Abstract
Background. Microbicide toxicity may reduce the efficacy of topical preexposure prophylaxis for human immunodeficiency virus (HIV) transmission. Noninvasive quantitative measures of microbicide toxicity would usefully inform microbicide development.
Methods. Ten subjects received 3 one-time interventions: 5 mL of Normosol-R fluid alone (negative control), 5 mL of 2% nonoxynol-9 (N-9) gel, and 5 mL of Normosol-R with coital simulation and sigmoidoscopic biopsy (CS + BX). Each dose of N-9 and Normosol-R contained 500 µCi of 99mtechnetium–diethylene triamine pentaacetic acid. Plasma and urine radioactivity was assessed over 24 hours.
Results. The plasma radioisotope concentration peaked 1 hour after N-9 dosing. The mean maximum radioisotope concentration after N-9 receipt was 12.0 times (95% confidence interval [CI], 6.8–21.0) and 8.4 times (95% CI, 5.2–13.5) the mean concentration after Normosol-R control receipt and CS + BX receipt, respectively; paired differences persisted for 24 hours. After N-9 dosing, the urine isotope level was 3.6 times (95% CI, 1.1–11.4) the level observed 8 hours after Normosol-R control receipt and 4.0 times (95% CI, 1.4–11.4) the level observed 4 hours after CS + BX receipt. Permeability after CS + BX receipt was greater than that after Normosol-R control receipt in 0–2-hour urine specimens only (mean permeability, 2.4; 95% CI, 1.0–5.8) but was not greater in blood.
Conclusions. Plasma sampling after rectal radioisotope administration provided quantitative estimates of altered mucosal permeability after chemical and mechanical stresses. Permeability testing may provide a useful noninvasive adjunct to assess the mucosal effects of candidate microbicides.
Clinical Trials Registration. NCT00389311.
Keywords: HIV rectal microbicide, epithelial disruption, mucosal permeability, Nonoxynol-9
Although the majority of newly reported cases of human immunodeficiency virus (HIV)/AIDS in the United States are among men who have sex with men (MSM) and up to 35% of women in the United States have engaged in receptive anal intercourse, little attention has focused on developing topical HIV microbicides for rectal use [1, 2]. Vaginal microbicides in development should also be evaluated for rectal safety (if not efficacy), since it is probable that any HIV microbicide developed for vaginal use will be used rectally. Experience from the COL-1492 trial, a large-scale vaginal microbicide trial using nonoxynol-9 (N-9), demonstrates that the failure to fully understand the potential local toxicities of an HIV microbicide formulation can impact efficacy and result in an increased risk of HIV transmission [3]. N-9 had been shown to cause mucosal erosions in the vagina and rectum, which has been postulated as a partial explanation for the increased HIV transmission rate in the COL-1492 study [4–8]. A large-scale trial of vaginally applied cellulose sulfate was halted when interim analysis indicated a higher HIV infection rate in the treatment group (although subsequent statistical analysis did not demonstrate the same degree of significance as the interim analysis) [9]. In vitro studies indicate that cellulose sulfate may disrupt cellular junctions in the mucosal epithelium, facilitating HIV translocation across the epithelium [10]. The possible role of microbicide-induced mucosal toxicity highlights the need to understand the potential for toxicity early in microbicide development.
To facilitate microbicide development, a simple, noninvasive, quantitative measure of altered colonic mucosal integrity is needed to assess the potential for altered mucosal integrity. Challenges to mucosal integrity may result during clinical studies, either from chemical stress due to the microbicide product (vehicle or active ingredient), physical stress (sigmoidoscopic biopsy), or sexual intercourse. These stresses may occur in microbicide development studies and have the potential to confound safety assessments.
N-9, used in this study as a chemical stressor, is associated with inflammatory changes evidenced by colposcopically visible erythema, proinflammatory cytokine release, and lamina propria CD8+ lymphocyte and macrophage infiltration [7, 8, 11]. These changes may occur in the absence of visible mucosal damage, although erosions are also seen. Phillips et al have demonstrated that rectal administration of N-9 is associated with shedding of sheets of epithelia (the single layer of cells lining the rectal mucosal surface) 15 minutes after dosing, possibly increasing the likelihood of HIV transmission [12, 13]. Epithelial repair from a single dose of N-9 also occurs rapidly, with an intact epithelial barrier observed 2 hours after N-9 administration and an epithelial appearance indistinguishable from baseline by 8 hours after administration. [13, 14].
