Abstract
Background. Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract illness (LRTI) in children. Several promising live-attenuated RSV vaccines are in development. Defining additional markers of attenuation could enhance clinical trials.
Methods. We used clinical data, virologic data, and nasal wash (NW) specimens from 20 RSV-naive children enrolled in studies of 4 live-attenuated RSV vaccines. Seven received minimally attenuated cpts248/955 or cpts530/1009 (group 1), 6 received moderately attenuated cpts248/404 (group 2), and 7 received highly attenuated rA2cp248/404/1030/ΔSH (group 3). NW specimens were tested for cytokines and chemokines via an electrochemiluminescence biosensor assay.
Results. Group 1 exhibited 1 instance of LRTI and significantly higher rates of fever than groups 2 or 3; there were no significant differences in peak titers of vaccine virus in NW specimens. In contrast, levels of interferon γ, interleukin 1β, interleukin 2, interleukin 6, and interleukin 13 were significantly greater in NW specimens from group 1, compared with those from group 3. Maximum increases in levels of most cytokines occurred after peak viral replication but coincided with clinical illness.
Conclusions. Substantial increases in proinflammatory, antiinflammatory, T-helper 1, T-helper 2, and regulatory cytokines were detected in children who received minimally attenuated live RSV vaccines but not in children who received highly attenuated vaccines. Levels of cytokines in NW specimens may be useful biomarkers of attenuation for live RSV vaccines.
Keywords: respiratory syncytial virus, live-attenuated vaccine, cytokine
Respiratory syncytial virus (RSV) is the primary cause of viral lower respiratory tract illness (LRTI) in infancy and early childhood [1–3]. Each year, RSV infection is estimated to lead to approximately 33 million cases of LRTI and up to 199 000 deaths in infants and children worldwide, [2] and approximately 2 million medically attended illnesses in infants and children in the United States [1]. Importantly, this burden of illness is not confined to infants: 78% of US children requiring medical attention for RSV infection are >1 year of age [1].
RSV vaccines are urgently needed to prevent RSV-associated illnesses in infants and young children. Live-attenuated RSV vaccines offer the greatest promise of providing durable local and systemic immunity to afford protection throughout early childhood [4, 5]. In addition, whereas inactivated RSV vaccines have primed for enhanced disease in RSV-naive recipients, this is not the case for live-attenuated RSV vaccines [6]. An ideal live RSV vaccine will successfully balance attenuation and immunogenicity: the host response to the vaccine will not cause illness but will provide protective immunity against RSV LRTI. Achievement of this balance has been challenging because attenuation generally reduces immunogenicity, due in part to the reduced antigen expression associated with reduced viral replication. Candidate vaccines evaluated during the 1960s–1990s were either insufficiently attenuated [7, 8] or overattenuated [9]. However, several promising candidate live-attenuated vaccines are currently in clinical development [10–12].
Assessment of the attenuation of live RSV vaccines has relied on clinical assessment and quantification of vaccine virus replication in nasal wash (NW) fluid [7–9, 13]. While these measurements provide critical clinical and virologic information, the contribution of local immune mediators to the clinical response to vaccination has not been assessed. Since host immune responses to RSV may contribute to disease pathogenesis [14–17], characterization of local cytokine and chemokine production following vaccination with a live-attenuated RSV vaccine might provide important additional information regarding attenuation. In addition, since administration of a live vaccine is a timed infection with an attenuated virus, longitudinal assessment of cytokine and chemokine levels with respect to baseline levels could provide important clues about the dynamics of the local immune response to natural RSV infection, particularly when evaluating data from the candidate vaccines that were insufficiently attenuated. Here, we provide a detailed assessment of the cytokines and chemokines detected in NW specimens obtained from RSV-naive children who received minimally attenuated, moderately attenuated, or highly attenuated live RSV vaccines.
Methods
Data and Specimens
This study made use of clinical data and NW specimens obtained from phase I clinical trials of 4 live-attenuated RSV vaccine candidates derived from the wild-type RSV A2 strain: cpts 248/955, cpts 530/1009, cpts 248/404, and r248/404/1030/ΔSH [7, 8, 10]. Informed, witnessed, written consent was obtained from parents of all children who participated in these studies [7, 8, 10]. The first 3 of these candidates were derived biologically by serial passage at low temperature and chemical mutagenesis [7, 8]. rA2cp248/404/1030/ΔSH was derived using recombinant technology [10]. Each virus contains the same set of cold-passage (cp) attenuating mutations, each is temperature sensitive (ts), and each is attenuated primarily by point mutations in the viral polymerase protein or in a transcription signal that reduces viral RNA synthesis.
