly enhance the efficacy of chemotherapy against tuberculosis and other infectious diseases, with implications for treatment duration, patient compliance, and more optimal resource allocation.
(See the editorial commentary by McMurray on pages 1193–4.)
Worldwide, the tuberculosis pandemic is associated with 1.7–2 million deaths annually, and the increase in multidrug-resistant tuberculosis (MDR-tuberculosis) further heightens this threat [1]. There is an urgent need for more effective therapeutic regimens to increase treatment compliance and decrease tuberculosis transmission [2–6]. The development of rationally designed molecular adjuvants that stimulate innate immune responses and shape the quality and strength of adaptive immunity, combined with select recombinant proteins, has enabled the development of a new generation of vaccines that can be used to treat and prevent infectious diseases.
Although development of more effective prophylactic vaccines for tuberculosis is a high priority, therapeutic approaches, such as postexposure vaccines, which could be used in combination with antibiotics to shorten treatment regimens, clear bacilli [7], and limit the spread of MDR-tuberculosis [8–10], should be explored. Using a combination of drugs plus vaccine, we showed therapeutic efficacy among patients infected with another macrophage pathogen of the genus Leishmania [11], supporting the potential of an immune-therapeutic approach. In this regard, few tuberculosis vaccine candidates have been evaluated for therapeutic efficacy [12–23].
We identified potent T cell antigens of Mycobacterium tuberculosis (M. tuberculosis), recognized by persons latently infected with M. tuberculosis, and used a synthetic nanoemulsion adjuvant, GLA-SE (a synthetic TLR-4 agonist [GLA], formulated in a stable oil-in-water emulsion [SE]), that adds an innate signal and potent Th1-inducing properties [24–26], to develop a vaccine candidate, ID93/GLA-SE [12–14]. ID93 combines 4 antigens belonging to families of M. tuberculosis proteins associated with virulence (Rv2608, Rv3619, and Rv3620) or latency (Rv1813) [27]. To adequately evaluate therapeutic vaccines, it is essential that long-term models of protection against diseases be developed and used. To do this, we have used both mouse and nonhuman primate (NHP) models. Unlike C57BL/6 and BALB/c mice, which exhibit stabilized pulmonary bacterial growth and survive for >1 year [7, 28–31], SWR/J mice exhibit extreme M. tuberculosis susceptibility, with progressive bacterial growth resulting in fatal disease, thus making them a good postexposure model to evaluate immunotherapeutic regimens [29]. The reasons for this increased susceptibility are unclear. Nevertheless, we have evidence that ID93/GLA-SE–immunized SWR/J mice mount effective immune responses and protection against M. tuberculosis (unpublished data). The cynomolgus monkey has been described as a good model for human tuberculosis, displaying a range of clinical and pathological changes [32], and may be efficiently protected against M. tuberculosis challenge [17, 33, 34]. To model synergy between chemo- and immune-therapy, we performed studies using ID93/GLA-SE, administered in combination with existing first-line antibiotics rifampicin (RIF) and isoniazid (INH) in SWR/J mice and cynomolgus monkeys. These data show that therapeutic immunization can be used to complement chemotherapy as an approach to treat tuberculosis.
MATERIALS AND METHODS
Mice, Treatment, and ID93/GLA-SE Immunization
Female, age-matched (4–6 weeks) SWR/J and C57BL/6 mice were purchased from Jackson and Charles River Laboratories, respectively. All mice were maintained in the animal facility of The Infectious Disease Research Institute (IDRI) and were treated in accordance with the guidelines of the Animal Care and Use Committee.
Mice were infected with a low dose (50–100 bacteria) aerosol (LDA) of M. tuberculosis H37Rv (ATCC #27294) with use of a University of Wisconsin–Madison aerosol chamber. At 15 or 30 days after infection, a subset of mice was started on a drug regimen of INH (at 85 mg/L of drinking water) and RIF (at 50 mg/L of drinking water) administered for 30, 60, or 90 consecutive days. Female mice are estimated to drink 0.15–0.37 mL/g [35]. Assuming a mean intake of 0.26 mL/g per day, animals would receive approximately 22 mg/kg of INH and 13 mg/kg of RIF per day. The minimum inhibitory concentrations for M. tuberculosis H37Rv are 0.25 μM for RIF and 1.0 μM for INH. A subset of groups receiving the 60–90-day RIF-INH combination regimen was also injected with either GLA-SE alone (referred to as Rx + GLA-SE) or the ID93/GLA-SE vaccine (referred to as Rx + ID93/GLA-SE), which were produced as previously reported [14, 24, 25]. Mice were immunized 3 times, 3 weeks apart, with 8 μg of protein plus 20 μg of GLA-SE either during (DTT; days 15, 36, and 57) or after antibiotic treatment (PTT; days 107, 128, and 149). Therapeutic efficacy was determined by tracking survival over time and by plating lung homogenates as previously described [13].
Cytokine Profiling Assay
Splenocytes (2 × 106/mL) were stimulated with ID93 (10 μg), purified protein derivative (10 μg; CSU), or phosphate-buffered saline. Cytokine concentrations (IFN-γ, IL-2, TNF, IL-5, IL-10, IL-13, and IL-17) were determined using a Procarta Luminex-based assay (Affymetrix).
Flow Cytometry
At various times after the last immunization (days 149 and 177), splenocytes were stained with fluorochrome-conjugated mAb anti-CD3, CD8, CD44, IFN-γ, TNF, IL-2 (eBioscience), and CD4 mAb (Invitrogen) and analyzed on a LSRII flow cytometer (BD Biosciences). Splenocytes were gated by forward and side scatter to identify intact round cells; 20 000 CD3+CD8+ events were acquired for each sample and analyzed using FlowJo (Treestar) and SPICE (courtesy of Mario Roederer, National Institutes of Health).
