Abstract
Increased protein synthesis is proposed as a mechanism of life-span extension during caloric restriction (CR). We hypothesized that CR does not increase protein synthesis in all tissues and protein fractions and that any increased protein synthesis with CR would be due to an increased anabolic effect of feeding. We used short- (4 hours) and long-term (6 weeks) methods to measure in vivo protein synthesis in lifelong ad libitum (AL) and CR mice. We did not detect an acute effect of feeding on protein synthesis while liver mitochondrial protein synthesis was lower in CR mice versus AL mice. Mammalian target of rapamycin (mTOR) signaling was repressed in liver and heart from CR mice indicative of energetic stress and suppression of growth. Our main findings were that CR did not increase rates of mixed protein synthesis over the long term or in response to acute feeding, and protein synthesis was maintained despite decreased mTOR signaling.
Key Words: Aging, Stable isotope, Protein metabolism.
CALORIC restriction (CR) without malnutrition is an intervention that consistently increases maximum life span across a variety of species. CR can increase mean and maximum life span by 40%, and because it is relatively simple and the outcomes robust, it is a good model to study the mechanisms of aging (1). Consistent with evolutionary theories of aging, it is believed that CR induces a state of somatic maintenance, which is antiaging, rather than the reproductive state that is proaging (2). Somatic maintenance implies an increase in repair processes, which is supported by evidence that CR inhibits the accumulation of oxidatively damaged proteins (3), increases respiratory control (4), increases mitochondria efficiency (5), and increases autophagy (6).
It has long been recognized that protein and energy metabolisms are dependent on each other (7–9). The process of protein synthesis is energetically expensive (10) and in isolated cells requires the greatest proportion (20.5%) of basal respiratory rate (11). In times of energy deficit, either absolute energy deficiency or decreased energetic flux, it is not energetically economical to build up large stores of protein. Given the dependence of protein turnover on energy status, it is surprising that during CR, protein turnover is observed to increase in some reports (4,12–14), and that CR increases transcription of genes associated with increased protein turnover (15).
Protein turnover is tissue specific. In a series of studies on male Sprague Dawley rats maintained on 50% CR from 3 weeks of age, there was an increase in whole body protein turnover (13), variable results in tibialis anterior or soleus muscle protein turnover (12), and no change in lung protein turnover (16). Recently, it has been reported that CR does not change actin and myosin turnover in either tibialis anterior or soleus muscles in male Wistar rats on a 40% restricted diet for 5 months (4) and that short-term (7 days) CR of 40% in male Wistar rats decreases protein turnover in liver and skeletal muscle but is maintained in heart (17). We (Price et al., unpublished data) used a liquid chromatography—tandem mass spectroscopy technique, in which in vivo tissue protein synthesis rates are measured by mass isotopomer distribution analysis (MIDA) of D2O incorporation into peptide fragments, to measure dynamics of the global proteome and found a marked reduction in synthesis rates of almost all proteins in the hepatic proteome, particularly mitochondrial proteins, in rats maintained on chronic CR. In our previous investigation into B6D2F1 mice on lifelong 40% CR, it was found that mitochondrial protein synthesis was maintained with CR compared to AL in heart and skeletal muscle when assessed over 6 weeks (39). Although some have summarized that CR increases protein turnover (18,19), the issue does not seem truly resolved, because tissue-specific responses are not consistent.
Much of what we currently know about the rates of protein turnover was obtained by the use of stable or radioactive isotopic tracers. In a series of experiments, it was determined that the commonly used method of using flooding doses of essential amino acid tracers were increasing protein synthesis rates (20,21), an intrinsic limitation based on principles of tracer methodology because the tracer changed the process that was being measured. Subsequent studies have identified leucine-stimulated mammalian target of rapamycin (mTOR) signaling and downstream activation of translation initiation factors as the means by which a large amount of essential amino acids stimulated protein synthesis (22). As discussed previously, in general, protein synthesis has been reported to increase with CR although changes are not uniform throughout tissues (4,12–14,16,18,19,23). The reports from which these conclusions are drawn (4,12–14,16,23) have all used a flooding dose of an essential amino acid. The flooding doses used in these studies were over twice what is observed to stimulate protein synthesis rates in humans (20,21,24). Therefore, the method itself may be simulating feeding, and the measured response is not only the effect of CR but also reflects the effects of CR on tissue-specific responses to feeding. Also, because some tissues, such as skeletal muscle, are sensitive to a feeding stimulus while others are not, the interpretation of tissue-specific protein turnover measurements over the long-term becomes complicated (25).