Sigmoidoscopic sampling of the colonic mucosa, with or without simulated coital activity, may be performed during microbicide development to understand the pharmacokinetics or toxicity of a candidate microbicide or vehicle. Even without the trauma of endoscopic biopsies, endoscopy has been observed to occasionally induce submucosal bruising, with elevation of the mucosal layer and submucosal hemorrhage. Furthermore, the shearing and compressive forces associated with rectal intercourse might alter the epithelial layer. During rectal microbicide development, it is essential to understand whether such procedures or coital shearing stress may adversely affect the rectum by altering mucosal permeability, so that one can appropriately interpret the effects of topical microbicides on the mucosal lining of the distal colon.
Altered permeability is commonly tested by measuring the differential intestinal absorption of solutes of varying molecular weight [15–20]. These tests have been used primarily to evaluate small intestine permeability after oral administration of the test agent, but they have not been used to assess permeability in the rectum, where absorption is very low. A few reports involving comparisons of healthy volunteers with individuals with inflammatory bowel disease or other conditions with altered intestinal permeability suggest that radioisotope chelates administered intrarectally can distinguish differences in distal colon permeability [20–24].
We sought to determine whether a small radiolabeled molecule, 99mtechnetium–diethylene triamine pentaacetic acid (99mTc-DTPA), can be used as a noninvasive measure of colonic mucosal permeability induced either by a known toxin, N-9, or by a combination of coital simulation and flexible sigmoidoscopy with multiple biopsies. We hypothesized that mucosal damage is measurable by simple tests of altered rectal permeability, whether due to inflammatory changes or the physical disruption of the mucosal barrier, both of which occur after N-9 administration, or due to the physical trauma of coital forces and colonic biopsy.
METHODS
Study Participants
After institutional review board approval was obtained, 10 MSM provided informed consent, were screened, and were enrolled in the study. Subjects were excluded if they reported any history of colonic disease or surgery; had recent diarrhea, defined as ≥3 loose stools per day ≤48 hours prior to entry; had coagulation abnormalities that would increase the risk for bleeding; or had any evidence of active symptomatic illness or a clinically significant finding on physical examination. All subjects agreed to refrain from receptive anal intercourse for 48 hours prior to and following each admission.
Study Interventions
Phase 1: Normosol-R Fluid (Negative Control)
Subjects were admitted to an inpatient clinical research unit and placed on a “liquids only” diet 16 hours prior to isotope-containing vehicle dosing. The following day, subjects received a 250-mL tap water enema 8 hours prior to dosing. The preparative enema had a type and volume reported to be commonly used prior to receptive anal intercourse and was administered 8 hours prior to dosing, to minimize trauma to the colonic epithelium that would be evident at the time of vehicle dosing [13, 14, 25, 26]. Subjects expelled the enema immediately after administration.
On day 1, 2 hours prior to dosing, subjects were restricted to receive nothing by mouth. Subjects voided urine to empty the bladder just before dosing. Subjects were administered a single intrarectal 5-mL application of Normosol-R (Hospira, Lake Forest, IL), an isotonic, salt-balanced solution used for intravenous fluid replacement, containing 500 µCi of 99mTc-DTPA as the negative control condition. All urine output was collected in separate containers at intervals of 0–2, 2–4, 4–8, 8–12, and 12–24 hours after dosing, and 1-mL aliquots were taken for gamma counting. Subjects were asked to void completely at the end of each interval; the time of their last void in the interval defined the end of that interval. Blood samples were obtained by venipuncture at the midpoint of each timed urine collection (ie, 1, 3, 6, 10, and 18 hours after dosing). Blood samples were immediately centrifuged, and 1-mL plasma aliquots were collected for gamma counting. Two hours after vehicle dosing, subjects resumed a regular diet. After the 24-hour urine collection was completed, subjects continued to phase 2. The time of any bowel movement occurring during the study admission was recorded.
Phase 2: N-9 Gel (Positive Control)
Subjects remained confined to the hospital, to allow a 2-day rest period between isotope dosing during phase 1 and initiation of phase 2. The evening prior to dosing, subjects were placed on the same dietary restrictions and underwent the same bowel preparation as described above. Within 10 minutes prior to dosing, a single tube of blood was obtained by venipuncture, and subjects were instructed to void as completely as possible to provide urine to confirm by gamma counting that no residual gamma activity remained in the urine.