Histopathology
Mice
At various times (days 106, 241, or 295 after infection), 1 lobe of each lung was fixed in 10% neutral buffered formalin. After fixation, tissues were embedded in paraffin and stained with hematoxylin and eosin (H&E), Fite's acid fast stain, or Trichrome stain by the Benaroya Research Institute Histology Core facility (Seattle, WA). Random images of each lung were taken for H&E and acid-fast bacteria (AFB) with use of a Nikon DS-L2 digital camera. Image Pro Plus was used for analysis of digitized images. Qualitative analyses were performed by a veterinary pathologist blinded to the source of specimens.
Non-Human Primates
A single lobe of the lung was fixed in 10% normal buffered formalin, embedded in paraffin, and stained with H&E, Fite's acid fast stain, or Trichrome stain. Qualitative analyses were performed by a veterinary pathologist and included comparing all fields in a group for organization of cellular infiltrate, number of granulomas, visual quantification of AFB, and pulmonary fibrosis (Biogenetics Research Laboratories).
Lesion Scoring
Lesions were scored as follows: 0, normal tissue morphology with no lesion present; 1, lesions involving < 10% of the tissue and minimal infiltration of inflammatory cells; 2, lesions affecting 10%–20% of the tissue and mild cellular infiltration; 3, lesions covering 21%–40% of the tissue and moderate cellular infiltration; and 4, lesions covering 41%–100% of the tissue and marked cellular infiltration.
Safety Evaluations in NHP
Details of experimental animals, M. tuberculosis infection, radiographic, clinical, necropsy, and bacterial burden procedures have been previously reported [17]. In brief, adult, cynomolgus macaques (Macaca fascicularis; 7 per group) were used and maintained in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. Before commencement and during the studies, the macaques underwent a rigorous battery of diagnostic and clinical procedures (eg, physical examination, complete blood count, erythrocyte sedimentation rate, serum chemistry, thoracic radiography, and tuberculin skin testing). Macaques were inoculated intratracheally with a high dose of approximately 5 × 102 CFU of virulent M. tuberculosis (Erdman strain) in a 1.0 mL volume to ensure the development of active tuberculosis. At 8 weeks after infection, a subset of macaques was started on a drug regimen of INH (15 mg/kg) and RIF (15 mg/kg) delivered by gavage (Rx) 3 times per week for a 4-week period or were left without further treatment (Mock). A subset of macaques was then immunized intramuscularly 3 times 2 weeks apart with 10 μg of ID93 plus 25 μg of GLA-SE (Rx + ID93/GLA-SE).
Statistical Analysis
Standard one-way analysis of variance (ANOVA), followed by Dunnett's Multiple Comparison Test, were used to determine statistical significance of CFU and other normally distributed sample populations. For nonnormally distributed sample populations, a nonparametric Wilcoxon rank-sum test was used to compare individual groups. Categorical data were analyzed using Fisher's exact test, and a log rank test was used to evaluate statistical differences between survival curves. P ≤ .05 was considered to be statistically significant; however, because sample sizes in the NHP studies were small, the statistical power of these studies was limited. Therefore, P < .05 for statistical significance should not necessarily be interpreted as precluding an important difference between groups. Statistical analyses were performed using GraphPad Prism, version 4.00, for Windows (GraphPad Software) or JMP, version 8.0 (SAS Institute).
RESULTS
Development of the SWR/J Mouse Model of tuberculosis Relapse and Reactivation
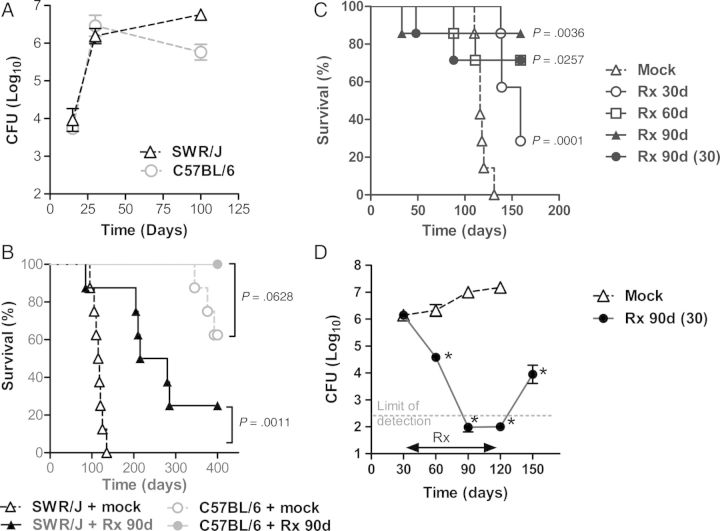
The purpose of this work was to establish a model for postexposure strategies and to elucidate the immunotherapeutic efficacy of ID93/GLA-SE. In contrast to C57BL/6 mice [36] and consistent with previous observations [29, 37], SWR mice failed to transition to a chronic state after M. tuberculosis infection (Figure 1A). Mock-treated SWR/J mice died of infection with a median survival time (MST) of 116.5 days, whereas those treated with RIF-INH for 90 days had an MST of 247.5 days (P < .001; log rank test) (Figure 1B).
Figure 1.
Bacterial burden and survival of SWR/J and C57BL/6 mice infected with Mycobacterium tuberculosis (M. tuberculosis) and treated with antibiotics. SWR/J and C57BL/6 mice were infected with an LDA of M. tuberculosis H37Rv. (A) The number of viable bacteria in the lungs (5 mice/group) were determined 15, 30, and 100 days after infection. Symbols indicate the mean ± the SD. (B) The survival of SWR/J and C57BL/6 mice was monitored in animals (8 mice/group) infected with M. tuberculosis H37Rv and mock or treated with a 90-day antibiotic regimen (Rx 90d) consisting of INH and RIF administered on days 30–120. (C) SWR/J mice were infected with a LDA of M. tuberculosis H37Rv and treated with 30, 60, or 90 days of antibiotics starting on day 15 (Rx 30d, 60d, 90d) or on day 30 (Rx 90d (30)). Survival of SWR/J mice (7 mice/group) is shown. (D) Number of viable bacteria in the lungs of animals (5 mice/group) mock or treated with a 90-day INH/RIF regimen (Rx 90d) administered on days 30–120 was determined 30, 60, 90, 120, and 150 days after infection. *P < .05 (one-way ANOVA followed by Dunnett's Multiple Comparison Test or log rank test) is considered to be statistically significant. One representative of 2 experiments is shown.