These 4 vaccine candidates were evaluated sequentially over 8 years and displayed a spectrum of attenuation when evaluated in RSV-naive infants and children ≤24 months of age. For the purposes of this study, we divided these specimens into 3 groups on the basis of clinical trials data. Group 1 included NW specimens from clinical trials of cpts 248/955 and cpts 530/1009. These 2 viruses were the least attenuated live RSV vaccine candidates: fever, upper respiratory tract illness, and/or otitis media were associated with shedding of vaccine virus, and LRTI (mild wheezing) was observed in 1 cpts 248/955 recipient [7]. In addition, cpts 248/955 and cpts 530/1009 were each transmitted to a single placebo recipient [7]. Group 2 included NW specimens obtained from clinical trials of cpts 248/404. cpts 248/404 was a moderately attenuated live RSV vaccine candidate. In phase I trials, this virus was restricted in replication, but caused significant nasal congestion in 1–3-month-old infant vaccinees [8]. Group 3 included NW specimens obtained from clinical trials of rA2cp248/404/1030/ΔSH, a highly attenuated RSV vaccine candidate that was well tolerated and moderately immunogenic in 1–3-month-old infants [10]. A nearly identical version of this virus, MEDI-559, is being evaluated in RSV-naive children (clinical trials registration NCT00767416). The doses of vaccine received were 104.0, 105.0, and 105.3 plaque-forming units (PFU) for subjects in groups 1, 2, and 3, respectively.
Information regarding respiratory and febrile illnesses and quantification of vaccine viruses in NW specimens was obtained from previous clinical trials. To participate in these studies, children had to be completely healthy, without signs or symptoms of illness at the time of vaccination.
Measurement of Cytokine and Chemokine Levels in NW Specimens
NW specimens were selected for evaluation on the basis of 3 criteria: (1) vaccine virus was detected in NW specimens on at least 1 day following inoculation, (2) no adventitious viral agents were detected by culture in children with febrile or respiratory illnesses [7, 8, 10], and (3) sequential NW specimens that had been stored at −80C and not previously thawed were available for testing. The NW specimens chosen for analysis included those obtained before inoculation, on the day of vaccination (study day 0); on the day of peak vaccine virus replication; and at 1 time point before (typically, 1–3 days before) and 2 time points after (typically, 3–10 days after) peak replication. Although the day of peak viral replication differed, with a later peak for the more attenuated candidate vaccines, all time points measured were within the first 18 days after inoculation. Cytokine and chemokine levels were measured in NW fluid using an electrochemiluminescence biosensor assay (Meso Scale Discovery, Gaithersburg, MD). Three types of kits were used, all in ultrasensitive format: the T-helper 1 (Th1)/T-helper 2 (Th2) 10-plex kit (which measures interferon γ (IFN-γ), interleukin 1β (IL-1β), IL-2, IL-4, IL-5, CXCL8 (IL-8), IL-10, IL-12 p70, IL-13, and tumor necrosis factor α (TNF-α), the chemokine 9-plex kit (which measures CCL11, CCL26, CXCL8 [IL-8], CXCL10, CCL2, CCL13, CCL22, CCL4, and CCL17), and a custom kit for measuring IFN-α2α, IFN-β, IL-17, IL-6, CCL3, and CCL5. Briefly, 25 µL of each NW sample was tested according to the manufacturer's instructions and read on a SECTOR Imager. Results were reported in picograms per milliliter.
Statistical Analysis
The median ages of children in each group were compared by the Mann–Whitney U test. Rates of illness were compared using the Fisher exact test. Mean log10 peak viral titers were compared by the Student t test. The Spearman rank correlation coefficient (ρ) was used to estimate correlations between fold increases in cytokine levels.