Subjects then received a single intrarectal application of 5 mL of Gynol II (containing 2% N-9 [1182 mosmol/kg]; Advanced Care Products, Raritan, NJ) with 500 µCi of 99mTc-DTPA incorporated. Urine and plasma samples were collected and evaluated as described above. Subjects resumed a regular diet 2 hours after dosing. Subjects were discharged home once the 24-hour urine collection interval was completed. Just prior to discharge, subjects were counseled to refrain from receptive anal intercourse for 48 hours following discharge and for 48 hours preceding the next study admission. Condom use for insertive partners was strongly advised.
Phase 3: Normosol-R Fluid With Coital Simulation Plus Endoscopic Biopsy (CS + BX)
Subjects were admitted to the clinical research unit once more and placed on the same restricted diet and underwent the same bowel preparation as described for prior phases. The following morning, subjects were taken to the endoscopy suite. Just prior to endoscopy, each subject was instructed to insert into the rectum and manipulate (via in/out cycles at 1 cycle per second for 5 minutes) a lubricated silicone vaginal dilator with the approximate size and shape of an erect human penis (product code 20; Milex Products, Chicago, IL). The dilator was then removed by the subject.
Flexible sigmoidoscopy was then performed by the study gastroenterologist (L. A. L.). A flexible endoscope (model CFQ160S; Olympus America, Center Valley, PA) was introduced per rectum and advanced to a distance of 8 cm beyond the anal verge. Biopsy forceps (Microvasive 1599; Boston Scientific, Natick, MA) were passed through the endoscope port, and 20 pinch biopsy specimens were obtained 8–25 cm beyond the anal verge. Subjects were immediately transported back to the inpatient unit and administered a single intrarectal application of 5 mL of Normosol-R with 500 µCi of 99mTc-DTPA incorporated. Subjects remained supine until completion of isotope dosing and were then allowed to ambulate freely.
Timed collection of urine and plasma specimens, resumption of regular diet, recording of bowel movements, and discharge counseling were performed as described above for phase 2.
Normosol-R Solution and N-9 Gel Test Vehicle Preparation and Administration
The test vehicles were compounded with radioisotope and loaded into dosing syringes by a commercial radiopharmacy (Cardinal Health, Beltsville, MD) and delivered to the nuclear medicine facility at The Johns Hopkins Hospital. The radioactivity of dosing syringes was measured in a dose calibrator (CRC 15-W; Capintec, Ramsey, NJ) and the time of measurement recorded. Subjects were placed in supine position, and the compounded isotope vehicle was injected intrarectally, using a lightly lubricated Luer applicator (product 35-1107; Professional Compounding Centers of America, Houston, TX). Following dosing, the residual radioactivity in each syringe was measured. This amount was decay corrected and subtracted from the initial syringe measurement, to determine the total dose administered to the subject.
Gamma Counting
Gamma activity in all blood specimens and interval-based urine specimens was counted on a gamma counter (Cobra-II Auto-Gamma counter; Packard Instrument Company, Meriden, CT) within a 110–150-keV energy window. Samples were corrected for background activity and instrument detector efficiency. To determine the cumulative urine gamma activity for a specified interval, gamma counts for urine aliquots were corrected for urine volume and sampling interval duration. Plasma and urine results were then decay corrected to account for time elapsed and were divided by the total amount of activity administered, to normalize for the dose retained by each subject. Plasma and urine results are expressed as a fraction of the initially administered isotope dose.
Statistical Analysis
The peak isotope concentration and time to peak concentration were assessed by visual inspection. Areas under the curve were calculated using the linear trapezoidal rule. For statistical analyses, subjects served as their own controls. Observations were summarized using geometric means and 95% confidence intervals (CIs). Comparisons between vehicle interventions were made for plasma and urine mucosal permeability isotope concentrations by using paired t testing of log-transformed data (SPSS; IBM, Somers, NY). Geometric mean ratios (GMRs) and 95% CIs between vehicle assignments were determined.
RESULTS
Ten MSM were enrolled and completed the study. Nine subjects were HIV seropositive, but they were otherwise healthy. All subjects tolerated the study procedures well. No adverse events greater than grade 1, as referenced in the Division of AIDS Toxicity Tables, were noted [27]. The mean isotope dose administered was 528 μCi for the N-9 intervention, compared with 496 μCi for the Normosol-R control intervention and 449 μCi for the CS + BX intervention (Table 1). In phase 3, the mean time from coital simulation and endoscopic biopsies to isotope dosing was 0.38 hours (95% CI, .30–.49). The mean time to first stool following dosing was 2.0 hours for the N-9 intervention, 4.5 hours for the Normosol-R control intervention, and 3.8 hours for the CS + BX intervention; no between-intervention comparisons were statistically significant. To control for these timing and dose-retention differences, all permeability calculations were made with radioisotope decay correction and dose-adjusted measurements.