An additional method to measure tissue protein turnover rates has been established that circumvents the problem of precursor labeling, namely stable isotopically labeled water (2H2O) (26–28). The 2H2O equilibrates throughout all tissues within an hour and decays with a half-life of 1 week (29). The long-term labeling design allows for the determination of average (or cumulative) effects over time, which is an advantage when studying long-term treatments. Importantly, the technique has been shown to be reliable and valid in a variety of tissues (28). Recently, others have adapted and validated the 2H2O method for acute interventions (17,30,31). This method relies on a flooding dose of 2H2O administered similarly to a flooding dose of amino acids but without the simultaneous stimulation of mTOR and translation initiation as with essential amino acids.
In this study, our aims were to understand the effects of CR on tissue-specific protein turnover. We hypothesized that CR does not increase protein synthesis in all protein fractions and tissues because of the energy requirements of messenger RNA (mRNA) translation. Further, we hypothesized that if a nutrient-sensitive tissue did increase protein synthesis, it would be due to increased anabolic responses to acute feeding. To address our hypothesis, we used a combination of 2H2O methods to measure in vivo protein synthesis in a variety of tissues in ad libitum (AL) and CR mice. Our use of 2H2O methods avoided the independent effect of flooding doses of essential amino acid that confound interpretation of previous studies.
Methods
Overall Study Design
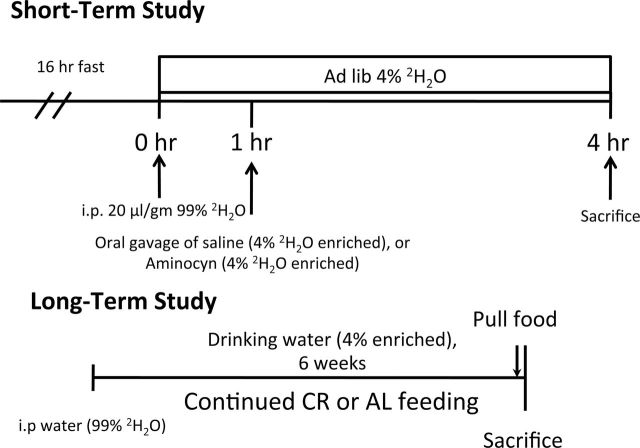
Male B6D2F1 mice from the National Institute of Aging (NIA) CR colony and age-matched AL controls were used for all aspects of the study. Lifelong CR mice were maintained at the NIA colony at 40% food restricted compared with AL. Mice were purchased at 6, 12, and 21 months of age into both AL and CR groups and were studied as one group. CR animals were maintained on NIH-31/NIA Fortified Diet, whereas AL animals were maintained on NIH-31 diet. Two separate cohorts were used for the study (Figure 1). The first cohort was used after a 1-week acclimatization to housing conditions, whereas the second cohort was used after a 1-week acclimatization and 6-week assessment of synthesis. The animals were individually housed and consumed the same absolute quantity of NIA diets while being maintained until experimentation at the CSU Laboratory Animal Resource Center, at 18–26°C (dry bulb), 30%–70% humidity, and a 12-hour light/dark cycle. All procedures at the facility meet or exceed the standards for facilities housing animals as described in the Animal Welfare Act regulations, the Guide for the Care and Use of Laboratory Animals and the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching and were approved by the CSU Animal Care and Use Committee (protocol #09-022A).
Figure 1.
Experimental design of short- and long-term studies.
Sixteen hours prior to sacrifice, all food, but not water, was removed from the animals’ cages (Figure 1). Animals were anesthetized with an intraperitoneal (i.p.) injection of sodium pentobarbital. Blood was then obtained by cardiac puncture (approximately 1mL) followed by rapid excision and cryopreservation of the heart, liver, and the posterior aspect of both the distal hind limbs (mixed skeletal muscle). All tissues were stored at −80°C until analysis.
Labeled Water
The use of heavy water (2H2O) allows simultaneous assessment of multiple synthetic processes. In this case, we assessed the synthesis of protein in three tissues: heart, liver, and skeletal muscle. Animals in Cohort 1 were studied in the short term by receiving an i.p. injection of 20 µL/gm body weight 99% 2H2O followed 1 hour later by an oral gavage of saline (Fasted) or an amino acid solution (Fed; Aminosyn, 0.05mg/gm body weight, Aminosyn II 15%, Hospira Inc, Lake Forest, IL). The amino acid dose was roughly equivalent to those used in previous studies of CR (4,12–14,16,23). Both saline and Aminosyn were enriched 4% with 2H2O. After 3 hours, the oral gavage tissues were collected. Animals in Cohort 2 were studied over the long term and received an i.p. injection of 99% enriched 2H2O calculated to enrich the body water pool (assumed 60% of body weight) to 5%. Animals were then allowed to drink ad libitum water enriched to 4% for the next 6 weeks.