To correct for the variability in levels of cytokines and chemokines observed at baseline (study day 0), the peak level of each analyte for each subject was compared to the value measured at baseline, with results of cytokine and chemokine measurements expressed as fold change over baseline. The maximum fold increase for each analyte was calculated for each subject. Differences in maximum fold increases between vaccine groups were compared using the Mann Whitney U test. Preliminary review of the data revealed that consistent increases over baseline were observed for 7 cytokines: IL-6, IFN-γ, IL-10, IL-13, IL1-β, IL-2, and TNF-α; these were selected for further analysis. A Bonferroni-adjusted significance level of 0.007 was calculated to adjust for the increased possibility of type-1 error in the comparisons of these cytokine measurements.
RESULTS
Subject Characteristics
NW specimens and clinical and virologic data from 20 RSV-naive young children were analyzed for this study. Group 1 included 7 children who received cpts 248/955 (n = 5) or cpts 530/1009 (n = 2), the minimally attenuated viruses. Group 2 included 6 children who received cpts 248/404, a moderately attenuated virus. Group 3 included 7 children who received rA2cp248/404/1030/ΔSH, a highly attenuated virus. The median ages of the children were 10 months (range, 7–14 months) for group 1, 1.75 months (range, 1–9 months) for group 2, and 2.5 months (range, 1.25–3.25 months) for group 3. Although NW specimens tested in this study were mostly from infants <12 months old, those in group 1 were significantly older than those in group 2 (P = .01) or group 3 (P = .002).
Clinical Response to Vaccines and Shedding of Vaccine Virus
Rhinorrhea and/or nasal congestion were observed frequently in each group of vaccinees (Table 1). These minor illnesses were also observed frequently in placebo recipients (data not shown). While the rates of fever, LRTI, cough, and otitis media were greater for children in group 1 than those in either group 2 or group 3, only the differences in the rate of fever were statistically significant (P = .02 for group 1 vs group 2, and P = .02 for group 1 vs group 3).
Table 1.
Clinical Responses to cpts248/955 or cpts 530/1009 (Group 1), cpts 248/404 (Group 2), or rA2cp248/404/1030/ΔSH (Group 3)
| Vaccine Group | Subjects, No. | Subjects With Indicated Symptom or Illness, % |
|||||
|---|---|---|---|---|---|---|---|
| Fever | URTI | LRTI | Cough | Otitis Media | Anya | ||
| 1 | 7 | 71 | 40 | 14 | 28 | 28 | 100 |
| 2 | 6 | 0 | 67 | 0 | 0 | 0 | 67 |
| 3 | 7 | 0 | 43 | 0 | 0 | 0 | 43 |
Clinical data are shown for vaccinees from whom nasal wash specimens were available for analysis. All data were obtained from previously published studies [7, 8, 10]. Definitions of illness are as previously described [7, 8, 10].
Abbreviations: LRTI, lower respiratory tract illness; URTI, upper respiratory tract illness.
a Any respiratory or febrile illness.
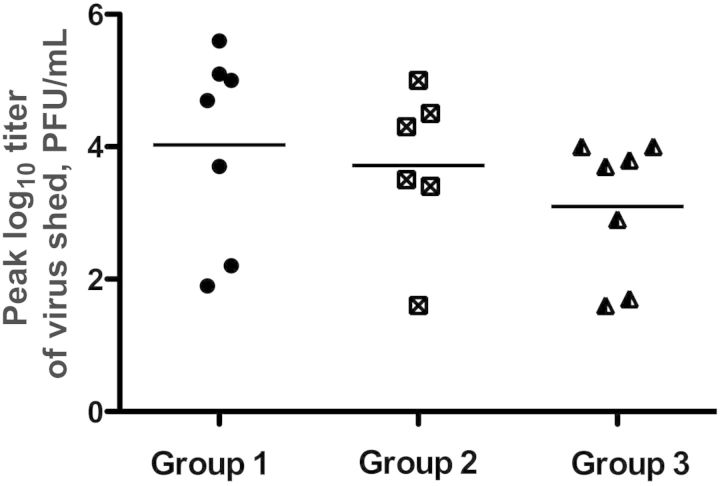
Mean peak log10 viral titers of vaccine virus shed were 4.03, 3.71, and 3.10 PFU/mL for children in groups 1, 2, and 3, respectively (Figure 1). While there was a trend toward reduced replication, these differences were not statistically significant, and considerable overlap in peak titers shed was observed among children in all 3 groups (Figure 1).