Table 1.
Pharmacokinetic Parameters for Isotope Assessment in Plasma and Urine
| Parameter | Intervention, Geometric Mean Value (95% CI) |
||
|---|---|---|---|
| Normosol-R | Nonoxynol-9 | CS + BX | |
| Plasma AUC, fraction of dose-h × 10–5 | 0.61 (.27–1.36) | 5.06 (3.61–7.10)a,b | 0.90 (.56–1.44) |
| Plasma Cmax, fraction of dose × 10–5 | 0.06 (.03–.14) | 0.74 (.50–1.09)a,b | 0.09 (.05–.14) |
| Plasma Tmax, h after intervention receipt | 5.3 (3.5–8.2)b | 1.1 (.9–1.4)a,b | 2.8 (1.8–4.3) |
| 99mTc dose retained after receipt, µCi | 496 (484–508)b | 528 (508–548)a,b | 449 (417–483) |
| Fraction of 99mTc detected in urine 0–24 h after intervention receipt, % of dose | 1.6 (.5–5.5) | 6.5 (4.2–9.9)a,b | 2.6 (1.2–5.3) |
| Time to first bowel movement, h after intervention receipt | 4.5 (1.7–11.9) | 2.0 (1.0–4.3) | 3.8 (1.6– 9.2) |
Abbreviations: AUC, area under the curve; CI, confidence interval; Cmax, maximum concentration; CS + BX, Normosol-R plus coital simulation and endoscopic biopsy; Tmax, time to maximum concentration; 99mTc, 99mtechnetium.
a P < .05, compared with Normosol-R, using the t test for pair-wise comparison.
b P < .05, compared with CS + BX, using the t test for pair-wise comparison.
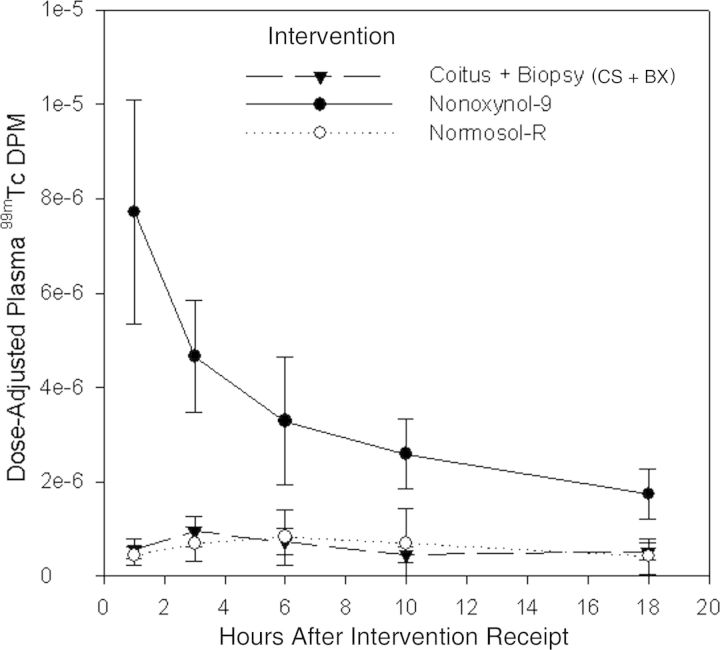
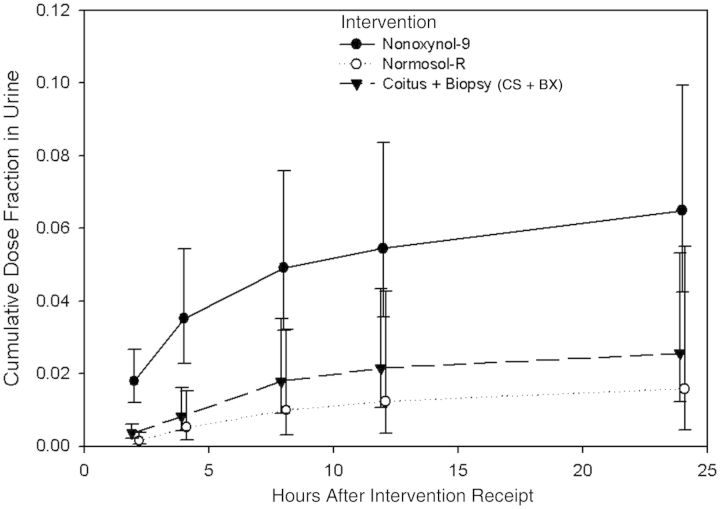
Following the test dose for all 3 conditions, the measurable radioisotope level increased in blood and urine before falling back toward background gamma activity (Figures 1 and 2). The radioisotope level in plasma, expressed as a fraction of the dose administered, peaked at the first observation time (1 hour) following N-9 dosing and declined thereafter. Plasma radioisotope levels peaked at lower levels and later after dosing for both the CS + BX intervention (peak, 3-hour specimen) and Normosol-R control intervention (peak, 6-hour specimen). Urine radioisotope levels demonstrated a similar pattern, with higher and earlier peak levels (detected in the 0–2-hour specimen) following N-9 dosing, compared with the other 2 test conditions, for which levels peaked in 2–4-hour specimens.