Tissue isolation.—Because of the heterogeneity of protein synthesis responses within a tissue, heart, liver, and skeletal muscle were fractionated according to our previously published procedures (32) as modified from Butz et al. (33). Tissue (50–70mg) was homogenized in 1mL isolation buffer (100mM KCl, 40mM Tris HCl, 10mM Tris Base, 5mM MgCl2, 1mM EDTA, 1mM ATP, pH = 7.5) with phosphatase and protease inhibitors (HALT, Thermo Scientific) using a bead homogenizer (Next Advance Inc, Averill Park, NY). The homogenate was centrifuged at 800g for 10 minutes to pellet a mixed protein fraction (Mixed). The supernatant from the low-speed spin was carefully removed and centrifuged (9000g) for 10 minutes to pellet a mitochondrial enriched fraction (Mito). The crude Mito pellet was washed and suspended in 200 µL of solution 2 (100mM KCl, 10mM Tris-HCl, 10mM Tris base, 1mM MgSO4, 0.1mM EDTA, 0.02mM ATP, and 1.5% BSA, pH 7.4), and then centrifuged (8000g, 10 minutes, 4°C). The pellet was washed a second time, suspended in 100 µL of solution 2 and centrifuged (6000g, 10 minutes, 4°C). The final Mixed and Mito pellets were washed with 500 µL of 100% ethanol, centrifuged (1000g, 30 seconds, 4°C), and rinsed with water (repeated twice). Protein pellets were solubilized in 1N NaOH (50°C, 15 minutes) and hydrolyzed into free amino acids (6M HCl, 120°C, 24 hours).
Preparation of analytes for mass spectrometric analyses.—The hydrolysates from the tissue isolations were ion-exchanged, dried under vacuum, and then suspended in 1mL of 50% acetonitrile, 50mM K2HPO4, pH 11. About 20 µL of pentafluorobenzyl bromide (Pierce Scientific, Rockford, IL) were added, and the sealed mixture was incubated at 100°C for 1 hour. Derivatives were extracted into ethyl acetate, and the organic layer was removed and dried by the addition of solid Na2SO4 followed by vacuum centrifugation.
GC-MS analysis of derivatized amino acids.—Using negative chemical ionization (NCI), derivatized amino acidswere analyzed on a DB225 gas chromatograph column. The starting temperature was 100°C, increasing 10°C per minute to 220°C. The mass spectrometry used NCI with helium as the carrier gas and methane as the reagent gas. The mass-to-charge ratios of 448, 449, and 450 were monitored for the pentafluorobenzyl-N,N-di(pentafluorobenzyl)alaninate derivative. In all cases, these mass-to-charge ratios represented the primary daughter ions that included all of the original hydrocarbon bonds from the given amino acid. 2H enrichment was calculated as described previously (34). The newly synthesized fraction (f) of muscle proteins was calculated from the true precursor enrichment (p) using MIDA (35,36). Protein synthesis was calculated as the change in enrichment of deuterium-labeled alanine (36) bound in muscle proteins over the entire labeling period and expressed as the common unit for protein synthesis rates (%/h).
Western Blot Analyses
From Animals in Cohort 1, approximately 30mg of frozen tissue was homogenized (Next Advance Inc, Averill Park NY) in 500 µL of ice-cold buffer (100mM KCl, 40mM Tris HCl, 10mM Tris Base, 5mM MgCl2, 1mM EDTA, 1mM ATP, pH 7.4) and commercial protease with phosphate inhibitor (Halt, Thermo Fisher, Rockford IL). Samples were centrifuged (10 minutes, 10,000g, 4°C), then the supernatant was removed, and the protein concentration determined using a bicinchoninic acid assay (Thermo Fisher, Rockford, IL). Samples were diluted to the same concentration, boiled with Laemmlli buffer, then 20 µg (heart and skeletal muscles) or 30 µg (liver) were separated using 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) at 200V. Proteins were transferred at 4°C (100V for 60 minutes in 20% w/v methanol, 0.02% w/v SDS, 25mM Tris, 192mM glycine, pH 8.3) to nitrocellulose paper and incubated in Superblock (Thermo Fisher, Rockford, IL) for 1 hour at room temperature. Antibodies were purchased from Cell Signaling Technologies (Boston, MA; RpS6 #2217, RpS6 phospho-Ser[240/244] #2215, 4E-BP-1 #9452, phospho-4E-BP-1[Thr37/46] #9459). Blots were incubated overnight with primary antibodies diluted 1:250 in Superblock reagent. Blots were washed in tris-buffered saline with Tween and incubated with anti-rabbit horse-radish peroxidase-conjugated secondary antibody diluted 1:2000 in Superblock with subsequent chemiluminescence detection (West Dura, Pierce, Rockford, IL). Images were captured, and densitometry was analyzed using a UVP Bioimaging system (Upland, CA). Blots were probed for phosphorylated proteins first, then stripped and reprobed for total protein. Equal loading was verified using ponceau-s staining, as well as actin antibodies (sc-8432, Santa Cruz Biotechnology, Santa Cruz, CA).