Figure 1.
Mean peak titer of vaccine virus shed by each subject in groups 1, 2, or 3. The mean peak log10 titer of vaccine virus detected in groups 1, 2, and 3 was 4.03, 3.71, and 3.10, plaque-forming units (PFU)/mL, respectively. These differences in mean peak log10 titer were not statistically significant.
Levels of Cytokines and Chemokines in NW Samples Obtained Prior to Vaccination
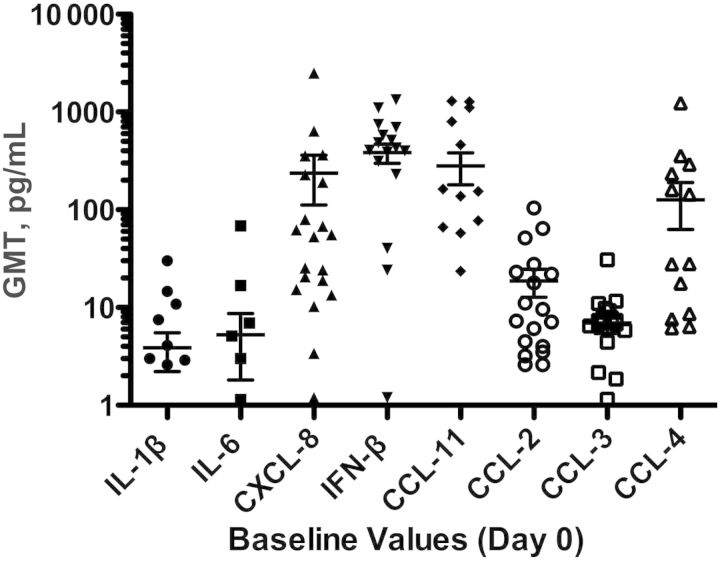
As described above, physical examinations were performed on all children prior to vaccination, and each child was required to be afebrile and free of respiratory symptoms at enrollment. Despite the absence of clinical illness, levels of several cytokines and chemokines were substantially elevated at baseline, most notably CXCL8 (IL-8), IFN-β, CCL11, and CCL4 (Figure 2). In addition, considerable variation in the range of baseline values was observed for 3 of these analytes: for CXCL8, 4–3576 pg/mL, for IFN-β, 1–1132 pg/mL, and for CCL 11, 1–1296 pg/mL. No age effect was evident for baseline values of any of these cytokines or chemokines (not shown). These findings confirmed the importance of comparing cytokine measurements obtained at time points following vaccination with those obtained at baseline, as described in Methods and below.
Figure 2.
Geometric mean titers (GMTs) at baseline for cytokines and chemokines with substantial variability at baseline. Ranges at baseline were the following for all analytes shown: interleukin 1β (IL-1β), 0–30 pg/mL; IL-6, 0–68.3 pg/mL; CXCL8, 4–3576 pg/mL; interferon β (IFN-β), 1–1132 pg/mL; CCL-11, 1–1296 pg/mL; CCL-2, 0–104.4 pg/mL; CCL-3 0–30.9 pg/mL; and CCL-4, 0–1238.1 pg/mL.
Longitudinal Analysis of Increases in Cytokine and Chemokine Levels in NW Specimens
The relationship between fold change in cytokine and chemokine levels, titer of vaccine virus shed, and clinical signs and symptoms (if any) was analyzed longitudinally for each subject. Data from a representative subject in each group are shown in Figure 3.
Figure 3.
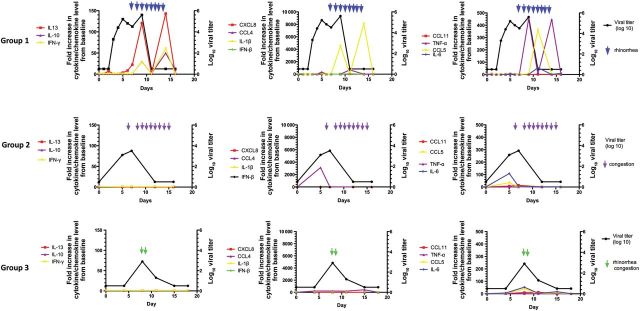
The relationship between levels of selected cytokines and chemokines, shedding of vaccine virus, and clinical symptoms for 3 representative children from groups 1, 2, and 3. Viral titers are indicated in black. Levels of 11 cytokines and chemokines are indicated in red, purple, yellow, and green, as shown in the key. Blue arrowheads indicate days of rhinorrhea for the subject in group 1, purple arrowheads indicate days of nasal congestion for the subject in group 2, and green arrowheads indicate days of nasal congestion for the subject in group 2. Abbreviations: IFN, interferon; IL, interleukin; TNF-α, tumor necrosis factor α.
