Abstract
Background.
The purpose of this study is to examine whether physical disability is associated with faster rate of decline in cognitive function.
Methods.
A longitudinal population-based cohort of 6,678 initially nondisabled older adults from a biracial urban community was interviewed at 3-year intervals from 1993 to 2012. Cognitive function was assessed using a standardized global cognitive score, and physical disabilities using activities of daily living (ADL) and instrumental activities of daily living (IADL).
Results.
During the follow-up period, 2,450 of 6,678 participants (37%) developed ADL and 2,069 of 4,287 participants (48%) developed IADL disability. After adjusting for demographic and physiologic confounders, cognitive function declined a mean of 0.048 unit per year before ADL disability and 0.047 unit per year before IADL disability. In comparison, the rate of cognitive decline accelerated further by 0.076 unit per year (156% increase) after ADL disability and 0.054 unit per year (115% increase) after IADL disability. Severity of ADL and IADL disabilities were also associated with faster cognitive decline following disability.
Conclusions.
In old age, cognitive function declines substantially faster following physical disability even after controlling for demographic and physiologic characteristics of participants.
Key Words: Cognitive decline, Physical disability, Activities of daily living, Instrumental activities of daily living
COGNITIVE decline in old age is common and is strongly associated with increased risk of cognitive impairment, Alzheimer’s disease, dementia, and death (1–5). With the U.S. population getting older, this problem of decline in cognitive function is expected to increase in future, underscoring the need to identify substrates contributing to cognitive decline and strategies to reduce their impact. Physical disability is one such adverse health condition that may be related to cognitive decline in older persons through neurodegenerative processes. Some studies have shown a relationship between self-reported physical disability and cognitive function (6,7), whereas others do not (8,9). In addition to physical disability, common chronic health conditions, such as diabetes (10,11) and pulmonary disease (12–15), and general indicators of demographic and anthropometric variables, such as education (16–18) and body mass index (19,20) have been shown to be associated with late-life cognitive decline.
The relationship between physical disability and cognitive function is not well established, and understanding this association is likely to require a thorough understanding of the temporal course of change in cognitive function with respect to the temporal change in physical disability. Previous research either used a set cutoff point to define cognitive decline or focused on the relationship between baseline physical disability and cognitive decline. However, physical disability is a dynamic process that can be defined in terms of transitions and progression toward more severe disability. In this article, we focus on one transition defined by the onset of physical disability and severity of physical disability at the time of reported onset.
Many previous studies have been limited in their ability to study temporal change in cognitive function in terms of temporal changes in physical disability due the insufficient longitudinal data. This study examines change in cognitive function before and after onset of disability in older persons using data from the Chicago Health and Aging Project (21) to test the hypothesis that onset of physical disability is associated with an increased rate of cognitive decline. Participants were 6,678 older residents of a geographically defined neighborhood in Chicago. During a mean of 12.6 years of observation of participants not disabled at baseline, cognitive function was assessed 2–6 times at 3-year intervals and their difficulties with activities of daily living (ADL) and instrumental activities of daily living (IADL) were ascertained at the same time. We used mixed-effects change point regression models to test the hypothesis that cognitive decline accelerates after onset of ADL and IADL disability.
Methods
Participants
The Chicago Health and Aging Project (CHAP) is an ongoing, population-based, longitudinal study of Alzheimer’s disease and other common health conditions among adults aged 65 years or older performed from 1993 to 2012. This study was performed in three adjacent neighborhoods on the south side of Chicago. The interviews were conducted in the participants’ homes in approximately 3-year cycles. Study participants who provided data for at least two cycles of follow-up data collection were included in this investigation.
Data Collection and Measures
Baseline data was collected beginning in 1993 using a survey that included questions on demographic variables, socioeconomic variables, physical and mental health, disability measures, and quality of life. The average time from baseline to first follow up was 3.29 (SD = 0.45) years, from baseline to second follow up was 6.37 (SD = 0.56) years, from baseline to third follow up was 9.35 (SD = 0.55) years, from baseline to fourth follow up was 12.35 (SD = 0.58) years, from baseline to fifth follow up was 15.32 (SD = 0.61) years, and from baseline to sixth follow up was 18.24 (SD = 0.59) years. Cognitive function and ADL data were collected at baseline and all subsequent follow ups. However, IADL data collection started at the second follow-up interview.