To determine optimal lengths of treatment in SWR/J mice, animals were treated with RIF-INH for either a 90-day regimen or for shortened periods of 30 or 60 days. Significant differences in survival and recoverable lung CFU between animals that were mock- or drug-treated for 30 (P < .0005; log rank test), 60 (P < .05; log rank test), or 90 days (P < .005; log rank test) were observed (Figure 1C). Changing the initiation of chemotherapy from 15 to 30 days after infection did not significantly alter the long-term efficacy of treatment (P > .50; log rank test) (Figure 1C). Although 60 or 90 days of chemotherapy was sufficient to decrease the number of viable lung bacteria below the limit of detection (Figure 1D), these treatment regimens were insufficient to achieve clearance of M. tuberculosis in SWR/J mice.
Therapeutic Vaccination with ID93/GLA-SE as an Adjunct to Chemotherapy Enhanced Survival and Shortened the Required Treatment Time
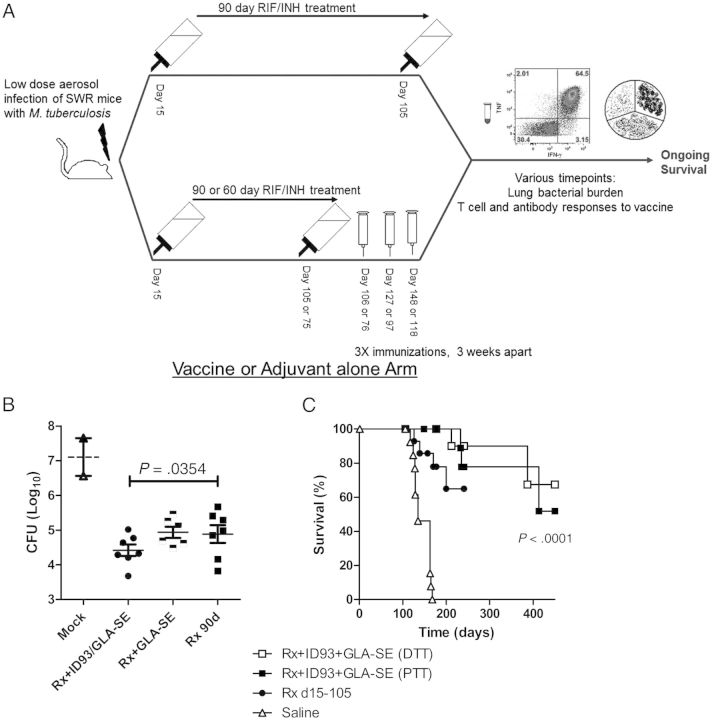
Three doses of the candidate tuberculosis vaccine fusion proteins ID83 and ID93 or their component antigens combined with GLA-SE as an adjuvant have previously provided prophylactic protection against tuberculosis in mouse and guinea pig models [12, 14]. We asked whether the ID93/GLA-SE vaccine would provide immunotherapeutic benefit as measured by reduction of CFU or improved survival (Figure 2A) and found that these vaccines increased the frequency of SWR/J survival after infection (P < .01).
Figure 2.
Colony-forming unit counts and survival of SWR/J mice infected with a LDA of Mycobacterium tuberculosis (M. tuberculosis) and treated with antibiotics and ID93/GLA-SE. SWR/J mice were infected with LDA of M. tuberculosis (day 0). Fifteen days later (day 15), mice were mock or antibiotic treated for 90 days (Rx 90d). A subset of antibiotic-treated mice in each group was also immunized 3 × 3 weeks apart with ID93/GLA-SE either during- (DTT; days 15, 36, 57) or after antibiotic treatment (PTT; days 107, 128, 149). (A) Scheme of immunotherapy experiments. (B) Number of viable bacteria in the lungs of animals (6 or 7 mice/group) was determined 177 days after infection. *P < .05 is considered to be statistically significant. (C) Protection was assessed by monitoring animal deaths (9 or 10 mice/group) caused by M. tuberculosis over time. One representative of 4 experiments is shown. P < .05 (log rank test) is considered to be statistically significant.
Experiments were undertaken to evaluate efficacy of the antibiotic regimen with ID93/GLA-SE or GLA-SE alone (Figure 2B). Efficacy was assessed on the bases of quantitative cultures of lung homogenates and survival over time. Compared with chemotherapy (Rx) alone, immunization with ID93/GLA-SE as an adjunct to chemotherapy further reduced CFU by 0.643 log10 (P < .05) (Figure 2B). No differences in lung CFU were observed between the groups administered GLA-SE adjuvant and chemotherapy (Rx + GLA-SE), compared with Rx (P > .05) (Figure 2B). Moreover, there was a significant difference between the postexposure efficacy induced by the Rx + ID93/GLA-SE and the Rx + GLA-SE groups (4.419 ± 0.17 vs 4.938 ± 0.16 log10; P < .05), showing that the adjunctive bactericidal effect observed in these studies is antigen dependent.