Statistics
Statistical analysis was performed using SPSS (V19, IBM SPSS, Somers, NY). Our initial goal was to also examine whether there were changes in protein turnover at three different ages. We did not detect a difference with age in our primary outcomes and therefore collapsed the ages into AL and CR groups to increase the number of animals in each comparison and increase statistical power. The effects of treatment (AL vs. CR) and condition (Fasted vs. Fed) were examined by two-by-two analysis of variance with a priori linear contrasts. In conditions where data were unequally variable, appropriate transformations were performed. For comparing AL and CR without respect to feeding (long-term and collapsed short-term data), a two-tailed unpaired t-test was used. Significance was set at p ≤ 0.05. Data are presented as mean ± standard error mean.
Results
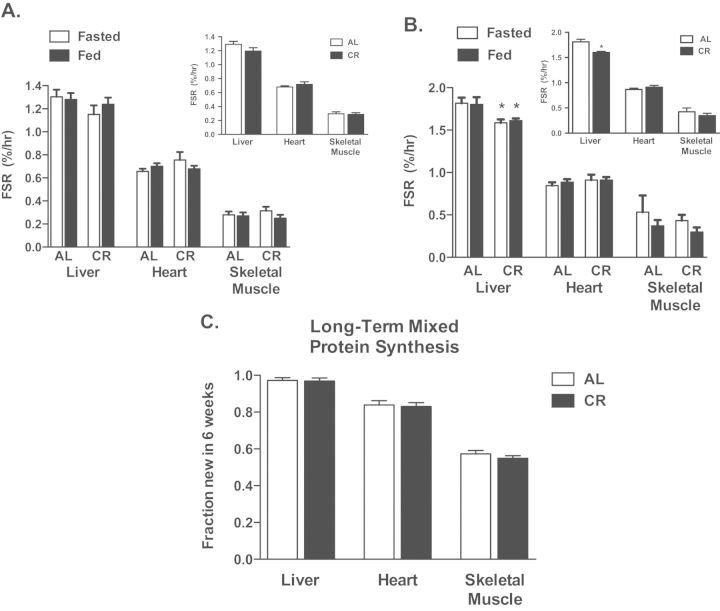
Liver, heart, and skeletal muscle–mixed protein synthesis were assessed in the 4-hour period after feeding an amino acid (or saline) solution and over a 6-week period. In the short-term cohort, there were no significant increases in mixed protein synthesis in all three tissues with feeding and no differences between AL and CR animals (Figure 2A). In liver, mitochondrial protein synthesis was lower in CR animals when compared with AL animals (Figure 2B) in both the fasted and the fed conditions. There were no differences between AL and CR animals for heart or skeletal muscle mitochondrial protein synthesis. When all three tissues were assessed over the long term, there were no significant differences between AL and CR animals for mixed protein synthesis (Figure 2C), although liver mixed protein was 100% newly replaced in this time period, and therefore, it is not possible to differentiate rates of synthesis between treatments.
Figure 2.
Rates of protein synthesis assessed over the short term (4 hours) (A and B) and long term (6 weeks) (C) in liver, heart, and skeletal muscle of lifelong CR or AL male B6D2F1 mice. Acute feeding of an amino acid solution did not increase mixed protein synthesis in any tissue and responses did not differ between AL and CR mice (A). Because no differences were noted with feeding, Fed and Fasted groups were collapsed into one group and compared (panel insets). Acute feeding did not increase mitochondrial protein synthesis (B), but the liver of CR mice had decreased rates of mitochondrial protein synthesis compared with AL mice in the fasted and fed conditions (B). Again, the only difference was slower rates of mitochondrial protein synthesis in the liver of CR animals. When animals were compared over the long term, there were no differences in mixed protein synthesis, although the liver protein was 100% replaced by this time point and therefore cannot be interpreted kinetically. FSR = fractional synthesis rate. *Significantly different from AL, p < .05. Mice were purchased at 6, 12, and 21 months of age into both AL and CR groups and were studied as one group. For short-term study, n = 15–17 for heart and liver (both fractions), 9–13 for skeletal muscle mixed, and 2–5 for skeletal muscle mitochondria. For long-term study, n = 23–24 for all groups.