Cognitive Function
Cognitive function was evaluated using a battery of four tests including two tests of episodic memory (immediate and delayed recall) derived from the East Boston Test (22,23), a test of perceptual speed (the Symbol Digits Modalities Test [24]), and a test of general orientation and global cognition (the Mini-Mental State Examination [25]). Because tests loaded on a single factor that accounted for about 75% of the variance in a factor analysis (26), we constructed a composite measure of global cognitive function based on all four tests. This measure combines variables with different ranges and floor-ceiling effects by averaging the four tests together after centering and scaling each to their baseline mean and standard deviation. Thus, a participant whose composite performance matches the average participant at baseline has a composite cognitive score of 0, and a person who performs one SD better than average on every test has a composite cognitive score of +1.
Physical Disability
This study included two widely used self-reported measures of self-care tasks. The ADL disability measure focused on the ability to perform six essential self-care tasks: bathing, dressing, eating, showering, toileting, and getting out of bed to chair (27). Responses contained three choices: “no help,” “help,” or “unable to do.” If the study participant could perform the activity with “no help,” questions were coded as “0” and the questions with choices of “help” or “unable to do” were coded as “1.” A summary score was computed by adding the scores on the six tasks and it ranged from 0 to 6.
The IADL focused on the ability to perform seven household tasks: using the phone, shopping for groceries, preparing meals, housekeeping, laundering, taking medication, and managing finances (28). Responses contained five choices: “no difficulty at all,” “a little difficult,” “some difficulty,” “a lot of difficulty,” and “just unable to do it.” If the participants could perform the activity with “no difficulty at all,” questions were coded as “0” and all other choices were coded as “1.” A summary score was computing by adding the scores on the seven tasks and it ranged from 0 to 7.
Covariates
The covariates of gender (males or females); race (blacks or nonblacks); education (measured in number of years of schooling completed); and time-varying measures of body mass index (kg/m2), number of comorbid conditions (cancer, heart condition, hypertension, stroke, diabetes, and hip fracture), and depression (Center for Epidemiological Studies-Depressive symptoms, CES-D [29]) were included because they might influence the association between cognitive function and physical disability.
Statistical Analysis
The descriptive measures were computed using mean and standard deviation for continuous variables and percentages for categorical variables. Linear mixed-effects regression models with onset of physical disability as the change point were used to study change in cognitive function (30). Each model allowed the rate of cognitive change to shift after the onset of disability measures. Random effects were included for the intercept and slopes to allow for individual differences in initial level of cognitive function and rates of predisability and postdisability change. Each model also included fixed effects for time before disability (in years since baseline); time after disability; and age, sex, race, education, depressive symptoms, number of medical conditions, body mass index, and their interactions with time before and after onset of disability. We retained only the significant interactions in subsequent models. We ran separate models for ADL and IADL disability measures. In addition to the earlier models, we also looked at the trajectory of cognitive function among incident disability to evaluate whether the patterns of temporal changes were different from our primary analysis. As a secondary question, we investigated whether the onset of ADL disability after the onset of IADL disability affected the rate of cognitive decline. For this purpose, we created two regression models: onset of IADL disability reported before onset of ADL disability and onset of ADL and IADL disabilities reported at the same interview. We used an indicator for each onset of disability and included an interaction with time after onset to study the rate of decline in cognitive function. All the models were fitted using SAS software (31).
Results
At the baseline assessment, cognitive function ranged from −2.68 to 1.73 (mean = 0.36, SD = 0.62). To characterize the change in cognitive function, we used a mixed-effects regression model that allowed the rate of cognitive function to shift following initial disability and controlled for the potentially confounding effects of demographic baseline measures of age, sex, race, education and time-varying measures of body mass index, number of chronic medical conditions, and depressive symptoms. We also performed regression models that accounted for the number of limitations at initial disability to see if the decline was faster depending on the number of limitations reported.