The subject from group 1 shed virus from days 3–9 after vaccination, with a peak log10 titer of 5.6 PFU/mL on day 8. This subject experienced rhinorrhea (Figure 3) from days 7–14. Substantial increases in IFN-γ, IL-10, IL-13, IL-1β, and IFN-β levels were observed; peak increases occurred on day 14 for the first 4 analytes and on day 11 for IFN-β (Figure 3). Thus, for this subject, vaccine virus shedding preceded but partially overlapped illness, and illness generally coincided with the period of maximum increases in these cytokines.
The results with this subject were generally representative of those in group 1. Viral shedding occurred in each of the 7 subjects, with a mean duration (last day of vaccine virus detection; ±SD) of 10.1 ± 2 days and a mean peak log10 titer (±SD) of 4.0 ± 1.5 PFU/mL. Rhinorrhea occurred in 6 of 7 subjects, with mean onset (±SD) on day 7 ± 2.9 and a mean end (±SD) on day 13.8 ± 2.9, thus overlapping with but extending beyond the time of viral shedding (data not shown). Increases in cytokine and chemokine expression similar to that shown in Figure 3 were observed in all individuals and generally coincided with illness (data not shown).
The subject from group 2 shed virus on study days 5 and 7, with a peak log10 titer of 3.5 PFU/mL on day 7 (Figure 3). This subject experienced nasal congestion (Figure 3) on day 6 and days 8–15. Thus, for this subject, the period of clinical illness also extended beyond the period of viral shedding. Increases in cytokine and chemokine levels were generally minimal or absent, with the exception of CCL4 and IL-6 levels, which coincided with viral shedding (Figure 3). The results observed in this subject were generally representative of the group. Viral shedding occurred in each of the 5 remaining individuals, with a mean duration (±SD) of 8.3 ± 1.4 days and a mean peak log10 titer (±SD) of 3.7 ± 1.2 PFU/mL. Nasal congestion occurred in 4 subjects (Table 1) and persisted beyond the period of viral shedding in 3 subjects. Increases in IL-6 and CCL4 levels occurred in 4 of 5 and 3 and 5 of these subjects, respectively.
The subject from group 3 shed virus on days 8 and 11 after vaccination, with a peak titer (±SD) of 2.9 log10 PFU/mL (Figure 3). This subject experienced rhinorrhea and mild congestion on study days 8 and 9 (green arrows). Minimal increases in cytokine and chemokine levels were observed in this subject (Figure 3). Viral shedding occurred in each of the subjects in this group, with a mean duration (±SD) of 8.8 ± 2.5 days, and a mean peak log10 titer (±SD) of 3.2 ± 0.9 PFU/mL Although 4 of the other 6 subjects had rhinorrhea or nasal congestion, onset for each of these children was ≥5 days after the last day vaccine virus was detected, suggesting a nonvaccine etiology of these symptoms. As noted for the subject shown in Figure 3, minimal increases in cytokine and chemokine levels were measured in nasal washes obtained for subjects in this group.