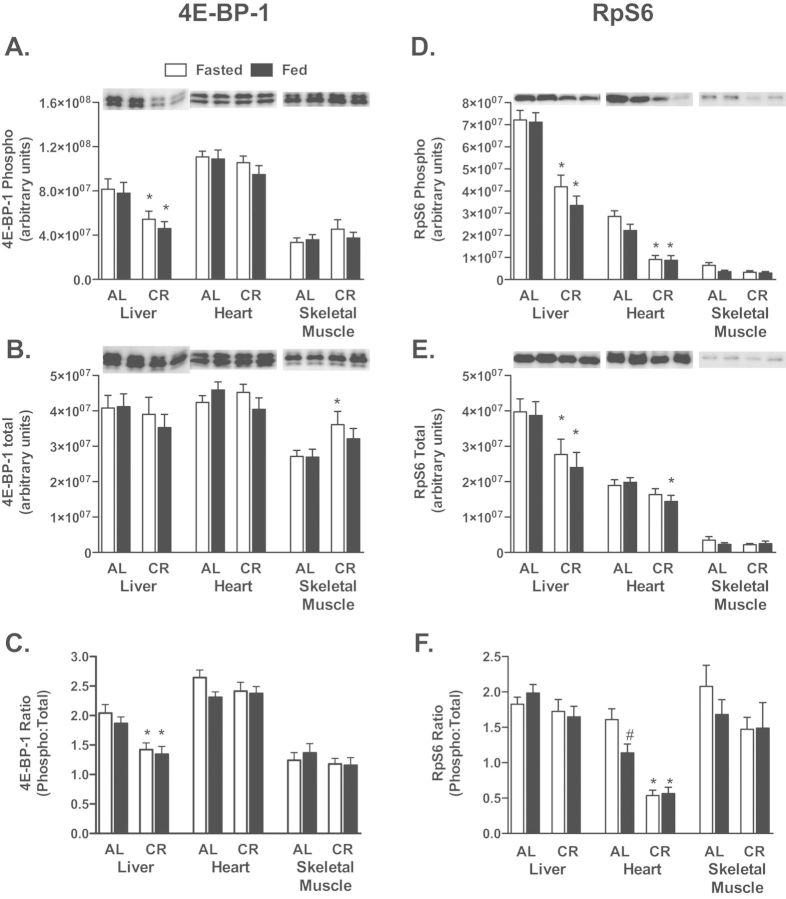
All three tissues were assessed for activation of the mTOR pathway by Western blotting for phospho and total 4E-BP-1 and RpS6. In liver, phospho-4E-BP-1 (Figure 3A) and phospho:total (Figure 3B) were decreased with CR as was phospho-RpS6 (Figure 3D) and total (Figure 3E). For heart, phospho-RpS6 (Figure 3D) and phospho:total (Figure 3F) were decreased with CR.
Figure 3.
4E-BP-1, phospho-4E-BP-1[Thr37/46], RpS6, and phospho-RpS6 [Ser240/244] were determined by Western blot for phospho (A and D), total (B and E), and phospho:total (C and F) in liver, heart, and skeletal muscle of AL and CR mice in the fasted and fed conditions. *Significantly different from AL, p < .05. #Significantly different from Fasted, p < .05. n = 7–10 for skeletal muscle RpS6 and 14–15 for all other quantifications.
Discussion
We hypothesized that CR does not increase protein synthesis in all protein fractions and tissues because of the energy requirements of mRNA translation. Further, we hypothesized that if a nutrient-sensitive tissue did increase protein synthesis, it would occur in the context of an increased anabolic effect to an acute feeding. Our main findings were that CR did not increase rates of mixed protein synthesis in response to an acute feeding or over the long term. Moreover, short-term responses indicate a potential decrease in mitochondrial protein synthesis in the liver during CR.
Short- and Long-term Protein Synthesis
Prior to investigation, we hypothesized that measurements of increased rates of protein synthesis with CR could have been due to an increased anabolic response to a flooding dose of essential amino acids. Others have recently adapted the labeled water method to assess short-term changes in protein synthesis (17,30,31), which allowed us to measure changes in protein synthesis to an amino acid bolus over a period of 4 hours. There was no detectable effect of feeding in any tissue, and this did not differ between lifelong AL and CR groups. This finding is consistent with what others have found in heart (17,37). In liver, however, rates of protein synthesis seem to differ based on the amino acid quantity infused (37), indicating a threshold of amino acid bolus. We were surprised not to find an anabolic effect of feeding in skeletal muscle, which is contrary to what others have previously reported (17). There are two potential reasons for the lack of a feeding response in the skeletal muscle. First, the mode of “feeding” is different among comparable experiments. In the current study, an oral gavage with an amino acid solution was used for the acute feeding. In other studies, free access to food over 20 hours (17), or intravenous infusion (21,22,38) was used. Although we modeled our feeding dose off the flooding dose of Smith et al. (21), it is possible that the mode of delivery and the necessity of crossing the gut could change metabolic responses to a given comparable amino acid dose. Second, in our hands, the incorporation of label over the 4-hour period into skeletal muscle was very low due to the slower rate (compared with liver and heart) of protein synthesis in skeletal muscle, thus increasing variability around a limit of detection. Therefore, the number of samples that we could obtain usable data were low. In the future, studies using the acute assessment techniques should be undertaken for longer periods of time (5 hours or more) to assess skeletal muscle protein synthesis.