Activities of Daily Living
The analytic sample for the primary ADL analysis consists of participants who reported no ADL disability at baseline. At baseline, the nondisabled sample was significantly younger; more educated, had higher body mass index, and higher cognitive function; and were more likely to be white and male than those excluded due to disability (Table 1). Those in the analytic sample also reported fewer depressive symptoms and chronic health conditions such as hypertension, diabetes mellitus, and stroke. During a mean of 12.6 years of observation, 2,450 of 6,678 persons (37%) reported an onset of ADL disability. The onset of ADL disability occurred at a mean of 7.1 (SD = 4.1) years after first assessment. The mean follow-up time after the onset of ADL disability was 4.6 (SD = 2.4) years.
Table 1.
Baseline Characteristics of Participants in a Random Sample of Population Aged 65 Years and Older
| Characteristics | ADL | IADL | ||||
| Nondisabled | Disabled | p value | Nondisabled | Disabled | p value | |
| N | 6,678 | 1,078 | 4,287 | 2,182 | ||
| Age (y) | 71.7 (5.8) | 78.9 (9.0) | <.001 | 72.5 (6.0) | 79.1 (8.3) | <.001 |
| Education (y) | 12.6 (3.4) | 10.8 (3.7) | <.001 | 13.1 (3.3) | 11.3 (3.5) | <.001 |
| Body mass index (kg/m2) | 28.1 (5.7) | 27.1 (7.6) | <.001 | 28.4 (5.5) | 27.7 (7.3) | <.001 |
| Cognitive function | 0.36 (.62) | −0.71 (1.1) | <.001 | 0.49 (.54) | −0.40 (1.1) | <.001 |
| Mini-Mental State Examination | 27.2 (3.1) | 22.3 (7.4) | <.001 | 27.7 (2.5) | 23.2 (6.7) | <.001 |
| Immediate recall | 8.9 (2.3) | 6.8 (3.4) | <.001 | 9.3 (2.1) | 7.3 (3.3) | <.001 |
| Delayed recall | 8.4 (2.7) | 6.0 (3.7) | <.001 | 8.9 (2.4) | 6.5 (3.8) | <.001 |
| Symbol digit test | 32.1 (13.1) | 18.0 (13.6) | <.001 | 34.4 (12.1) | 21.1 (13.1) | <.001 |
| Gender, % | <.001 | .003 | ||||
| Female | 60.6 | 73.7 | 62.2 | 66.0 | ||
| Male | 39.4 | 26.3 | 37.8 | 34.0 | ||
| Race, % | .01 | .02 | ||||
| Nonblacks | 36.4 | 32.9 | 35.3 | 32.2 | ||
| Blacks | 63.6 | 67.1 | 64.7 | 67.7 | ||
| Depressive symptoms, % | <.001 | <.001 | ||||
| 0–1 | 80.8 | 50.6 | 85.0 | 56.6 | ||
| 2–3 | 7.0 | 12.6 | 5.6 | 12.2 | ||
| 4+ | 12.2 | 36.8 | 9.2 | 31.1 | ||
| Chronic diseases, % | ||||||
| Heart disease | 11.4 | 23.2 | <.001 | 10.5 | 22.7 | <.001 |
| Diabetes mellitus | 6.3 | 13.5 | <.001 | 6.3 | 13.7 | <.001 |
| Stroke | 6.6 | 28.5 | <.001 | 6.6 | 23.2 | <.001 |
Notes: ADL = activities of daily living; IADL = instrumental activities of daily living.
As shown in Table 2, the composite measure of global cognition declined a mean of 0.048 unit per year prior to disability and in those never disabled with ADL. Following ADL disability, the rate accelerated by 0.076 unit per year, a more than 158% increase relative to aging-related cognitive decline. This effect is shown in Figure 1A for a person with ADL disability in year 4 (dashed line) compared with a person not disabled (solid line).
Table 2.