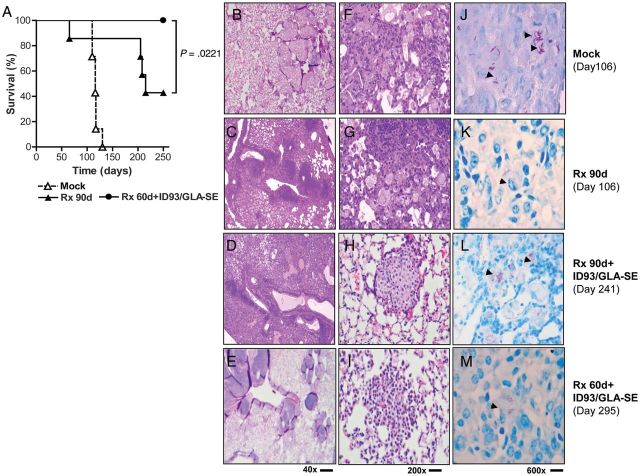
Vaccine administered as an immunotherapeutic after (PTT) or during (DTT) 90 days of treatment prevented death in 52% and 67% of M. tuberculosis-infected mice, respectively, (P < .0001) (Figure 2C). Whereas 40% of the animals receiving 90 days of chemotherapy alone survived M. tuberculosis infection (MST, 214 days), 100% of the animals receiving vaccine immunotherapy after 60 days of chemotherapy survived for at least 250 days (P < .05) (Figure 3A). Of importance, these studies demonstrate that vaccine immunotherapy could reduce the duration of therapy by at least one-third while preventing death for an extended period after chemotherapy was withdrawn. Our data thus suggest that the ID93/GLA-SE vaccine administered in conjunction with antibiotics could be used to shorten standard chemotherapy regimens (Figure 3A), with a possible positive impact on compliance.
Figure 3.
Survival of SWR/J mice infected with Mycobacterium tuberculosis (M. tuberculosis) and treated with the ID93/GLA-SE vaccine and reduced antibiotic chemotherapy. SWR/J mice were infected with an LDA of M. tuberculosis H37Rv. Fifteen days later, mice were treated for 60 or 90 days with antibiotics (Rx 60d and Rx 90, respectively). After the completion of the 60-day antibiotic regimen, mice were immunized 3 × 3 weeks apart with ID93/GLA-SE. (A) Protection was assessed by monitoring animal deaths (7 mice/group) due to M. tuberculosis over time. P < .05 (log rank test) is considered to be statistically significant. (B–M) Histopathological evaluation of lung tissues after challenge with M. tuberculosis H37Rv. Inflammatory responses and granuloma (g) formation are shown in H&E sections (B–I), and the presence of AFB (arrows) (J–M) was evaluated. (B, F and J) Mock-treated mice, day 106; (C, J and K) 90-day antibiotic therapy, day 106; (D, H and L) 90-day antibiotic therapy + ID93/GLA-SE, day 241; (E, I and M) 60-day antibiotic therapy + ID93/GLA-SE, day 295 Data shown are representative of 5 mice/group. One representative of 3 experiments is shown.
Therapeutic Vaccination with ID93/GLA-SE as an Adjunct to Antibiotic Treatment Reduces Tuberculosis Lung Pathology
To characterize the effect of antibiotics combined with ID93/GLA-SE on lung pathology, sections from mock-, Rx-, and Rx + ID93/GLA-SE–treated mice were taken for histological analysis on various days until experiments were terminated (Table 1, Figure 3B–M). The lungs of mock-treated mice had diffuse alveolar edema (Figure 3B and F) with grade 3–4 (40%–100%) involvement of the lung parenchyma appearing greatly inflamed and necrotic as previously reported [29, 38], with numerous acid-fast bacilli (>30/600x high power field) (Figure 3J). The lung sections of the Rx90d group showed obvious resolution of inflammatory lesions (Figure 3C and G) with only rare bacilli (<1/HPF) (Figure 3K; Table 1). At day 241, the lungs of Rx 90d + ID93/GLA-SE mice had numerous granulomas (Figure 3D and H) and few bacilli (≤6 organisms/HPF, 600x) (Figure 3L; Table 1). At day 295, lungs of mice treated with 60 days of antibiotics and immunized with ID93/GLA-SE showed no significant lesions (Figure 3E and I; Table 1) and few bacilli (Figure 3M).
Table 1.
Effects of ID93/GLA-SE Immunotherapy on Lung Pathology of Mycobacterium tuberculosis–Infected SWR/J
| Groupa | Lesion Grade | Lung (%)b | Lung AFBc | Granuloma structure | Diagnosis |
|---|---|---|---|---|---|
| Mock (Day 106) | 3–4 Moderate-Marked | 40–100 | 6–30 | Coalescing macrophage nodules, with syncytial giant cells | Histiocytic alveolar and interstitial pneumonia, moderate to marked; granulomatous lobar bronchopneumonia. |
| Numerous AFB in lesions | |||||
| Rxd (Day 106) | 0–2 Mild-Moderate | 0%–40% | <1 | No nodular granulomas, Few macrophages | Histiocytic alveolar and interstitial pneumonia, mild to moderate. |
| Resolution of large lesions | Minimal AFB in lesions | ||||
| Rxd (Day 241, 295) | 4 Marked | 41–100 | ≤30 | No significant histiocytic granulomas, no syncytial macrophages | Histiocytic alveolar and interstitial pneumonia, marked. |
| Many AFB in lesions | |||||
| Rxd + ID93/GLA-SEe (Day 241) | 2 Mild-Moderate | 11–40 | ≤6 | Histiocytic granulomas with syncytial macrophages | Histiocytic alveolar and interstitial pneumonia, mild-moderate. |
| Several small dense lymphoid aggregates | Few or no AFB in lesions | ||||
| Rxf + ID93/GLA-SEe (Day 295) | 1–3 Minimal-Moderate | 0–40 | ≤1–6 | Histiocytic granulomas with syncytial macrophages | Histiocytic alveolar and interstitial pneumonia, minimal-moderate. |
| Minimal, multifocal, infiltration of lymphocytes | Few AFB in lesions |
a Data are representative of 3–5 animals per group.
b Percent of lung tissue involved: Minimal (grade 1 or <10%); Mild (grade 2 or 11%–20%); Moderate (grade 3 or 21%–40%); Marked (grade 4 or 41%–100%).
c Number of AFB/HPF, 600x.
d 90 day INH/RIF chemotherapy initiated 15 days following infection with M. tuberculosis.
e Mice were immunized 3 times, 3 weeks apart after the administration of a chemotherapy treatment.
f 60 day INH/RIF chemotherapy initiated 15 days following infection with M. tuberculosis.