The ability to measure long-term protein synthesis (eg, days to weeks) is a particular advantage of the labeled water approach. Here, we report that over the long term, there was no effect of CR on mixed protein synthesis in three different tissues. Long-term synthesis rates were greatest in liver, in which protein was already replaced within the 6-week period, followed by heart and skeletal muscles. Previously we assessed the synthesis rates of just the mitochondrial protein fraction (mitochondrial biogenesis) over the long term in liver, heart, and skeletal muscle (39). In that report, liver mitochondrial proteins were 100% new, making these data uninterpretable kinetically, with slower rates of synthesis for heart and skeletal muscle. The relative synthesis rates between tissues in both the current and our previous publication are consistent with those published in neonatal pigs (37). In the current study, we assessed short-term mitochondrial protein synthesis and found slower rates of synthesis in CR compared with AL mice. This finding substantiates that there may be decreased mitochondrial protein synthesis during CR in the liver as others have reported. The current findings, in combination with our previously published report (39) illustrate the importance of assessing multiple tissues when exploring the systemic effects of CR because responses seem to vary by tissue.
Methodological Considerations
Recently, we proposed that the assessment of rates of protein synthesis is important when determining age-related changes (38). It is therefore important to discuss how well our short-term- and long-term-labeled water methods qualitatively and quantitatively support each other. With our short-term measurement, liver protein synthesis was greater than heart protein synthesis, which was greater than skeletal muscle. Qualitatively, these rates are supported by our long-term outcomes and are in line with what we expect for synthetis rates relative to each other in these tissues (37). Quantitatively, the results of short-term and long-term studies do not necessarily match. As an example, we can use data from short-term and long-term skeletal muscle proteins. Long-term measurements calculate to 0.055%/h, whereas short-term measurements average 0.28%/h. It is therefore apparent that values measured over 4 hours do not quantitatively predict the long-term values. It is worth noting that values calculated from the long-term experiment are reflective of what others have found with short-term incorporation of labeled amino acids (32,40,41). Further, when others (31) simultaneously measured protein synthesis with a radioactive phenylalanine tracer and 2H2O methods similar to ours, they also found that there were quantitative differences between the methods, but not qualitative differences.
The differences between short- and long-term measurements could be explained by differences in turnover between the protein pools of the tissue. It could be that one pool, representing 75% of the proteins, is rapidly turned over. In the current study, the short-term value of 0.28%/h would equate to 100% new protein in approximately 14 days. A second pool representing 25% of the proteins could be synthesized more slowly with minimal turnover in 6 weeks (eg, extracellular matrix). If the 75% pool is at a plateau of 100% for 4 weeks and a second pool’s synthesis is minimal, a 6-week integrated value could equate to 0.055 %/h for the entire period.
Finally, the observation of higher synthesis rates over the short term is not unique to labeled water and may be a result of flooding doses in general. In rats given a flooding dose of [13C6]phenylalanine, skeletal muscle mitochondrial protein synthesis was about 0.6%/h (42), a value even higher than our values. Other studies have found similar values in rat mixed muscle (31,43,44), and all have used some form of a flooding dose. It has been well documented that in skeletal muscle, the flooding dose of amino acid was likely causing a feeding response (20,21). However, that should not be the case when using 2H2O (30). Future studies would need to consider multiple time points or further tissue fractionation to determine whether this is true. This variation of synthesis rates over time reiterates the importance of both short-term and long-term measurements of protein turnover when investigating CR or other antiaging interventions.
Translation Regulation of Protein Synthesis
In our previous publication using long-term CR mice, we reported a general suppression of mTOR signaling in CR animals (39). We confirm that finding in the present study because, although tissue specific, there is a general decrease in 4E-BP-1 and RpS6 phosphorylation. In this report, as well as our previous, we chose to present phospho, total, and phospho:total ratios because it is unclear which is the driving factor in the degree of mTOR activation. For example, in liver, there is a decrease in 4E-BP-1 phosphorylation and no change in total 4E-BP-1, resulting in a decreased phospho:total ratio. However, in RpS6, there is a decrease in phosphorylation and a concomitant decrease in total protein resulting in no change in the ratio. We are unsure whether the phosphorylation or the ratio of phosphorylation to total ultimately dictates mTOR regulation. However, responses in liver and heart are consistent with decreased activation of the mTOR pathway because any changes are consistently in the direction of decreased activation. The observed decrease in mTOR activation with CR, at least in liver and heart, is consistent with a stress response in which energetic constraints, either total energy or energy flux, lead to decreased translation initiation (45,46). It is therefore interesting that protein synthesis rates are maintained in these tissues despite the energetic constraints. In our previous report (39), we demonstrated that cellular proliferation in the liver and heart of CR mice were decreased during lifelong CR. It is therefore apparent that in times of energetic constraints, there are tradeoffs between energy consuming cellular processes such as synthesis and proliferation.