Annual Rate of Change in Global Cognitive Function After Activities of Daily Living (ADL) Disability Measure
| Model Term | Model 1† | Model 2‡ |
| Estimate (SE) | Estimate (SE) | |
| Time since baseline | −0.048 (.002)§ | −0.048 (.002)§ |
| Time since baseline × age | −0.003 (.001)§ | −0.003 (.001)§ |
| Time since disability | −0.076 (.008)§ | −0.038 (.007)§ |
| Time since disability × age | −0.006 (.001)§ | |
| Time since disability × male | −0.021 (.008)|| | −0.020 (.008)¶ |
| Time since disability × limitations | −0.038 (.005)§ | |
| Time since disability × limitations × age | −0.002 (.0005)§ | |
| Time since disability × limitations × black | 0.019 (.006)|| | |
| Age | −0.026 (.002)§ | −0.026 (.002)§ |
| Male | −0.115 (.024)§ | −0.116 (.024)§ |
| Black | −0.347 (.028)§ | −0.344 (.028)§ |
| Education | 0.069 (.003)§ | 0.069 (.003)§ |
| Depression | −0.009 (.005)§ | −0.009 (.005)§ |
| Body mass index | 0.013 (.002)§ | 0.013 (.002)§ |
| Comorbid conditions | −0.039 (.009)§ | −0.037 (.009)§ |
| Intercept | 0.272 (.032)§ | 0.272 (.032)§ |
Notes: †Association between rate of cognitive decline and time since disability.
‡Association between rate of cognitive decline, time since disability, and number of limitations.
§ p < .001, || p < .01, ¶ p < .05.
Figure 1.
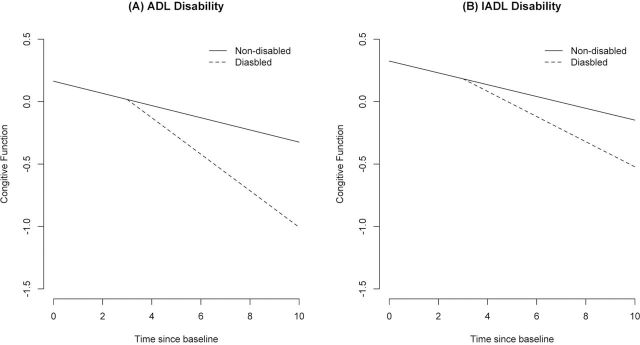
Change in cognitive function among nondisabled and those disabled at fourth year of observation. (A) Activities of daily living (ADL) disability. (B) Instrumental activities of daily living (IADL) disability.
Older age was associated with lower level of cognition at baseline and more rapid decline before and after ADL disability. In addition, males had a more rapid decline after ADL disability than females. Following ADL disability, the rate accelerated by 0.021 unit per year for males relative to females. We also found that depressive symptoms were associated with decline in cognitive function before ADL disability but did not change the rate of decline in cognitive function after disability.
A larger number of limitations reported at the time of onset of ADL disability were associated with a more rapid decline in cognitive function. At the time of ADL disability, the rate accelerated by 0.038 unit per year for each limitation. This effect is shown in Figure 2A for a person with no limitation (solid line), one limitation (10th percentile, dashed line), and three limitations (90th percentile, dotted line). Number of limitations accelerated decline more among black participants than white participants. Number of limitations also accelerated decline more at older ages than younger ages.
Figure 2.
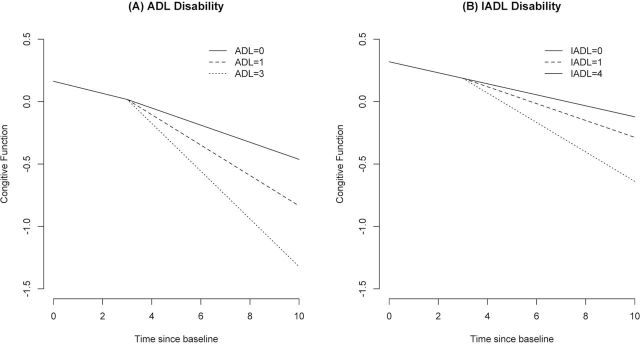
Change in cognitive function among those with no limitations and those with disability at fourth year of observation with onset in 10th percentile (activities of daily living [ADL] = 1 and instrumental activities of daily living [IADL] = 1) and 90th percentile (ADL = 3, IADL = 4). (A) ADL disability. (B) IADL disability.