Figure 1.
Plasma 99mtechnetium (99mTc) level over time following a rectal dose with Normosol-R plus coital simulation and sigmoidoscopic biopsy (CS + BX), 2% nonoxynol-9 gel, and Normosol-R fluid alone (negative control). The y-axis denotes 99mTc disintegrations per minute (DPM) adjusted (ie, divided) by the dose retained. Circles and triangles denote mean values, and whiskers denote 95% confidence intervals.
Figure 2.
Cumulative fraction of 99mtechnetium (99mTc) measured in urine following isotope injection, relative to the dose administered rectally. Time points represent the total activity measured for the urine collection interval. Circles and triangles denote mean values, and whiskers denote 95% confidence intervals (CIs). Values for Normosol-R fluid alone (negative control) and Normosol-R plus coital simulation and biopsy (CS + BX) are offset slightly from the actual time point so that CIs appear distinct for each study intervention.
The mean cumulative fraction of the administered dose detected in urine was higher for the N-9 intervention (at 6.5%), compared with the CS + BX (at 2.6%) and Normosol-R control (at 1.6%) interventions (Table 1 and Figure 2). The GMRs for the N-9 intervention relative to both Normosol-R control (4.10 [95% CI, 1.6–10.0]) and CS + BX (2.5 [95% CI, 1.2–5.2]) interventions were statistically significant. There was no significant difference when comparing the dose fraction in urine between the Normosol-R control and CS + BX interventions.
The GMRs of the maximum plasma concentration of radioisotope for the N-9 intervention relative to the Normosol-R control and CS+BX interventions were 12.0 (95% CI, 6.8–21.0; Table 2) and 8.4 (95% CI, 5.2–13.5), respectively. The ratios for the N-9 intervention relative to the Normosol-R intervention and for the N-9 intervention to the CS + BX intervention declined with time, but each remained statistically significant up to and including the final observation, at 18 hours. There was no significant difference in plasma GMRs between the CS + BX and Normosol-R interventions at any time point.
Table 2.
Geometric Mean Ratios (GMRs) of Paired Data From Study Interventions
| Variable | Intervention Comparison, GMR (95% CI) |
||
|---|---|---|---|
| N-9 vs Normosol-R | N-9 vs CS + BX | CS + BX vs Normosol-R | |
| Plasma 99mTc AUC | 8.3 (4.7–15.6)a | 5.7 (3.4–9.4)a | 1.5 (.7–3.3) |
| Plasma 99mTc Cmax | 12.0 (6.8–21.0)a | 8.4 (5.2–13.5)a | 1.4 (.7–3.1) |
| Plasma 99mTc Tmax | 0.21 (.15–.28)a | 0.40 (.25–.66)a | 0.52 (.29–.94)a |
| Urine 99mTc level, h after intervention receipt | |||
| 0–2 | 12.2 (6.3–23.6)a | 5.0 (3.1–8.2)a | 2.4 (1.0– 5.8)a |
| 2–4 | 5.0 (2.1–11.8)a | 4.0 (1.4–11.4)a | 1.3 (.5–3.8) |
| 4–8 | 3.6 (1.1–11.4)a | 1.6 (.7–3.4) | 2.3 (.5–9.6) |
| 8–12 | 2.8 (.7–10.6) | 1.7 (.7–4.1) | 1.7 (.4–7.8) |
| 12–24 | 1.9 (.8–4.7) | 1.7 (.6–4.8) | 1.2 (.4–4.3) |
Abbreviations: AUC, area under the curve; CI, confidence interval; Cmax, maximum concentration; CS + BX, Normosol-R plus coital simulation and endoscopic biopsy; N-9, nonoxynol-9; Tmax, time to maximum concentration; 99mTc, 99mtechnetium.
a P < .05, using the t test for pairwise comparison.