ID93/GLA-SE Elicits Robust TH1 Immune Responses in SWR/J Immunized Mice
Experiments were conducted to characterize the immunogenicity of ID93/GLA-SE in SWR/J mice after M. tuberculosis infection. In response to in vitro restimulation with ID93, a subset of cytokines representing pro-inflammatory and TH1 and TH2 functional groups was significantly up-regulated (Figure 4A). TNF, a soluble mediator of M. tuberculosis-specific immunity in infected individuals, was significantly up-regulated at day 241 in the group immunized with ID93/GLA-SE (P < .05). In addition, ID93-specific IFN-γ, IL-2, and IL-17 responses were detected, which were significantly higher in vaccinated animals, compared with unvaccinated animals. No statistically significant difference in the concentration of the TH2-type IL-5 cytokine was detected, but significant ID93-specific IL-10 and IL-13 responses were measured at day 241.
Figure 4.
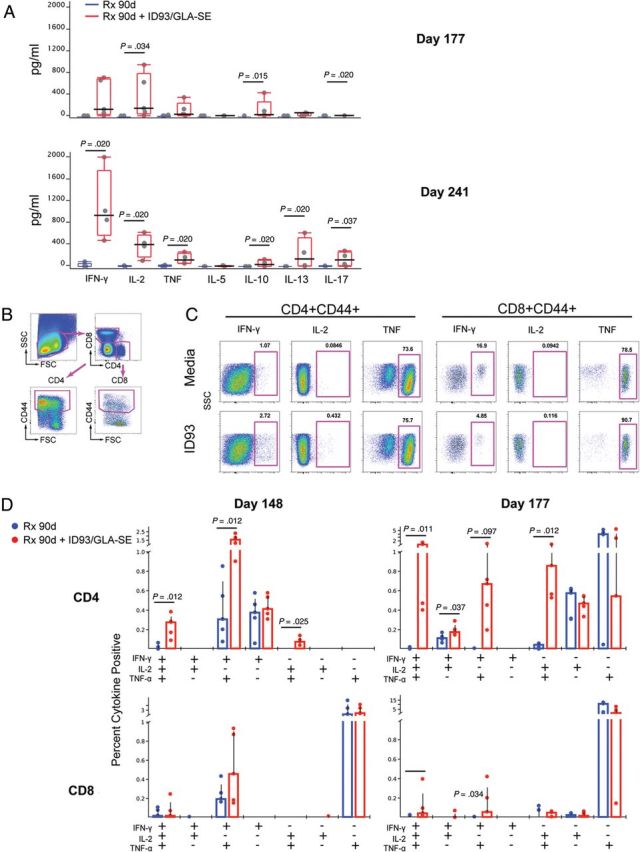
ID93-specific cytokine responses in SWR mice after immunotherapy. SWR mice were infected with an LDA Mycobacterium tuberculosis (M. tuberculosis) H37Rv and treated with either 90 days of antibiotics alone or antibiotics followed by immunization with ID93/GLA-SE 3 × 3 weeks apart. (A) Cytokine profile of ID93-stimulated splenocytes recovered at either day 177 or 241 after infection. Cells were incubated for 24 hours in the presence of antigen or media control, and supernatants were collected and analyzed by multiplex bead array for IFN-γ, IL-2, TNF, IL-5, IL-10, IL-13, and IL-17. Box plots show median and interquartile range after background subtraction. P values from Wilcoxon rank-sum test. (B–D) Intracellular cytokine staining for ID93-specific T cell responses at days 149 and 177 after infection. Cells were stimulated with ID93 or media control in the presence of brefeldin A for 8–12 hours and stained with fluorochrome-conjugated antibodies against CD3, CD4, CD8, CD44, IFN-γ, IL-2, and TNF. (B and C) The panels show the gating scheme for FACS analysis. (D) Box plots in lower panel show median and interquartile range after background subtraction. P values from Wilcoxon rank-sum test. One representative of 2 experiments is shown.
Polyfunctional CD4+ TH1 cells have recently been described as a correlate of protection against Leishmania major and have been implicated in limiting disease progression in human tuberculosis [39, 40]. Frequencies of CD4+ and CD8+ T cells producing IFN-γ, IL-2, and TNF were thus examined to determine the phenotype of ID93-specific T cell responses (Figure 4B–D;). Higher frequencies of ID93-specific polyfunctional triple-positive and IFN-γ+TNF+ double-positive CD4+ T cells were observed in mice receiving adjunctive immunotherapy, compared with mice receiving only chemotherapy (P < .05), (Figure 4B–D). High background responses of ID93-specific TNF in both the CD4+ and CD8+ T cell subsets were observed, which was likely attributable to the increased immune activation of an ongoing M. tuberculosis infection in these animals. Although ID93-specific responses in CD8+ T cells were lower in magnitude than those observed in the CD4+ compartment, there were significantly higher frequencies of double (IFN-γ+TNF+) and triple positive (IFN-γ+IL-2+TNF+) CD8+ T cells in mice receiving adjunctive ID93/GLA-SE vaccination. All together, these data show that, although there are many antigens present after M. tuberculosis infection that could be potentially primed and boosted continuously, ID93/GLA-SE administered adjunctively with antibiotics was successful at stimulating a significantly more robust, high-quality (polyfunctional), and durable TH1-type anti-ID93 CD4+ T cell response.