Comparison of Increases in Cytokine and Chemokine Levels Between Groups
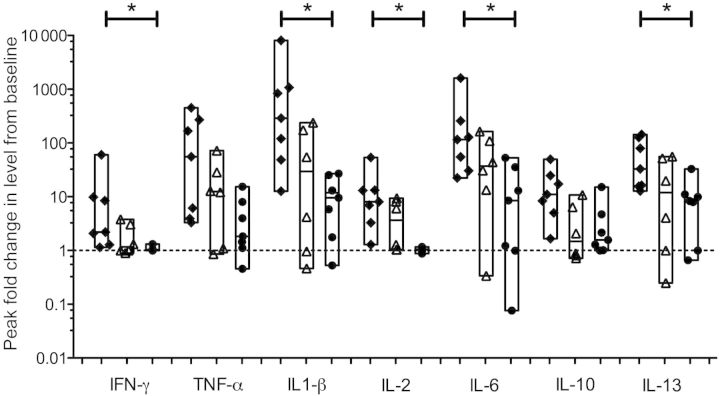
To compare data between subjects in groups 1, 2, and 3, we used the peak fold increase for each analyte and each subject and calculated the median peak fold increase for all cytokines and chemokines. Results from analyses of cytokines in which substantial differences between groups were observed are shown in Figure 4. For each cytokine except IL-10, a stair-step diminution was observed that corresponded to increasing vaccine attenuation: subjects in group 1, who received the least attenuated RSV vaccine, had the greatest fold increase (black diamonds), followed by subjects in group 2, who received the moderately attenuated RSV vaccine (open triangles), and then by subjects in group 3, who received a highly attenuated RSV vaccine (black circles). When data from subjects in group 1 and subjects in group 3 were compared, median peak fold increases differed significantly for IFN-γ (P = .005) IL-1β (P = .002) IL-2 (P = .006), IL-6 (P = .007), and IL-13 (P = .007). Differences for TNF-α and IL-10 did not meet the Bonferroni-adjusted significance level (P = .038 and .018, respectively). No consistent differences in median peak fold increases were observed for IFN-β or for any of the chemokines (data not shown).
Figure 4.
Peak fold change from baseline in levels of selected cytokines. To correct for the variability in levels of cytokines and chemokines observed at baseline (study day 0), the peak level of each analyte for each subject was compared to the value measured at baseline. Results are expressed as fold change over baseline (dotted line). For each cytokine, the black diamonds represent data from group 1, the open triangles represent data from group 2, and the black circles represent data from group 3. The floating bars represent the range from minimum to maximum values; the median values for each subject are shown. Asterisks indicate a P value of ≤ .007 for the difference between group 1 and group 3. Abbreviations: IFN-γ, interferon γ; IL, interleukin; TNF-α, tumor necrosis factor α.
In general, the levels of measured cytokines increased or decreased in parallel; as examples, the Spearman rank correlation coefficients for peak levels of IL-10 and TNF-α, IL-10 and IFN-γ, IFN-γ and IL-13, and IL-10 and IL-1β were 0.68, 0.76, 0.85, and 0.88, respectively. In contrast, peak increases in levels of these cytokines were weakly correlated with peak viral titer: ρ for peak viral titer and TNF-α, IL-10, IFN-γ, IL-13, and IL-1β were 0.14, 0.39, 0.36, 0.37, and 0.33, respectively.
DISCUSSION
In this study, we demonstrated an inverse relationship between the levels of cytokines measured in NW fluid in RSV-naive children and the levels of attenuation of several live RSV vaccines based on RSV strain A2. These vaccine viruses each contained the same set of attenuating cp mutations derived by serial passage at low temperature and were attenuated primarily by ts point mutations affecting RNA synthesis. Thus, direct comparisons were appropriate. Children in group 1, who received either of the 2 least attenuated viruses, cpts 248/955 or cpts 530/1009, had significantly greater fold increases in IFN-γ, IL-1β, IL-2, IL-6, and IL-13 levels than children in group 3, who received the highly attenuated rA2cp248/404/1030/ΔSH. While not statistically significant, differences in levels of TNF-α and IL-10 were also observed. Children in group 2, who received the moderately attenuated cpts 248/404 virus, had levels of NW cytokines that were intermediate in magnitude between those if children who received the least attenuated and highly attenuated vaccine candidates.
Although the peak increases in levels of several cytokines in NW specimens differed significantly between groups 1 and 3 (Figure 4), the differences in peak titers of vaccine virus shed were not significant (Figure 1). These findings suggest that clinical attenuation of live RSV vaccines is the combined result of restriction of virus replication and diminution of the inflammatory host response. The reduction in viral replication likely reduces the amount of direct virus-induced epithelial cell damage. In addition, the ts point mutations, which are found in the viral polymerase and a transcription gene-start signal, are thought to reduce viral RNA synthesis. This would be most pronounced with rA2cp248/404/1030/ΔSH, which contains the greatest number of attenuating mutations and is the most attenuated of these viruses. The reduction in the synthesis of viral RNA and proteins likely reduces the stimulation of innate immune responses mediated by Toll-like and cellular cytoplasmic receptors. It is possible that these attenuating mutations may have additional and independent effects on the host response. Further work is needed to explore this possibility.