Perspectives and Conclusions
The current report along with our previous report (39) collectively illustrates the antagonistic pleiotropy between growth and somatic maintenance (2). At the center of these decisions is the mTOR pathway due to its role in cell growth and survival. In skeletal muscle, strategies such as amino acid feeding serve to increase protein synthesis by activation of the mTOR pathway (47) and have been strongly advocated for an older population to stimulate growth and slow sarcopenia. However, suppressed mTOR signaling seems to be a common feature of longevity models (48), and chronic rapamycin feeding, which inhibits mTOR signaling, decrease cellular proliferation (39) and increase longevity (49). Because of differences between a cell’s propensity to replicate, it is likely that tissue-specific responses to energetic constraints or other cellular stresses will play a large role in determining the success of antiaging treatments. We therefore suggest approaches that consider multiple tissues for systemic antiaging treatments.
Funding
This projected was funded by NIH 1K01AG031829-01.
Acknowledgments
Elise Donovan and Dan Heusinger are thanked for their help with the experiments. We thank Ryan Yee and Jon Bahn for their help with GC-MS analyses. Finally, Michael Pagliassotti, Greg Cartee, and Manfred Diehl are acknowledged for their initial assistance and experimental advice.
References
- 1. Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002; 34: 1340–1354 [DOI] [PubMed] [Google Scholar]
- 2. Kapahi P. Protein synthesis and the antagonistic pleiotropy hypothesis of aging. Adv Exp Med Biol. 2010; 694: 30–37 [DOI] [PubMed] [Google Scholar]
- 3. Youngman LD, Park JY, Ames BN. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci USA. 1992; 89: 9112–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zangarelli A, Chanseaume E, Morio B, et al. Synergistic effects of caloric restriction with maintained protein intake on skeletal muscle performance in 21-month-old rats: a mitochondria-mediated pathway. FASEB J. 2006; 20: 2439–2450 [DOI] [PubMed] [Google Scholar]
- 5. Civitarese AE, Carling S, Heilbronn LK, et al. CALERIE Pennington Team Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007; 4: e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergamini E, Cavallini G, Donati A, Gori Z. The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol. 2004; 36: 2392–2404 [DOI] [PubMed] [Google Scholar]
- 7. Butterfield GE, Calloway DH. Physical activity improves protein utilization in young men. Br J Nutr. 1984; 51: 171–184 [DOI] [PubMed] [Google Scholar]
- 8. Todd KS, Butterfield GE, Calloway DH. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J Nutr. 1984; 114: 2107–2118 [DOI] [PubMed] [Google Scholar]
- 9. Cuthbertson DP, McGirr JL, Munro HN. A study of the effect of overfeeding on the protein metabolism of man: The effect of muscular work at different levels of energy intake, with particular reference to the timing of the work in relation to the taking of food. Biochem J. 1937; 31: 2293–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997; 77: 731–758 [DOI] [PubMed] [Google Scholar]
- 11. Buttgereit F, Brand MD. A hierarchy of ATP-consuming processes in mammalian cells. Biochem J. 1995; 312 (Pt 1): 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. el Haj AJ, Lewis SE, Goldspink DF, Merry BJ, Holehan AM. The effect of chronic and acute dietary restriction on the growth and protein turnover of fast and slow types of rat skeletal muscle. Comp Biochem Physiol A Comp Physiol. 1986; 85: 281–287 [DOI] [PubMed] [Google Scholar]
- 13. Lewis SE, Goldspink DF, Phillips JG, Merry BJ, Holehan AM. The effects of aging and chronic dietary restriction on whole body growth and protein turnover in the rat. Exp Gerontol. 1985; 20: 253–263 [DOI] [PubMed] [Google Scholar]
- 14. Merry BJ, Holehan AM, Lewis SE, Goldspink DF. The effects of ageing and chronic dietary restriction on in vivo hepatic protein synthesis in the rat. Mech Ageing Dev. 1987; 39: 189–199 [DOI] [PubMed] [Google Scholar]
- 15. Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999; 285: 1390–1393 [DOI] [PubMed] [Google Scholar]
- 16. Goldspink DF, Merry BJ. Changes in protein turnover and growth of the rat lung in response to ageing and long-term dietary restriction. Mech Ageing Dev. 1988; 42: 253–262 [DOI] [PubMed] [Google Scholar]
- 17. Yuan CL, Sharma N, Gilge DA, et al. Preserved protein synthesis in the heart in response to acute fasting and chronic food restriction despite reductions in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2008; 295: E216–E222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tavernarakis N, Driscoll M. Caloric restriction and lifespan: a role for protein turnover? Mech Ageing Dev. 2002; 123: 215–229 [DOI] [PubMed] [Google Scholar]
- 19. Holehan AM, Merry BJ. The experimental manipulation of ageing by diet. Biol Rev Camb Philos Soc. 1986; 61: 329–368 [DOI] [PubMed] [Google Scholar]