ID93/GLA-SE as an Adjunct to Antibiotic Treatment in Cynomolgus Macaques
To demonstrate the safety of ID93/GLA-SE when administered as an adjunct to antibiotics in NHP, macaques were administered 3 doses of the vaccine after 1 month of RIF-INH therapy (Figure 5A). Injection-site reactions were minimal, with no more than barely perceptible erythema and edema (Draize scale range, 0–1), and there were no significant changes in body weight and temperature (data not shown). All 7 (100%) of the Rx + ID93/GLA-SE–immunized NHP survived to the last time point evaluated, whereas 6 NHP (85.7%) in the antibiotics alone group and 3 NHP (42.8%, P = .44) in the mock-treated group survived to this point (Figure 5B). Four monkeys treated with Rx + ID93/GLA-SE either had no radiological changes or resolved the M. tuberculosis infection before the end of the experiment (as evidenced by lung infiltrates on previously positive chest radiographs), whereas none of the macaques receiving Rx alone or mock treatments resolved their M. tuberculosis infection and remained chest radiograph positive (Figure 5C). Forty percent of the macaques treated with Rx + ID93/GLA-SE responded dramatically to adjunctive immunotherapy by showing quantitative differences in M. tuberculosis bacterial numbers, compared with the Rx alone group; (P < .05), (Figure 5D). Of interest, the Rx + ID93/GLA-SE macaques that had lower CFU counts also had negative chest radiograph findings at the end of the experiment. There was also a correlation by histopathology between group assignment and the presence of diseased tissue, with animals receiving ID93/GLA-SE containing the most healthy organs and the saline group having the most diseased organs (P = .003) (Figure 5E). Overall, these results show that an ID93/GLA-SE vaccine was well tolerated as a postexposure immunotherapeutic agent in cynomolgus macaques.
Figure 5.
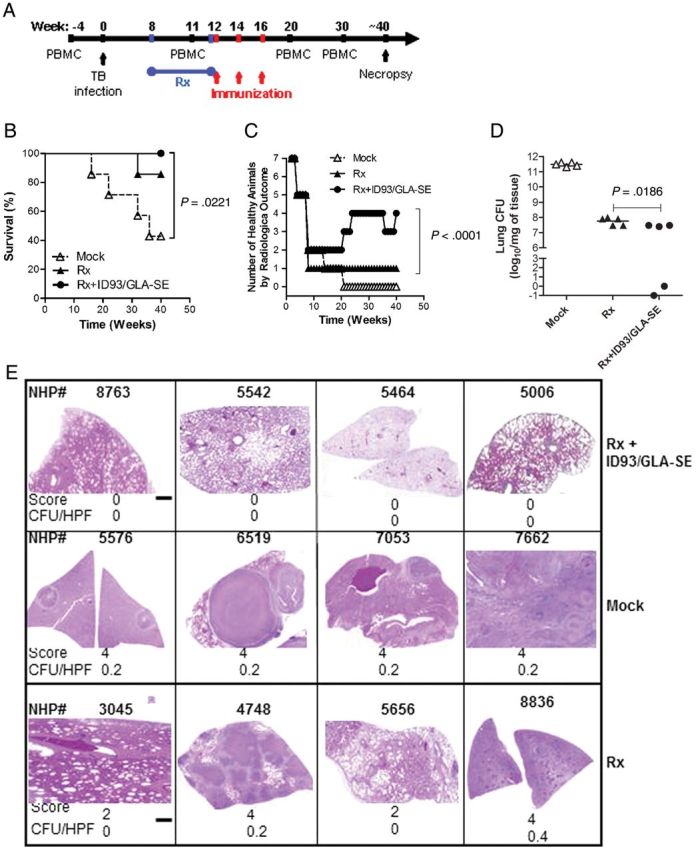
Survival, clinical parameters, and bacterial burden of NHP infected with Mycobacterium tuberculosis (M. tuberculosis) and treated with antibiotics. Cynomolgus macaques were inoculated intratracheally with 1000 CFU of virulent M. tuberculosis (Erdman strain). The infection was allowed to proceed for 60 days, followed by treatment with 30 days of INH-RIF antibiotics delivered by gavage or saline (mock). Monkeys (7 per group) were injected with ID93/GLA-SE (Rx + ID93/GLA-SE) administered 3 times 2 weeks apart or did not receive further treatment (Mock, Rx). (A) Scheme of NHP immunotherapy experiment. (B) Survival was monitored for 50 weeks after exposure. (C) CXR changes were also evaluated monthly for 50 weeks after exposure. (D) At necropsy, bacteria were quantified by enumerating the bacteriological burden (CFU) in monkey lungs. (E) Histologic appearance of H&E-stained sections of lung tissues harvested from NHP.
DISCUSSION
When delivered as an adjunct to chemotherapy in mice and monkeys, the ID93/GLA-SE vaccine [12–14] was successful in inducing robust TH1 immune responses, shortening the chemotherapy time required for protection, reducing bacterial burden, or extending survival time, compared with antibiotics only. Although the concept of effective postexposure immunization has been demonstrated in tuberculosis models [22, 41], previous studies have not shown the potential of augmenting or shortening chemotherapy regimens with a defined subunit vaccine, such as ID93/GLA-SE.
Conventional anti-tuberculosis drugs target biosynthetic processes involved in bacterial growth, including RNA transcription (RIF), protein translation (streptomycin), and cell wall biogenesis (INH). The required 6-month regimen of treatment with first-line drugs is plagued with noncompliance, contributing to the emergence of MDR-tuberculosis. For several weeks after the cessation of 90 days of antibiotic treatment, M. tuberculosis CFU could not be recovered from the lungs of treated SWR/J mice, suggesting that, when left without additional treatment, nondetectable bacteria become cultivable and could cause a relapse. The drugs that are currently in common use preferentially target replicating organisms; therefore, a nonreplicative subpopulation of the bacteria present in persistent or chronic infections will show resistance to drugs. Relapse rates are higher in those who do not complete their full antibiotic course and those who are concomitantly infected with HIV [42], revealing the need for a pertinent animal model for developing postexposure treatment strategies.