When compared to cytokine levels at baseline, infection with either cpts 248/955 or cpts 530/1009 was associated with increased levels of proinflammatory, antiinflammatory, regulatory, Th1-type, and Th2-type cytokines, including TNF-α, IFN-γ, IL-1β IL-2, IL-6, IL-10, and IL-13. Interestingly, elevated levels of IL-4 were not found, as has been shown in other studies of children with RSV infections [18]; however, these results are consistent with those of studies that have shown increases in TNF-α, IL-1β [19] IL-6 [19–21], and IL-10 [21] levels in respiratory secretions from children infected with RSV.
This study also provided an opportunity for a longitudinal assessment of the relationships between clinical symptoms, shedding of RSV, and cytokine and chemokine production. An important limitation of this analysis is that all of the viruses in this study were attenuated, compared with wild type RSV; even with the minimally attenuated cpts 248/955 and cpts 530/1009 viruses, only 1 child in this study experienced LRTI. For this reason, the findings presented here do not represent the full spectrum of disease pathogenesis observed during natural RSV infections. Nevertheless, children in groups 1 and 2 exhibited a high frequency of clinical disease that appeared to be vaccine related, and these timed infections with attenuated viruses may provide important clues as to the mechanisms of RSV disease and viral clearance in RSV-naive children.
In reviewing these data, 2 features became apparent. First, viral shedding generally preceded clinical symptoms, and symptoms persisted after virus was no longer detectable by culture (Figure 3). These data are consistent with those from preclinical studies [22, 23] and a clinical treatment trial of the RSV F monoclonal antibody palivizumab [24], which suggest that the host response contributes to RSV disease. Second, increases in levels of proinflammatory and antiinflammatory cytokines (IL-1β and IL-10, respectively) and of Th1- and Th2-type cytokines (IFN-γ and IL-13, respectively) were all highly correlated. Thus, there was no evidence of a cytokine imbalance; rather, these cytokines appeared in concert as viral replication diminished.
An additional important feature of this study was the availability of a baseline (preinoculation) NW sample from each child. While many cytokines (eg, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, and IL-13) were virtually undetectable in the baseline specimens, CXCL8, IFN-β, CCL11, and CCL4 were present in substantial and highly variable amounts. This was surprising, since children were required to be symptom-free as a prerequisite for enrollment. It may be that collection of the NW specimen, which was performed using saline and a bulb syringe, induced mild local inflammation, leading to the release of preformed mediators such as CXCL8. If this is the case, it is likely that any method of collection from the respiratory tract might produce this type of response in some children. From a practical standpoint, this suggests that caution should be used in interpreting levels of these particular analytes when measured in clinical studies that rely on samples obtained at the time of acute illness [25, 26].
This study has several important limitations. First, most illnesses were confined to the upper respiratory tract, and samples were obtained from the upper respiratory tract, so no inferences can be drawn about RSV LRTI. In addition, infants who received cpts 248/404 and rA2cp248/404/1030/ΔSH were significantly younger than those who received cpts 248/955, so we cannot say with certainty that the near absence of detectable nasal cytokine responses in recipients of rA2cp248/404/1030/ΔSH was vaccine virus related, rather than age related. However, the facts that nearly all participants were <12 months old and that other studies have shown exuberant cytokine responses in young infants [27] suggest that inherent properties of the vaccine viruses, rather than age of the vaccinees, determined the pattern of cytokine response.
In summary, we have shown that insufficiently attenuated RSV vaccines elicit a complex pattern of cytokine responses in RSV-naive infants and children and that these responses are mostly absent in recipients of an appropriately attenuated live RSV vaccine. Data from this study suggest that measurement of nasal cytokine levels is an additional useful tool for the assessment of attenuation of live RSV vaccines.
Notes
Acknowledgments. We thank Roberta Casey, Barbara Burns, and Jennifer Marron, for their clinical research expertise; Jason Morsell, for assistance with manuscript preparation; and the families who participated in these studies.