- 20. Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol. 1992; 262(3 Pt 1):E372–E376 [DOI] [PubMed] [Google Scholar]
- 21. Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998; 275: E73–E78 [DOI] [PubMed] [Google Scholar]
- 22. Vary TC, Lynch CJ. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007; 137: 1835–1843 [DOI] [PubMed] [Google Scholar]
- 23. Goldspink DF, Kelly FJ. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J. 1984; 217: 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rennie MJ, Smith K, Watt PW. Measurement of human tissue protein synthesis: an optimal approach. Am J Physiol. 1994; 266(3 Pt 1):E298–E307 [DOI] [PubMed] [Google Scholar]
- 25. Miller BF. Human muscle protein synthesis after physical activity and feeding. Exerc Sport Sci Rev. 2007; 35: 50–55 [DOI] [PubMed] [Google Scholar]
- 26. Previs SF, Fatica R, Chandramouli V, Alexander JC, Brunengraber H, Landau BR. Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am J Physiol Endocrinol Metab. 2004; 286: E665–E672 [DOI] [PubMed] [Google Scholar]
- 27. Hellerstein MK. New stable isotope-mass spectrometric techniques for measuring fluxes through intact metabolic pathways in mammalian systems: introduction of moving pictures into functional genomics and biochemical phenotyping. Metab Eng. 2004; 6: 85–100 [DOI] [PubMed] [Google Scholar]
- 28. Belloto E, Diraison F, Basset A, Allain G, Abdallah P, Beylot M. Determination of protein replacement rates by deuterated water: validation of underlying assumptions. Am J Physiol Endocrinol Metab. 2007; 292: E1340–E1347 [DOI] [PubMed] [Google Scholar]
- 29. Raman A, Schoeller DA, Subar AF, et al. Water turnover in 458 American adults 40-79 yr of age. Am J Physiol Renal Physiol. 2004; 286: F394–F401 [DOI] [PubMed] [Google Scholar]
- 30. Dufner DA, Bederman IR, Brunengraber DZ, et al. Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am J Physiol Endocrinol Metab. 2005; 288: E1277–E1283 [DOI] [PubMed] [Google Scholar]
- 31. Gasier HG, Riechman SE, Wiggs MP, Previs SF, Fluckey JD. A comparison of 2H2O and phenylalanine flooding dose to investigate muscle protein synthesis with acute exercise in rats. Am J Physiol Endocrinol Metab. 2009; 297: E252–E259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinson MM, Richards JC, Hickey MS, et al. Acute {beta}-adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. Am J Physiol Regul Integr Comp Physiol. 2010; 298: R25–R33 [DOI] [PubMed] [Google Scholar]
- 33. Butz CE, McClelland GB, Brooks GA. MCT1 confirmed in rat striated muscle mitochondria. J Appl Physiol. 2004; 97: 1059–1066 [DOI] [PubMed] [Google Scholar]
- 34. Fanara P, Turner S, Busch R, et al. In vivo measurement of microtubule dynamics using stable isotope labeling with heavy water. Effect of taxanes. J Biol Chem. 2004; 279: 49940–49947 [DOI] [PubMed] [Google Scholar]
- 35. Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol. 1999; 276(6 Pt 1):E1146–E1170 [DOI] [PubMed] [Google Scholar]
- 36. Busch R, Kim YK, Neese RA, et al. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta. 2006; 1760: 730–744 [DOI] [PubMed] [Google Scholar]
- 37. Wilson FA, Suryawan A, Orellana RA, Gazzaneo MC, Nguyen HV, Davis TA. Differential effects of long-term leucine infusion on tissue protein synthesis in neonatal pigs. Amino Acids. 2011; 40: 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller BF, Hamilton KL. A perspective on the determination of mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2012; 302: E496–E499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell. 2012; 11: 150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miller BF, Olesen JL, Hansen M, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol (Lond). 2005; 567(Pt 3):1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith GI, Patterson BW, Mittendorfer B. Human muscle protein turnover–why is it so variable? J Appl Physiol. 2011; 110: 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jaleel A, Short KR, Asmann YW, et al. In vivo measurement of synthesis rate of individual skeletal muscle mitochondrial proteins. Am J Physiol Endocrinol Metab. 2008; 295: E1255–E1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vary TC, Siegel JH, Tall BD, Morris JG, Smith JA. Inhibition of skeletal muscle protein synthesis in septic intra-abdominal abscess. J Trauma. 1988; 28: 981–988 [DOI] [PubMed] [Google Scholar]
- 44. Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980; 192: 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003; 115: 577–590 [DOI] [PubMed] [Google Scholar]
- 46. Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell. 2010; 40: 228–237 [DOI] [PubMed] [Google Scholar]
- 47. Glynn EL, Fry CS, Drummond MJ, et al. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr. 2010; 140: 1970–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaeberlein M, Kennedy BK. Hot topics in aging research: protein translation and TOR signaling, 2010. Aging Cell. 2011; 10: 185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009; 460: 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]