Aliquots from the urine collections showed that the mean radioisotope level after the N-9 intervention was 12.2 (95% CI, 6.3–23.6) times that of the Normosol-R control intervention at 0–2 hours and still 3.6 (95% CI, 1.1–11.4) times the control intervention during the 4–8-hour period. The urine radioisotope level following the N-9 intervention was also greater than that for the CS + BX intervention at both 0–2 hours (GMR, 5.0 [95% CI, 3.1–8.2]) and 2–4 hours (GMR, 4.0 [95% CI, 1.4–11.4]); the urine measurements did not show any differences between study interventions at later intervals. The CS + BX intervention urine isotope level was greater than that for the Normosol-R control intervention only during the 0–2-hour interval (GMR, 2.4 [95% CI, 1.02–5.8]).
DISCUSSION
Measuring the radiolabel 99mTc-DTPA in plasma demonstrated the ability to detect greater colonic mucosal permeability following a single rectal dose of N-9, compared with either the Normosol-R control or CS + BX interventions. The difference in permeability was most pronounced at the first observation point, 1 hour after rectal dosing, but continued throughout the 18-hour observation period. There was no difference in the plasma radiolabel level between the Normosol-R control and CS + BX interventions at any time. The radiolabel level in urine after the N-9 intervention was greater than that following both the Normosol-R control and CS + BX intervention, but the duration of the difference was shorter (up to 8 hours and up to 4 hours, respectively) and of lesser magnitude, compared with blood.
This permeability testing method could be used as an adjunctive measure to rapidly and noninvasively evaluate some types of mucosal alteration caused by rectal microbicide candidates before proceeding to larger-scale clinical trials. It allows frequent collection of permeability data over time. Furthermore, it provides permeability data for the entire mucosal surface exposed to a radiolabeled microbicide candidate. By contrast, biopsies cannot be as frequent as blood or urine sampling, cannot be so numerous as to cover the entire surface exposed to study product, and are more logistically complex. The trade-off is that biopsies provide samples that can be tested for numerous mechanism-specific readouts (eg, histologically assessed mucosal integrity, inflammatory chemokines, or susceptibility to HIV infection), whereas permeability testing provides an undifferentiated composite measure of what may be multiple types of mucosal changes. For example, the mucosal N-9 effects may result from both physical loss of epithelial integrity in clinical studies [11, 28] and deleterious effects on both tight and adherens junctions in in vitro studies [29]. Whether one or both of these N-9 effects are relevant to the HIV-enhancing effect of N-9 is unproven [9]. Since multiple mechanisms of toxicity are possible, additional work is needed to correlate this permeability measure with other, more mechanistically specific assays, especially susceptibility to HIV infection, to strengthen its value as a marker of toxicity that is relevant to HIV prevention with topical microbicides.
In our study, plasma samples indicated greater differences in signal intensity and less variability than urine samples when comparing N-9 permeability to Normosol-R–related permeability. This demonstrates that the benefit associated with urine interval collection (ie, the phlebotomy-free collection method) is counterbalanced by the benefits of plasma sampling, which requires less complex sample handling and yields a superior dynamic range. Since the earliest sampling times observed—a 1-hour interval for plasma and a 0–2-hour interval for urine—showed the greatest difference between N-9 and Normosol-R, far shorter collection intervals could be used. The time to peak permeability, however, is partly dependent on the release rate of radioisotope from the vehicle, the permeability characteristics of the radiolabeled molecule, and the toxicokinetics of the permeability-altering mechanism that may vary across candidate microbicide agents and vehicles. Furthermore, the time to peak radioisotope concentration is a function of the 99mTc-DTPA plasma pharmacokinetics (clearance and distribution), which will vary with the selection of other radiolabeled compounds. Given this uncertain time to maximal permeability effect, caution is warranted in selecting shorter observation periods with other microbicide candidates, vehicles, and radiolabeled probes.
On the basis of the variability observed and our sample size, we should be able to detect increases in permeability that are twice the magnitude of the Normosol-R control. This twice-background magnitude represents a permeability-altering effect that is only one-sixth the magnitude of the 12-fold peak concentration differences observed with N-9 in our study. Whether isotope detection 2 times above background is sufficiently sensitive to detect important changes relevant for HIV infection remains to be established through correlation with HIV challenge ex vivo and seroconversion outcome studies.