As part of our secondary analyses, we looked at the rate of decline in cognitive function only among those who reported onset of ADL disability. The composite measure of global cognition declined a mean of 0.065 unit per year prior to disability. Following ADL disability, the rate accelerated by 0.058 unit per year, a more than 89% increase relative to decline before onset. Older age and males were still associated with more rapid decline in cognitive function. At the time of ADL disability, the rate accelerated by 0.017 unit per year for each additional limitation (data not shown).
Instrumental Activities of Daily Living
The analytic sample for IADL consisted of those with no IADL disability at the second follow-up interview. The onset of IADL disability occurred at 4.8 (SD = 2.6) years after first assessment, respectively. The average follow-up time was 3.8 (SD = 2.6) years after the reported onset of IADL disability. Those who developed disability were older and less educated and had lower baseline levels of cognitive function and depression than those who did not develop any disability (data not shown).
As shown in Table 3, the composite measure of global cognition declined a mean of 0.047 unit per year prior to disability and in those never disabled with IADL. Following IADL disability, the rate accelerated by 0.054 unit per year, a more than 115% increase relative to decline prior to IADL disability. This effect is shown in Figure 1B for a person with IADL disability in year 4 (dashed line) compared with a person not disabled (solid line).
Table 3.
Annual Rate of Change in Global Cognitive Function After Instrumental Activities of Daily Living (IADL) Disability Measure
| Model Term | Model 1† | Model 2‡ |
| Estimate (SE) | Estimate (SE) | |
| Time since baseline | −0.047 (.003)§ | −0.044 (.003)§ |
| Time since baseline × age | −0.003 (.0004)§ | −0.003 (.0004)|| |
| Time since disability | −0.054 (.006)§ | −0.027 (.007)§ |
| Time since disability × age | −0.002 (.001)¶ | −0.002 (.001)¶ |
| Time since disability × limitations | −0.017 (.003))§ | |
| Time since disability × limitations × black | 0.010 (.004)|| | |
| Age | −0.047 (.003)§ | −0.020 (.002)§ |
| Male | −0.091 (.014)§ | −0.091 (.014)§ |
| Black | −0.312 (.016)§ | −0.307 (.016)§ |
| Education | 0.051 (.002)§ | 0.050 (.002)§ |
| Depression | −0.021 (.004)§ | −0.020 (.004)§ |
| Body mass index | 0.007 (.001)§ | .007 (.001)§ |
| Comorbid conditions | −0.017 (.006)§ | −0.016 (.007)§ |
| Intercept | 0.416 (.038)§ | 0.411 (.038)§ |
†Association between rate of cognitive decline and time since disability.
‡Association between rate of cognitive decline, time since disability, and number of limitations.
§ p < .001, || p < .01, and ¶ p < .05.
Older age was associated with lower level of cognition at baseline and more rapid decline before and after IADL disability. Sex, education, race, medical conditions, depressive symptoms, and body mass index were related to level of cognitive function but not to change before or after IADL disability (data not shown). The number of limitations reported at the time of onset of IADL disability was associated with faster decline in cognitive function. At the time of IADL disability, the rate accelerated by 0.017 unit per year for each limitation. This effect is shown in Figure 2B for a person with no limitation (solid line), one limitation (10th percentile, dashed line), and four limitations (90th percentile, dotted line). In addition, number of limitations accelerated decline more among black participants than white participants.
We also looked at the rate of decline in cognitive function only among those who reported onset of IADL disability. The composite measure of global cognition declined a mean of 0.048 unit per year prior to disability. Following IADL disability, the rate accelerated by 0.034 unit per year, a more than 89% increase relative to aging-related cognitive decline. Older age and male gender were still associated with more rapid decline in cognitive function. The rate accelerated by 0.017 unit per year for each additional limitation at the time of onset (data not shown).