We and others have shown that combining antigens in recombinant fusions, such as ID93, Mtb72f, ID83, CSU-F36, H56, and H1, induces multi-antigen-specific immune responses and increased protection against M. tuberculosis [12, 14, 15, 17–20, 41]. The ID93 fusion protein was designed with antigens from EsX, virulence factor, and latency-associated families and was formulated with a potent TLR4 agonist-containing adjuvant, GLA-SE, which induces a TH1-type CD4+ T cell response [12, 14, 43, 44]. GLA also stimulates and directs innate and adaptive immune responses by inducing DC maturation and the release of pro-inflammatory cytokines and chemokines associated with immune cell trafficking [25, 26, 45]. Low-dose M. tuberculosis infection and subsequent chemotherapy induced relatively few ID93-specific T cells. However, postinfection immunization with ID93/GLA-SE administered with antibiotics induced a strong ID93-specific TH1 immune response, as measured by IFN-γ CD4+ T cells and CD8+ T cell responses at later times, perhaps indicating the ability of M. tuberculosis infection to prime CD8+ T cell responses that are boosted by immunization-dependent CD4+ T cell help.
It has been hypothesized that cellular immunity induced by immunization could enhance pathology or trigger reactivation of persistent lung bacteria. However, we found that ID93/GLA-SE was safe when administered after M. tuberculosis exposure with antibiotics in both mice and monkeys. Similar to human tuberculosis, substantial monkey-to-monkey variability in the clinical outcome of M. tuberculosis-infected macaques has been previously reported [1]. Thus, the dramatic quantitative reduction in M. tuberculosis numbers and negative radiograph findings observed in a subset of the monkeys administered Rx + ID93/GLA-SE adjunctive immunotherapy was anticipated. Furthermore, dramatic differences in lung histopathology were observed in animals treated with ID93/GLA-SE as an adjunct to chemotherapy, compared with mock and Rx controls; this combination minimized the formation of large coalescing macrophages and/or reduced necrosis. The potential value of the SWR model for modeling postexposure treatment of tuberculosis is emphasized by these studies. The results of the NHP studies show that treatment is effective in this model but that adjunctive immunotherapy with ID93/GLA-SE provided an improvement over current standards of care. Although the natural history of infection in cynomolgus monkeys mimics human disease, the cost of housing NHP for studies lasting multiple years can be prohibitive. The SWR mouse model presented here would allow more rapid screening of potential candidates, especially in light of the paucity of rodent models showing granulomatous pathology due to experimental M. tuberculosis infection.
Mathematical modeling of tuberculosis to evaluate the expected benefits of shortening the duration of effective chemotherapy for active pulmonary tuberculosis have shown that the introduction of new, shorter treatment regimens are associated with significant cost savings and higher compliant rates, which could dramatically accelerate reductions in tuberculosis incidence and mortality [46]. These models predict up to 2- or 3-fold increases in rates of decrease if shorter regimens are accompanied by enhanced case detection [6]. Taken together, these data demonstrate the potential to optimize therapy regimens that we already have and to enhance chemotherapeutic efficacy by boosting the immune response with an appropriately adjuvanted vaccine, such as ID93/GLA-SE. Our results suggest that a vaccine and chemotherapy strategy could be an efficient approach to induce immunity and antibacterial effects by 2 independent and complementary mechanisms to shorten the duration, toxicity, and cost of lengthy chemotherapy regimens, thus increasing patient compliance, limiting the creation of MDR strains, and possibly preventing disease relapse by reducing residual bacteria that survive initial chemotherapy.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank D. Argilla, I. Zharkikh, A. Bernard, K. Bernards, N. Stride, T. Dutill, T. Evers, K. Durgan, E. Kristalinskaia, J. Laurance, L. Bogatzki, I. Tukacovic, W. Wicomb, and J. Zheng, for their technical expertise, and D. Roberts, J. Ahn, and T. Parish, for minimum inhibitory concentration data.
Financial support. This work was supported by National Institutes of Health (grants AI-044373 and UC1AI-067251 to S.G.R. and contracts AI-25479 and HHSN272200800045C and grant AI-078054 to R.N.C.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed..
References
- 1.Dye C. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat Rev Microbiol. 2009;7:81–7. doi: 10.1038/nrmicro2048. [DOI] [PubMed] [Google Scholar]
- 2.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1900–2000. Bull.World Health Organ. 1994;72:213–20. [PMC free article] [PubMed] [Google Scholar]
- 3.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. Jama. 2005;293:2767–75. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg AM, Spigelman M. Challenges in tuberculosis drug research and development. Nat Med. 2007;13:290–4. doi: 10.1038/nm0307-290. [DOI] [PubMed] [Google Scholar]
- 5.Lobue P, Menzies D. Treatment of latent tuberculosis infection: an update. Respirology. 2010;15:603–22. doi: 10.1111/j.1440-1843.2010.01751.x. [DOI] [PubMed] [Google Scholar]
- 6.Salomon JA, Lloyd-Smith JO, Getz WM, et al. Prospects for advancing tuberculosis control efforts through novel therapies. PLoS Med. 2006;3:e273. doi: 10.1371/journal.pmed.0030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15:211–4. doi: 10.1038/nm.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy E, Lowrie DB, Jolles SR. Current strategies in TB immunotherapy. Curr Mol Med. 2007;7:373–86. doi: 10.2174/156652407780831557. [DOI] [PubMed] [Google Scholar]
- 9.Wejse C, Gustafson P, Nielsen J, et al. TBscore: signs and symptoms from tuberculosis patients in a low-resource setting have predictive value and may be used to assess clinical course. Scand J Infect Dis. 2008;40:111–20. doi: 10.1080/00365540701558698. [DOI] [PubMed] [Google Scholar]
- 10.Andersen P. Tuberculosis vaccines—an update. Nat Rev Microbiol. 2007;5:484–7. doi: 10.1038/nrmicro1703. [DOI] [PubMed] [Google Scholar]