The mechanical stress of receptive anal intercourse on colonic mucosa has been suggested to be an important background variable for measuring the toxicity of rectally administered microbicides. Similarly, colonic biopsies are commonly used in rectal microbicide development studies. At least with regard to permeability changes to small molecules, the combined stress of sex and biopsy appear to be trivial in contrast to the effect of N-9, since we demonstrated no differences between the Normosol-R control and CS + BX interventions in plasma isotope levels and only brief and small (relative to the N-9 intervention) differences in urine isotope levels.
The study had several other limitations. In this pilot study, we performed only a few blood collections, to establish the feasibility of this method for detecting permeability. Future studies could collect blood with more frequency just after the toxic challenge. This would enable more accurate model-based estimates of permeability changes over time, providing a more detailed toxicokinetic profile beyond the absorption and elimination rate constants of the radiolabel. The reported results were not controlled for bowel activity, which might increase colonic clearance of isotope, resulting in effectively lower isotope doses; however, the mean time to first bowel movement was not statistically different among study interventions. Given that 9 of 10 subjects in the study were HIV positive, one might question the generalizability of results to healthy volunteers if HIV infection affects mucosal permeability. However, the cumulative percentage of isotope observed in urine under the negative control condition was similar to other reports in healthy volunteers [21, 23, 24]. More importantly, since subjects served as their own controls, beyond background differences, the magnitude of the N-9 effect would have to differ in HIV-positive subjects in order for HIV infection to falsify our observations.
Having established the feasibility of this quantitative rectal permeability assessment, further correlation of permeability changes due to diverse mucosa altering mechanisms relevant to HIV transmission are essential to establish the relevance of this small molecule permeability measure to microbicide development. Vaginal permeability feasibility is also needed since the mucosal surfaces are more complex in the vagina and endocervix. Similarly, understanding the effect of hormonal fluctuation on the rectal mucosa in women may be important. If these HIV transmission–relevant correlations are established, then studying the permeability effects of universal placebo, microbicide formulations that have failed in clinical studies, and the effects of seminal fluid may aid in the design and interpretation of future microbicide trials.
Notes
Acknowledgments. We thank the research subjects, for participating in this study; and Dr Teresa Parsons and Mr James Johnson (Division of Clinical Pharmacology, Johns Hopkins University School of Medicine), for their technical support of this project.
Disclaimers. Use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention or the US Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and HIV Prevention (contract 200-2001-08015) and the National Institutes of Health National Center for Research Resources (grant M01-RR000052 to the Johns Hopkins General Clinical Research Center).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.Centers for Disease Control and Prevention. Subpopulation estimates from the HIV incidence surveillance system—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:985–9. [PubMed] [Google Scholar]
- 2.Mosher WD, Chandra A, Jones J. Hyattsville, MD: National Center for Health Statistics; 2005. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Report no. 362. [PubMed] [Google Scholar]
- 3.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 4.Watts DH, Rabe L, Krohn MA, Aura J, Hillier SL. The effects of three nonoxynol-9 preparations on vaginal flora and epithelium. J Infect Dis. 1999;180:426–37. doi: 10.1086/314881. [DOI] [PubMed] [Google Scholar]
- 5.Rustomjee R, Abdool Karim Q, Abdool Karim SS, Laga M, Stein Z. Phase 1 trial of nonoxynol-9 film among sex workers in South Africa. AIDS. 1999;13:1511–5. doi: 10.1097/00002030-199908200-00011. [DOI] [PubMed] [Google Scholar]