ADL Disability Following IADL Disability
In this sample, 552 (12.5%) participants reported IADL disability before ADL disability and 485 (10.9%) reported both ADL and IADL at the same interview. The rate of cognitive decline among participants who reported IADL before or at the same interview as ADL was 0.040 unit per year. Following IADL disability, the rate accelerated by 0.040 unit per year, about 100% increase relative to decline before onset. This rate of cognitive decline accelerated further by 0.023 following ADL disability, about 58% increase relative to decline before IADL disability. In addition, when participants reported ADL and IADL disability at the same time, the rate of cognitive function accelerated by 0.061, which turns out to be 153% increase in the rate of cognitive decline. Also, the sum of the rates of decline from the two previous models adds up to the combined model. This might lead us to believe that ADL and IADL independently increase the rate of cognitive decline in older persons.
Discussion
As part of a longitudinal population-based study, change in cognitive function was assessed in older people before and after onset of disability. During a mean of more than 9 years of observation, 37% of the participants developed at least one ADL limitation and 48% developed IADL limitations. The cognitive function declined by 158% after onset of ADL disability and 115% after IADL disability onset. These findings persisted after controlling for demographic variables, chronic health conditions, and depression. The results support the hypothesis that disability in old age is associated with accelerated cognitive decline.
Onset of ADL and IADL disabilities represent the impact of age-related comorbid conditions that can affect a person’s ability to maintain an independent lifestyle. The ability of older persons to function independently depends on their ability to perform certain physical and cognitive functions. Basic and instrumental ADLs have a significant cognitive component in addition to a physical component that might be a strong predictor of future cognitive decline. Therefore, it is possible that basic and instrumental ADLs act as a substrate that accelerates cognitive decline from the existence of neurodegenerative processes. Therefore, understanding the relationship between physical and cognitive function can provide us with better mechanisms for preventing cognitive decline in older adults.
The association between self-reported disability and cognitive decline has not been extensively investigated. One population-based study of 365 older persons found that physical disability was associated with cognitive decline after a 2-year follow up (6), consistent with the results of this study. Yet decline was estimated from two measurement points, making it difficult to distinguish from level of function or to know whether it preceded or followed disability. Another population-based study found that physical disability was not associated with future cognitive decline (9). However, their study did not use a global standardized cognitive function and censored older persons who performed poorly and were susceptible to floor effects. Also, their power to detect an association between physical disability and cognitive decline was limited possibly due to insufficient postdisability data and inclusions of prevalent cases with incident cases. Therefore, we believe that these studies may not have been sufficient to securely estimate the relation of physical disability to cognitive decline.
In essence, the findings of this research result from the strengths of the study design. Participants were drawn from a geographically defined population making it likely that a broad spectrum of disability and paths of cognitive change were represented and that results were generalizable. Cognition was assessed with four well-known scales and measured on three–six occasions with good participation in follow up. This made it possible to precisely estimate person-specific rate of change in cognitive function related to onset of disability.
Limitations
The main limitation is the length of time between cognitive tests. A 3-year interval in cognitive tests limited our ability to track short-term changes in cognition around the time of physical disability. Also, participants had to survive at least 6 years from the first assessment to be included in analyses. In terms of physical disability, we only looked at two aspects, namely, onset of physical disability and number of physical disabilities at onset. However, other aspects of physical disability such as faster progression or recovery from disability can also impact the rate of cognitive decline in older adults. These factors may have led us to underestimate the rate of change in cognitive decline associated with physical disability.
Conclusions
In conclusion, we found that onset of physical disability and number of physical disabilities at onset was associated with rate of decline in cognitive function. The trajectories of cognitive decline before and after physical disability showed significant differences in older persons. The findings of this research suggest that physical disability is an important risk factor that affects the future cognitive decline in older persons. Therefore, minimizing the risk of physical disability may also minimize the risk of cognitive decline in older age.
Funding
This work was supported by grants from the National Institutes for Health (R01-AG-11101 and R01-AG-09966).