Financial support. This work was supported by the National Institutes of Health (NIH; contracts N01-AI-15444 and HHSN272200900010C). U. J. B. and P. L. C. were supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, NIH.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hall CB, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins PL, et al. Rational design of live-attenuated recombinant vaccine virus for human respiratory syncytial virus by reverse genetics. Adv Virus Res. 1999;54:423–51. doi: 10.1016/s0065-3527(08)60374-7. [DOI] [PubMed] [Google Scholar]
- 5.Collins PL, Murphy BR. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc Am Thorac Soc. 2005;2:166–73. doi: 10.1513/pats.200501-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright PF, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25:7372–8. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karron RA, et al. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus (RSV) vaccines in chimpanzees, adults, infants and children. J Infect Dis. 1997;176:1428–36. doi: 10.1086/514138. [DOI] [PubMed] [Google Scholar]
- 8.Wright PF, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–42. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 9.Wright PF, et al. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J Infect Dis. 2006;193:573–81. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- 10.Karron RA, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 11.Jin H, et al. Evaluation of recombinant respiratory syncytial virus gene deletion mutants in African green monkeys for their potential as live attenuated vaccine candidates. Vaccine. 2003;21:3647–52. doi: 10.1016/s0264-410x(03)00426-2. [DOI] [PubMed] [Google Scholar]
- 12.Luongo C, et al. Increased genetic and phenotypic stability of a promising live-attenuated respiratory syncytial virus vaccine candidate by reverse genetics. J Virol. 2012;86:10792–804. doi: 10.1128/JVI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein DI, et al. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr Infect Dis J. 2012;31:109–14. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 14.Graham BS, Rutigliano JA, Johnson TR. Respiratory syncytial virus immunobiology and pathogenesis. Virology. 2002;297:1–7. doi: 10.1006/viro.2002.1431. [DOI] [PubMed] [Google Scholar]
- 15.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–55. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotz MT, Peebles RS., Jr Mechanisms of Respiratory Syncytial Virus Modulation of Airway Immune Responses. Curr Allergy Asthma Rep. 2012;12:380–7. doi: 10.1007/s11882-012-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg HF, Domachowske JB. Inflammatory responses to respiratory syncytial virus (RSV) infection and the development of immunomodulatory pharmacotherapeutics. Curr Med Chem. 2012;19:1424–31. doi: 10.2174/092986712799828346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mobbs KJ, et al. Cytokines in severe respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2002;33:449–52. doi: 10.1002/ppul.10101. [DOI] [PubMed] [Google Scholar]
- 19.Laham FR, et al. Differential production of inflammatory cytokines in primary infection with human metapneumovirus and with other common respiratory viruses of infancy. J Infect Dis. 2004;189:2047–56. doi: 10.1086/383350. [DOI] [PubMed] [Google Scholar]
- 20.McNamara PS, et al. Pro- and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur Respir J. 2004;23:106–12. doi: 10.1183/09031936.03.00048103. [DOI] [PubMed] [Google Scholar]
- 21.Bennett BL, et al. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007;195:1532–40. doi: 10.1086/515575. [DOI] [PubMed] [Google Scholar]
- 22.Graham BS, et al. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–33. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prince GA, et al. Treatment of respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model wit systemically administered monoclonal antibody (palivizumab) and glucocorticosteroid. J Infect Dis. 2000;182:1326–30. [Google Scholar]
- 24.Malley R, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J Infect Dis. 1998;178:1555–61. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 25.Assefa D, et al. Attenuated interleukin-8/leukocyte immunoresponse in preterm infants compared with term infants hospitalized with respiratory syncytial virus bronchiolitis: a pilot study. Hum Immunol. 2011;72:708–11. doi: 10.1016/j.humimm.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Somers CC, et al. Effect of dexamethasone on respiratory syncytial virus-induced lung inflammation in children: results of a randomized, placebo controlled clinical trial. Pediatr Allergy Immunol. 2009;20:477–85. doi: 10.1111/j.1399-3038.2009.00852.x. [DOI] [PubMed] [Google Scholar]
- 27.Kristjansson S, et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol. 2005;116:805–11. doi: 10.1016/j.jaci.2005.07.012. [DOI] [PubMed] [Google Scholar]