- 6.Gagné N, Cormier H, Omar RF, et al. Protective effect of a thermoreversible gel against the toxicity of nonoxynol-9. Sex Transm Dis. 1999;26:177–83. doi: 10.1097/00007435-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Van Damme L, Niruthisard S, Atisook R, et al. Safety evaluation of nonoxynol-9 gel in women at low risk of HIV infection. AIDS. 1998;12:433–7. doi: 10.1097/00002030-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Stafford MK, Ward H, Flanagan A, et al. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–31. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 10.Mesquita PM, Cheshenko N, Wilson SS, et al. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J Infect Dis. 2009;200:599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin HL, Jr, Stevens CE, Richardson BA, et al. Safety of a nonoxynol-9 vaginal gel in Kenyan prostitutes. A randomized clinical trial. Sex Transm Dis. 1997;24:279–83. doi: 10.1097/00007435-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Phillips DM, Taylor CL, Zacharopoulos VR, Maguire RA. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception. 2000;62:149–54. doi: 10.1016/s0010-7824(00)00156-6. [DOI] [PubMed] [Google Scholar]
- 13.Phillips DM, Sudol KM, Taylor CL, Guichard L, Elsen R, Maguire RA. Lubricants containing N-9 may enhance rectal transmission of HIV and other STIs. Contraception. 2004;70:107–10. doi: 10.1016/j.contraception.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Tabet SR, Surawicz C, Horton S, et al. Safety and toxicity of nonoxynol-9 gel as a rectal microbicide. Sex Transm Dis. 1999;26:564–71. doi: 10.1097/00007435-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Bjarnason I, Maxton D, Reynolds AP, Catt S, Peters TJ, Menzies IS. Comparison of four markers of intestinal permeability in control subjects and patients with coeliac disease. Scand J Gastroenterol. 1994;29:630–9. doi: 10.3109/00365529409092484. [DOI] [PubMed] [Google Scholar]
- 16.Zamora SA, Hilsden RJ, Meddings JB, Butzner JD, Scott RB, Sutherland LR. Intestinal permeability before and after ibuprofen in families of children with Crohn's disease. Can J Gastroenterol. 1999;13:31–6. doi: 10.1155/1999/457315. [DOI] [PubMed] [Google Scholar]
- 17.Hallemeesch MM, Lamers WH, Soeters PB, Deutz NE. Increased lactulose/rhamnose ratio during fluid load is caused by increased urinary lactulose excretion. Am J Physiol Gastrointest Liver Physiol. 2000;278:G83–8. doi: 10.1152/ajpgi.2000.278.1.G83. [DOI] [PubMed] [Google Scholar]
- 18.Maxton DG, Bjarnason I, Reynolds AP, Catt SD, Peters TJ, Menzies IS. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, L-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Colch) 1986;71:71–80. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- 19.Teahon K, Somasundaram S, Smith T, Menzies I, Bjarnason I. Assessing the site of increased intestinal permeability in coeliac and inflammatory bowel disease. Gut. 1996;38:864–9. doi: 10.1136/gut.38.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins RT, Ramage JK, Jones DB, Collins SM, Goodacre RL, Hunt RH. Small bowel and colonic permeability to 51Cr-EDTA in patients with active inflammatory bowel disease. Clin Invest Med. 1988;11:151–5. [PubMed] [Google Scholar]
- 21.Obinna FC, Cook G, Beale T, et al. Comparative assessment of small intestinal and colonic permeability in HIV-infected homosexual men. AIDS. 1995;9:1009–16. doi: 10.1097/00002030-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 22.O'morain CA, Abelow AC, Chervu LR, Fleischner GM, Das KM. Chromium 51- ethylenediaminetetraacetate test: a useful test in the assessment of inflammatory bowel disease. J Lab Clin Med. 1986;108:430–5. [PubMed] [Google Scholar]
- 23.Rask-Madsen J, Schwartz M. Absorption of 51Cr-EDTA in ulcerative colitis following rectal instillation. Scand J Gastroenterol. 1970;5:361–8. [PubMed] [Google Scholar]
- 24.Jorgensen VL, Nielsen SL, Espersen K, Perner A. Increased colorectal permeability in patients with severe sepsis and septic shock. Intensive Care Med. 2006;32:1790–6. doi: 10.1007/s00134-006-0356-6. [DOI] [PubMed] [Google Scholar]
- 25.Feil W, Lacy ER, Wong YM, Burger D, Wenzl E, Starlinger M, Schiessel R. Rapid epithelial restitution of human and rabbit colonic mucosa. Gastroenterology. 1989;97:685–701. doi: 10.1016/0016-5085(89)90640-9. [DOI] [PubMed] [Google Scholar]
- 26.Hylton J, Fuchs EJ, Hendrix CW. Microbicides 2004. London, United Kingdom: 2004. An assessment of sexual practices affecting the feasibility of microbicide development among MSM; pp. 28–31. Poster# 02667. [Google Scholar]
- 27.Division of AIDS table for grading the severity of adult and pediatric adverse events; Version 1.0. December 2004. Clarification August 2009 http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.doc . Accessed 5 November 2012. [Google Scholar]
- 28.Fuchs EJ, Lee LA, Torbenson MS, Parsons TL, Bakshi RP, Guidos AM, Wahl RL, Hendrix CW. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis. 2007;195:703–10. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 29.Wilson SS, Cheshenko N, Fakioglu E, Mesquita PM, Keller MJ, Herold BC. Susceptibility to genital herpes as a biomarker predictive of increased HIV risk: expansion of a murine model of microbicide safety. Antivir Ther. 2009;14:1113–24. doi: 10.3851/IMP1463. [DOI] [PMC free article] [PubMed] [Google Scholar]