References
- 1. Anstey KJ, Mack HA, von Sanden C. The relationship between cognition and mortality in patients with stroke, coronary heart disease or cancer. European Psychologist. 2006; 11: 182–195 [Google Scholar]
- 2. Berg S. Aging, behavior, and terminal decline. In: Birren JE, Schaie KW, eds. Handbook of the Psychology of Aging. 4th ed. New York: Academic Press; 1996; 323–337 [Google Scholar]
- 3. Dixon RA, Garrett DD, Lentz TL, MacDonald SW, Strauss E, Hultsch DF. Neurocognitive markers of cognitive impairment: exploring the roles of speed and inconsistency. Neuropsychology. 2007; 21: 381–399 [DOI] [PubMed] [Google Scholar]
- 4. Dixon RA, de Frias CM. The Victoria Longitudinal Study: from characterizing cognitive aging to illustrating changes in memory compensation. Aging Neuropsych Cog. 2004; 11: 346–376 [Google Scholar]
- 5. MacDonald SW, Hultsch DF, Dixon RA. Aging and the shape of cognitive change before death: terminal decline or terminal drop? J Gerontol B Psychol Sci Soc Sci. 2011; 66: 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Black SA, Rush RD. Cognitive and functional decline in adults aged 75 and older. J Am Geriatr Soc. 2002; 50: 1978–1986 [DOI] [PubMed] [Google Scholar]
- 7. Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. J Am Geriatr Soc. 2005; 53: 1966–1972 [DOI] [PubMed] [Google Scholar]
- 8. Payette H, Gueye NR, Gaudreau P, Morais JA, Shatenstein B, Gray-Donald K. Trajectories of physical function decline and psychological functioning: the Quebec longitudinal study on nutrition and successful aging (NuAge). J Gerontol B Psychol Sci Soc Sci. 2011; 66 Suppl 1: i82–i90 [DOI] [PubMed] [Google Scholar]
- 9. Chodosh J, Miller-Martinez D, Aneshensel CS, Wight RG, Karlamangla AS. Depressive symptoms, chronic diseases, and physical disabilities as predictors of cognitive functioning trajectories in older Americans. J Am Geriatr Soc. 2010; 58: 2350–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregg EW, Yaffe K, Cauley JA, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. 2000; 160: 174–180 [DOI] [PubMed] [Google Scholar]
- 11. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004; 61: 661–666 [DOI] [PubMed] [Google Scholar]
- 12. Grant I, Heaton RK, McSweeny AJ, Adams KM, Timms RM. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med. 1982; 142: 1470–1476 [PubMed] [Google Scholar]
- 13. Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009; 180: 134–137 [DOI] [PubMed] [Google Scholar]
- 14. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007; 69: 483–489 [DOI] [PubMed] [Google Scholar]
- 15. Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008; 56: 1845–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yaffe K, Fiocco AJ, Lindquist K, et al. ; Health ABC Study Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009; 72: 2029–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tervo S, Kivipelto M, Hänninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004; 17: 196–203 [DOI] [PubMed] [Google Scholar]
- 18. Koster A, Penninx BW, Bosma H, et al. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann Epidemiol. 2005; 15: 564–571 [DOI] [PubMed] [Google Scholar]
- 19. Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005; 65: 892–897 [DOI] [PubMed] [Google Scholar]
- 20. Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008; 70: 360–367 [DOI] [PubMed] [Google Scholar]
- 21. Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003; 5: 349–355 [DOI] [PubMed] [Google Scholar]
- 22. Albert M, Smith LA, Scherr PA, et al. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991; 57: 167–178 [DOI] [PubMed] [Google Scholar]
- 23. Wilson RS, Bennette DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002; 59: 1910–1914 [DOI] [PubMed] [Google Scholar]
- 24. Smith A. Symbol Digits Modalities Test, Los Angeles:Western Psychological Services;1982 [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189–198 [DOI] [PubMed] [Google Scholar]
- 26. Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999; 54: P155–P160 [DOI] [PubMed] [Google Scholar]
- 27. Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976; 6: 493–508 [DOI] [PubMed] [Google Scholar]
- 28. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969; 9: 179–186 [PubMed] [Google Scholar]
- 29. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993; 5: 179–193 [DOI] [PubMed] [Google Scholar]
- 30. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982; 38: 963–974 [PubMed] [Google Scholar]
- 31. SAS Institute Inc SAS 9.3 Software. Cary, NC: SAS Institute Inc.; 2012. [Google Scholar]