- 11.Nascimento E, Fernandes DF, Vieira EP, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1 + MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28:6581–7. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin SL, Bertholet S, Kahn M, et al. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27:3063–71. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertholet S, Ireton GC, Kahn M, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. J Immunol. 2008;181:7948–57. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertholet S, Ireton GC, Ordway DJ, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich J, Aagaard C, Leah R, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005;174:6332–9. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet. 2010;375:2110–9. doi: 10.1016/S0140-6736(10)60393-5. [DOI] [PubMed] [Google Scholar]
- 17.Reed SG, Coler RN, Dalemans W, et al. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci USA. 2009;106:2301–6. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skeiky YA, Ovendale PJ, Jen S, et al. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J Immunol. 2000;165:7140–9. doi: 10.4049/jimmunol.165.12.7140. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Henao-Tamayo M, Harton M, et al. A toll-like receptor-2 directed fusion protein vaccine against tuberculosis. Clin Vaccine Immunol. 2007 doi: 10.1128/CVI.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinrich Olsen A, van Pinxteren LA, Meng Okkels L, Birk Rasmussen P, Andersen P. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect Immun. 2001;69:2773–8. doi: 10.1128/IAI.69.5.2773-2778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowrie DB, Tascon RE, Bonato VL, et al. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–71. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 22.Cardona PJ, Amat I, Gordillo S, et al. Immunotherapy with fragmented Mycobacterium tuberculosis cells increases the effectiveness of chemotherapy against a chronical infection in a murine model of tuberculosis. Vaccine. 2005;23:1393–8. doi: 10.1016/j.vaccine.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich J, Weldingh K, Andersen P. Prospects for a novel vaccine against tuberculosis. Vet Microbiol. 2006;112:163–9. doi: 10.1016/j.vetmic.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RC, Fox CB, Dutill TS, et al. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75:123–32. doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Coler RN, Bertholet S, Moutaftsi M, et al. Development of glucopyranosyl lipid A, a synthetic TLR4 agonist, as a vaccine adjuvant. PloS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pantel A, Cheong C, Dandamudi D, et al. A new synthetic TLR4 agonist, GLA, allows dendritic cells targeted with antigento elicit Th1 T-cell immunity in vivo. Eur J Immunol. 2011 doi: 10.1002/eji.201141855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertholet S, Ireton GC, Kahn M, et al. Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis. Journal of immunology. 2008;181:7948–57. doi: 10.4049/jimmunol.181.11.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musa SA, Kim Y, Hashim R, Wang GZ, Dimmer C, Smith DW. Response of inbred mice to aerosol challenge with Mycobacterium tuberculosis. Infection and Immunity. 1987;55:1862–6. doi: 10.1128/iai.55.8.1862-1866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner OC, Keefe RG, Sugawara I, Yamada H, Orme IM. SWR mice are highly susceptible to pulmonary infection with Mycobacterium tuberculosis. Infection and Immunity. 2003;71:5266–72. doi: 10.1128/IAI.71.9.5266-5272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen PE. [Endovascular interventional treatment of hemoptysis] Ugeskr Laeger. 2005;167:3160–3. [PubMed] [Google Scholar]
- 31.Ravn P, Brock I, Andersen P, Weldingh K. [A possible successor of the Mantoux test after 97 years] Ugeskr Laeger. 2005;167:2905–6. [PubMed] [Google Scholar]
- 32.Walsh GP, Tan EV, dela Cruz EC, et al. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med. 1996;2:430–6. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 33.Langermans JA, Andersen P, van Soolingen D, et al. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc Natl Acad Sci USA. 2001;98:11497–502. doi: 10.1073/pnas.201404898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langermans JA, Doherty TM, Vervenne RA, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23:2740–50. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 35.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–43. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell DG, Barry CE, 3rd, Flynn JL. Tuberculosis: what we don't know can, and does, hurt us. Science. 2010;328:852–6. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikonenko BV, Einck L, Nacy CA. Anti-tuberculosis drug therapy in mice of different inbred strains. Infect Genet Evol. 2010;10:1151–4. doi: 10.1016/j.meegid.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Turner OC, Sugawara, Yamada H, Cummings B, Orme IM. Crystalloid inclusions in the cytoplasm of alveolar macrophages of the SwR/J mouse. A possible cause of susceptibility to mycobacterium tuberculosis? Journal of Submicroscopic Cytology and Pathology. 2001;33:217–9. [PubMed] [Google Scholar]
- 39.Wilkinson KA, Wilkinson RJ. Polyfunctional T cells in human tuberculosis. Eur J Immunol. 2010;40:2139–42. doi: 10.1002/eji.201040731. [DOI] [PubMed] [Google Scholar]
- 40.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 41.Aagaard C, Hoang T, Dietrich J, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17:189–94. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 42.Mak A, Thomas A, Del Granado M, Zaleskis R, Mouzafarova N, Menzies D. Influence of multidrug resistance on tuberculosis treatment outcomes with standardized regimens. Am J Respir Crit Care Med. 2008;178:306–12. doi: 10.1164/rccm.200802-240OC. [DOI] [PubMed] [Google Scholar]
- 43.Baldwin SL, Shaverdian N, Goto Y, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–63. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 44.Bertholet S, Goto Y, Carter L, et al. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–45. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiley SR, Raman VS, Desbien A, et al. Targeting TLRs expands the antibody repertoire in response to a malaria vaccine. Sci Transl Med. 2011;3:93ra69. doi: 10.1126/scitranslmed.3002135. [DOI] [PubMed] [Google Scholar]
- 46.Demissie A, Wassie L, Abebe M, et al. The 6-kilodalton early secreted antigenic target-responsive, asymptomatic contacts of tuberculosis patients express elevated levels of interleukin-4 and reduced levels of gamma interferon. Infect Immun. 2006;74:2817–22. doi: 10.1128/IAI.74.5.2817-2822.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]